Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Bianca Galateanu.
Organ-on-chips (OOCs) are microfluidic devices used for creating physiological organ biomimetic systems. OOC technology brings numerous advantages in the current landscape of preclinical models, capable of recapitulating the multicellular assemblage, tissue–tissue interaction, and replicating numerous human pathologies.
- organ-on-chip
- tumor-on-chip
- polymeric microfluidic devices
1. Introduction
Regardless of the therapeutic area of new emerging therapies and novel agents entering the market, nephrotoxicity is a major challenge, as the kidney is the second target of drugs and chemicals after the liver. More than 25% of the adverse effects in today’s pharmacotherapy are caused by nephrotoxic effects [1]. Of these, 20% are reported during postmarket surveillance [2], as early stages of drug development fail to deliver relevant output with this respect [3]. Consequently, the poor correlation between the preclinical and clinical outcomes has led to the failure of most drugs before reaching the patient [4]. Preclinically approved drugs have been withdrawn a few times due to the side effects observed in the clinical trials [5,6][5][6]. Therefore, the pharmaceutical industry is under high pressure to speed up the drug-development process and to design new cures that are very effective in humans with reasonable costs [7].
The traditional in vivo tests on animal models are costly and often fail to accurately predict the efficiency and toxicity in humans due to the species’ different metabolic responses to specific agents and the variations in some genes’ expressions, such as cytochrome P450 genes [8]. Consequently, the poor similarity between the physiological environment in animals and human bodies, which may alter the results of drug efficiency in various diseases, is a major barrier for future use of in vivo tests on animals [9]. With respect to cancer research, animal models in particular lack predictability [10] since they do not recreate the exact human tumor microenvironment (TME) and may exhibit different cell biology and cancer behavior when tumorous cells interact locally with stromal cells. In addition, the ethics concerns of sacrificing animals are a significant barrier in testing many discovered drugs on animals [11,12][11][12]. In 2021, the European Parliament agreed with a large majority to ban experiments on animals, which have killed about 12 million animals in 2017, revealing the importance of finding alternatives for biological assays developed with other procedures than sacrificing animals [13].
The main difficulties in cancer research are forming an effective in vitro TME able to accurately recapitulate the local tissue in which the tumor is forming [16][14]. Conventional preclinical in vitro models for anticancer drug screening are generally classified in 2D cell cultures and 3D cell architectures and have been extensively exploited as simple and cost-efficient methods to simulate cancer propagation and drug response [17][15]. The 3D cancer models deliver a helpful substitute to animals, but they still do not consider the dynamic environment of the human tissues or organs. However, they do not reproduce the complex assemblage of the human 3D cells from living organs to properly elucidate the cancer cell migration and invasion, also taking into consideration the mechanical forces (such as hydrostatic pressure, fluid shear stress, breathing motions in lung) naturally occurring in human bodies. Nonetheless, neither of these systems is transporting a blood or nutrient-rich medium through an endothelium-lined vasculature, limiting the real prediction of tissue–tissue interactions and circulating immune cells during therapeutic drug dosage [18,19][16][17].
Recently, new devices known as organ-on-chips (OOCs), which are able to recapitulate the multicellular assemblage, tissue–tissue interactions, and to replicate human pathologies and the appropriate physical TME, have emerged as a practical cost-efficient solution for tumor-growth investigation and anticancer-drug screening by combining the microfluidic technology with 3D cell-culture procedure to simulate the entanglement of the cells as in their native environment [19,22,23][17][18][19]. OOCs are compact and easy-to-use microphysiological functional units that recapitulate the native function and the mechanical strain that the cells experience in the human bodies, allowing the development of a wide range of applications such as disease modeling or even the development of diagnostic devices. However, important features of the membranes involved in the fabrication of OOC compartments to allow cells’ structural support and nutrient transportation are often poorly investigated. Nowadays, both synthetic and natural polymers are explored for the manufacturing process of advanced OOC microdevices, being able to replicate various organ bionic pathophysiological models. Poly(dimethylsiloxane) is one of the most employed synthetic polymers used for lung, liver, heart, and multi-organ-on-chip (MOOC) membranes in microfluidic devices due to its extraordinary high transparency and flexibility. However, it is not a degradable material able to contribute to the formation of the natural extracellular matrix (ECM). Alternative biopolymers with higher biocompatibility, such as collagen-based materials containing cell-growth factors and hormones, have been used for OOC fabrication to simulate the physiological behavior of living organs. Despite significant advances, many polymeric materials still do not meet the mechanical properties of the in vivo organs and do not exhibit optimal cytocompatibility suitable for accurate pharmaceutical screening or dynamic simulation of cancer cell behavior.
2. Fabrication of Organ-on-Chip
2.1. Principle and Manufacture of Organ-on-Chips
Microfluidic technologies have rapidly developed in the past years in terms of fabrication methodology, materials involved, and complexity of the systems to faithfully respond to the medical requirements. OOCs are microfluidic cell-culture micromachines that can recapitulate an organ-level response to medical treatment and reconstruct physiological dynamics observed in native human tissues, for instance, physiological flow, biomechanical motions, nutrient transportation, and drug delivery [22][18], in a convenient manner, delivering a platform with a new opportunity for oncology research. As biology-inspired engineered microdevices [24][20], the OOCs for tumor investigation must enable a series of possibilities: (i) the introduction of pharmaceutical compounds or reagents as fluids with the similar dynamic flow as for biological fluids; (ii) the ability to perfuse these fluids around on the chip, and to combine and mix them; (iii) the introduction of other sensors or devices for monitoring the results, such as detectors for bioanalysis. By incorporating tumor organoids in microfluidic devices, “tumor-on-chip” (TOCs) models that allow the reconstruction of the TME are created. These chips enable a deeper understanding of the tumor mechanism in vivo, which runs to enhanced preclinical evaluation of drug efficiency. Human solid tumors are highly heterogeneous [16][14], owning a complex microenvironment with a dense ECM, abnormal vessels, various stromal cells, or different immune-type cells [22][18]. Additionally, the nearby stromal tissues of the tumor act as an active source (and reservoir) of different cytokines and growth factors that affect the tumor development and pharmacological feedback. Several studies have shown that the complex variety of the cellular microenvironment may impact in some respect the tumor behavior, including tumorigenesis, angiogenesis, tumor invasion, metastasis, and endurance to therapeutic products. Many compounds generated by tumorous or stromal cells determine the propagation dynamics of solid tumors. Moreover, the 3D nature and the size of the tumor proved to be an utmost concern in the proper understanding of tumor dynamics showing a direct connection between the size of a tumor and its aggressiveness and the ability of a drug to be delivered to it [25,26,27][21][22][23]. Consequently, cancer is a true suite of complex pathologies that share some common elements and presents few differences that seriously complicate the choice of satisfactory treatment. Unlike in vivo tumor-growth microenvironments, in vitro cancer models are typically investigated under atmospheric conditions not specific to the living organs. In an in vitro metastasis cell culture, migrated tumor cells have to be subjected to varying microenvironments and oxygen gradients to mimic the activity of in vivo intravasation [28][24]. Tumor chips combine micromachining and cell biology to manipulate the external conditions and precisely mimic physiological environments, such as dynamic mechanical stress, fluid shear, oxygen, and drug concentration gradients and cell patterning to reflect the full picture of tumor formation and growth mechanism [29,30][25][26]. As TOCs resulted from the need to investigate appropriately cancerous cells in their specific TME, the fabrication technology is the same as for conventional OOCs, whereas the healthy organ cells were replaced by diseased cells. The microdevices are named chips since they were originally engineered using micromanufacturing techniques used in computer-microchip production [20][27]. Microfluidic systems can be employed to form tumor chips with a single-line channel by engaging cells from a single source, or more complex organ chips that associate two or more tissue categories that can be interposed right through a porous ECM-coated membrane or an ECM gel that fills one or more micronetworks [31][28]. The viability of the cells can be preserved over prolonged time intervals (up to several months) by perfusing the culture medium either across the endothelium-lined vascular microchannels, parenchymal microchannels, or both. More importantly, the culture medium can be substituted by blood for several hours through endothelialized vascular channels [32][29]. There are two steps required to be taken into consideration for the proper design of a tumor chip: (1) to comprehend the fundamentals essential for the physiological function of the aimed organ, and afterward to establish the key factors such as various cell types, structures, and the organ’s particular physiological microenvironment; (2) to construct a cell-culture device relying on the identified features. Different procedures have been embraced to build tumor-chip kits, among which the most extensively engaged are photolithography and soft lithography [33[30][31],34], replica molding [35][32], microcontact printing, and bioprinting techniques [36,37,38][33][34][35].2.1.1. Lithography
Lithography represents an etching process applied in microfabrication to project parts on a thin layer or the form of a substrate (also named a wafer) and is commonly divided into three categories: photolithography (or UV lithography), soft lithography, and replica molding. Photolithography employs light to transfer a geometric shape from a photomask to a photosensitive chemical photoresist on the wafer. In the first step, masks are required that correspond to the targeted constructions [22,34][18][31]. The manufacturing process continues with the deposition of a spin-coated photoresist layer on a wafer that can be corroded by chemical reagents, for instance, silicon, glass, or quartz, and the photoresist is subjected to UV photopolymerization. Afterward, the pattern is moved to the wafer and is etched to achieve a microfluidic chip with microflow channels. Although extensively employed, the lithography-manufacturing processes are costly due to the cleanroom requirements, the necessity of multiple masks, and time-consuming multiple processing steps. Recently, Kasi and coworkers proposed a rapid-prototyping organ-chip device using maskless photolithography [39][36]. The reseauthorchers reported a simplified method that describes a rapid and cleanroom-free microfabrication compatible with soft lithography for fast-prototyping organ-chip devices in a maximum of 8 h. Soft lithography used for tumor-chip microfabrication involves in the first stage the preparation of a microchannel mold on a silicon substrate by photolithography [40][37], followed by the use of a liquid polymer (commonly polydimethylsiloxane, PDMS) to discharge the mold to acquire an optically transparent elastomeric stamp with microstructures. In the end, different complex 3D microchannels are achieved on various polymer wafers by transferring the pattern from the stamp. Ferreira et al. have recently developed a fast alternative to soft lithography to manufacture OOCs based on PDMS with integrated microactuators. The novel protocol decreases the complexity and number of steps, and is more time and cost-efficient compared to complex multilayered microfluidic devices [41][38]. Replica molding represents the technology in which a patterned silicon mold is employed, followed by the pouring step of a liquid polymer (usually PDMS) onto the mold for thermal crosslinking [42][39]. Later, the PDMS instrument is removed from the substrate and fixed on a clean, smooth wafer (for instance glass) to achieve a microfluidic chip with microfluidic networks. The microcontact-printing technology is very similar to the replica-molding technique [36][33]. It is differentiating only by the supplementary steps further used to manipulate the pattern of cultured cells by printing the PDMS stamp on the wafer with biomolecules (such as proteins) in a designed pattern so that the cells on the membrane can be modeled as well by adjusting the pattern of the printed proteins [43,44][40][41]. However, even though microfluidic devices have been successfully fabricated by lithography or related techniques, the procedures are still costly and time-consuming. Additionally, these procedures are able only to produce the microchip itself, while other components such as microtissues, mechanical stimuli, or result detectors need to be separately produced. Recent advances in lithography-based fabrication techniques have emerged in high-throughput technologies with a standardized format, being more user-friendly and affordable for large preclinical research studies. The OrganoPlate system (Mimetas, Leiden, The Netherlands) is a commercial compact microfluidic device that can process 96 independent cultures using a standard microtiter plate. The microdevice replaces the inner membrane with a gel-media boundary for transepithelial transportation showing good results for the investigation of fluidic diffusion, tumor-cell invasion, and aggregation and toxicity assays [2]. The main technologies used for the microfabrication of OOCs are shown in Figure 1.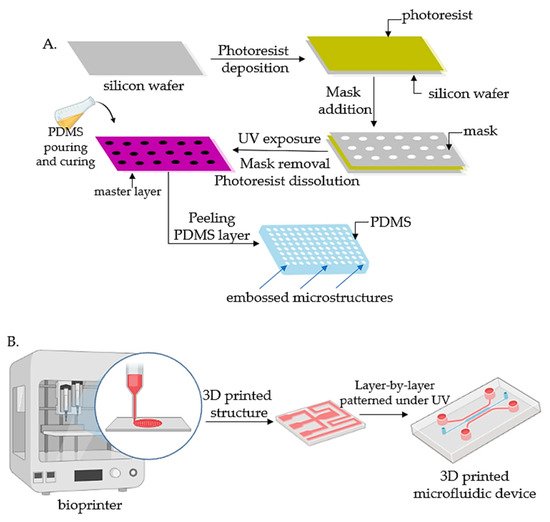
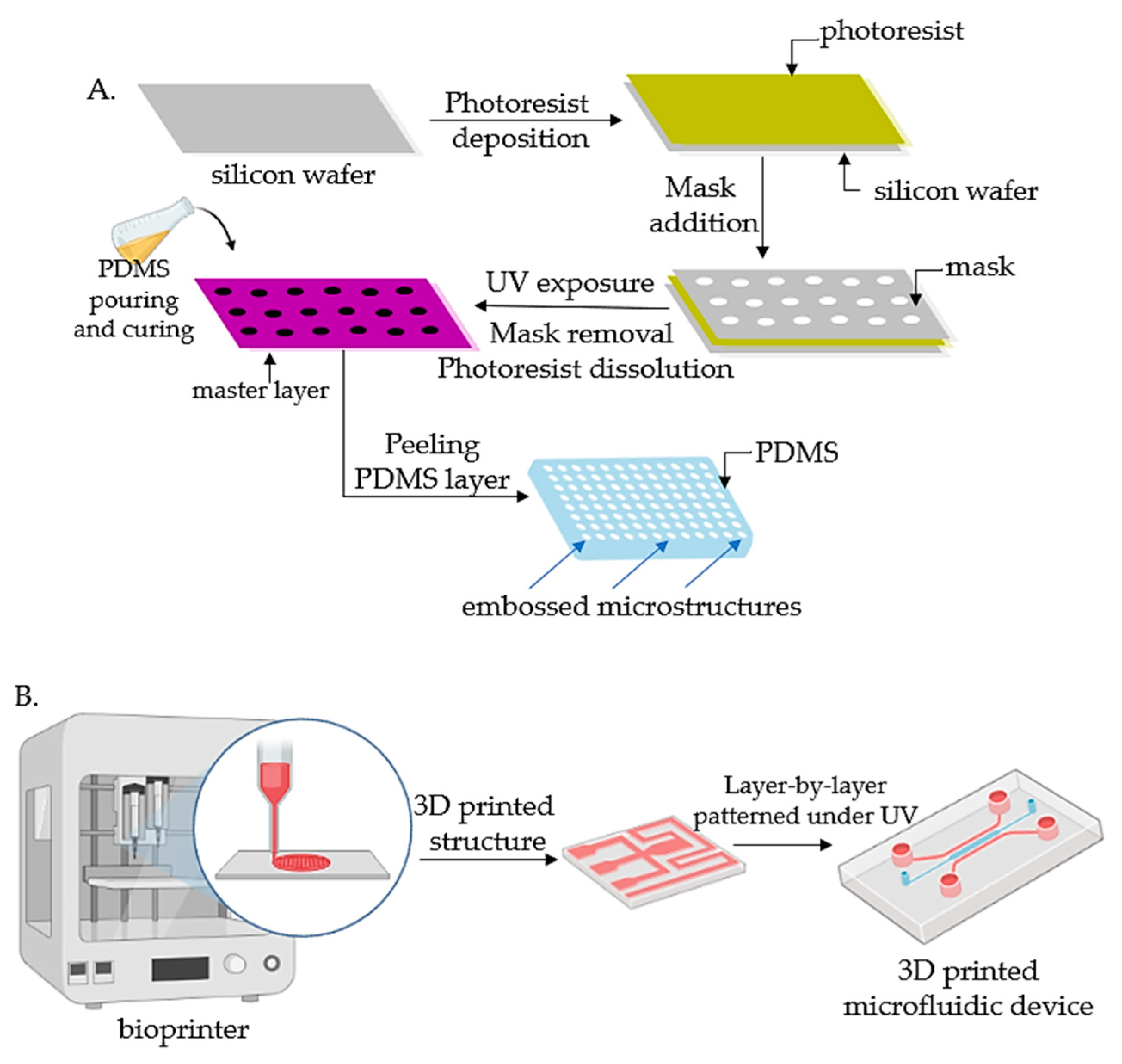 Figure 1. Fabrication technologies for OOCs: (A) The fabrication of micropatterned slabs of PDMS through photolithography; (B) Schematic 3D-printing process for the fabrication of microfluidic devices.
Figure 1. Fabrication technologies for OOCs: (A) The fabrication of micropatterned slabs of PDMS through photolithography; (B) Schematic 3D-printing process for the fabrication of microfluidic devices.
2.1.2. Bioprinting
Bioprinting technology has emerged in the last years as a versatile tool based on layer-by-layer addition that can be easily used for tumor-chip manufacturing [48,49,50][45][46][47]. A significant advantage is related to a wider range of materials and cells that can be printed simultaneously onto a substrate of cell-compatible biomaterials to construct 3D composite blocks with good spatial resolution and reproducibility [51,52][48][49]. Bioprinting also comprises a large group of procedures that can be divided into fused-deposition modeling (FDM), stereolithography, inkjet printing, and laser-assisted bioprinting [53][50]. Bioprinting procedures are appealing due to numerous advantages that accommodate the tumor-chip fabrication. Bioprinting technology can reconstruct the diverse TME and the 3D nature of the tumor by using bioinks that form cell conglomerations that can include various cell types, such as tumor-related fibroblasts, immune cells, and endothelial cells, to create vascular systems [54][51]. In addition, layer-by-layer bioprinting enables the formation of the biomimetic microenvironment for a heterogenous supply of biologically important proteins and growth factors that are essential to managing cancer-cell signaling, growth, and invasion [55][52]. Furthermore, the bioprinting technique allows for the printing of cells quickly and precisely in microfluidic chips, pattern vasculature, and model biological barriers. Vascularization channels play a significant role as they are vital to preserve tissue activities or to differentiate tissue parts. The tumor vasculature is much distinct from the blood vessels of healthy tissues, showing alterations in heterogeneity, multidirectional blood flow, permeability, and unordered distribution all over the diseased cells [56][53]. Proper vascularization of specific cancer-type cells has constantly been questioned in the production of functional in vitro tumor-cell cultures. Bioprinting technology manages to overcome these difficulties, showing the ability to replicate the abnormalities encountered in tumor tissue by building miniaturized pathophysiological models and offering control of features at the same size scale of living cells [57,58,59][54][55][56]. The main polymers used as bioinks for OOCs by bioprinting are summarized in Table 1.Table 1.
[68].
Additionally, the hydrophilicity of the polymeric membrane represents an important factor in determining the protein adsorption and, therefore, cell adhesion. It was observed that the hydrophobicity of the polymeric membrane could cause the absorption of small nonspecific molecules such as drugs or biomolecules from the cellular media, misleading the experimental results [72][69]. For this reason, most synthetic polymers have been submitted to chemical or physicochemical surface modifications to increase the hydrophilicity of the materials by enhancing their wettability properties. PMMA structures have been modified with different hydrophilic functionalities such as aminated polyethyleneglycol [73][70] or submitted to oxygen plasma treatment for the activation of the PMMA surface [74][71] to control the cell adhesion. The cellular adhesion is strongly determined by the surface wettability and roughness of the polymer, while the cell attachment is influenced by the cell type [75][72]. Premnath and colleagues [76][73] developed a simple approach to laser-modify the surface of a silicon chip to adjust cell adhesion and proliferation, and showed that the cervical cancer cells’ behavior is modulated to migrate onto untreated sites. Transparent polyethersulfone (PES) membranes have proven to be with optimized morphology, and have showed improved adhesion and cell viability compared to the commercial hydrophilic polytetrafluoroethylene (PTFE) [77][74]. Moreover, the increase in hydrophilicity of the polymeric layers enhanced the cell adhesion as well as the cell-adhesive proteins. It is worth mentioning that hydrophilicity/hydrophobicity balance can also control the essential nutrient diffusion through the polymeric membrane from the OOCs compartments and generally, water contact-angle measurements are performed to determine the wettability properties of the polymers (Table 2).
The main polymers used as bioinks for OOCs by bioprinting.
| Bioink Composition | Bioprinting Method | OOC Model | References | |
|---|---|---|---|---|
| Collagen | extrusion | Lung, gut | [60,61] | [57][58] |
Table 2.
As the surface properties such as surface roughness and wettability influence the first stages of cell adhesion and migration, the mechanical properties of the polymeric membrane are modulated by the material’s stiffness and influence the later stages of cell growth. Investigations such as tensile strength, compressive stress, and wettability are generally achieved to determine if the polymer properties are suitable for the application. Thus, the mechanical performances of the interface membrane from the OOCs should be chosen accordingly to the native organ characteristics and to mimic the occurring physiological stimuli present in the replicated environment. Moreover, the porosity of the membrane layer represents an essential feature as it provides cell communication between the chip compartments and allows nutrient transportation to the cells. Pore size and shape also determine the space available for the cells to migrate, and small pores mainly reduce the cell adhesion, but increase the barrier function of cells.
The main polymers employed in the fabrication of membranes in OOCs microdevices.
| Polymer | Chemical Structure | Contact Angle with Water | Young’s Modulus | Application | |||||
|---|---|---|---|---|---|---|---|---|---|
| Polydimethylsiloxane (PDMS) | 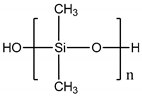 |
107° [78] | 107° [75] | Variable from kPa to MPa | Cardiovascular [79] Kidney [80] Liver [81] Lung [82] | Cardiovascular [76] Kidney [77] Liver [78] Lung [79] |
|||
| Gelatin | extrusion | kidney | |||||||
| Poly (bisphenol-A- carbonate) (PC) | 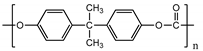 |
~85° [83] | ~85° [80] | [62,63] | [59][60] | ||||
| ~1.2 GPa [ | 84 | ] | ~1.2 GPa [81] | Tumor vasculature [85] Colon and breast cancer [86] | Tumor vasculature [82] Colon and breast cancer [83] |
Methacrylate gelatin (GelMa) | extrusion | Vessel, liver | |
| Poly (ethylene terephthalate) (PET) | 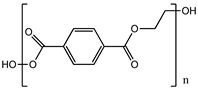 |
83° [87] | 83° [84] | [64,65] | [61][62 | 4.7 GPa [88 | ] | ||
| ] | 4.7 GPa | [ | 85] | Gut [89] Kidney [90] Liver [91] | Gut [86] Kidney [87] Liver [88] |
Alginate | extrusion | Heart | [66, |
| Polylactic acid (PLA) | 67 | ] | 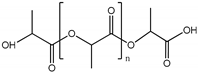 |
~75° [92] | ~75° [89][63] | 3.1 GPa [93] | 3.1 GPa [90][64] | ||
| Endothelial barrier [ | 94 | ] | Endothelial barrier | [91] | Cellulose | extrusion | tumor, liver | ||
| Poly (ε-caprolactone) (PCL) | 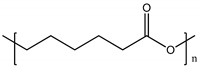 |
120° [95] | 120° [92] | [68,69] | [65] | ~400 MPa [96] | ~400 MPa [[66] | ||
| Polyethyleneglycol (PEG) Poly ε caprolactone (PCL) |
inkjet, extrusion | Colon tumor | [59,65] | [56][62] |
2.2. Material Requirements for the Fabrication of Organ-on-Chips
Many biomaterials and fabrication methods have been recently developed to meet the OOCs’ functions. While designing the OOC microdevice, the organ or tissue characteristics and behavior must be considered to accurately simulate the in vivo motions, cell proliferation, or drug responses. Considering the organ characteristics aimed to be mimicked, different types of OOCs can be created. However, although each type of OOCs may function differently, the main components remain the same. The OOC system is generally equipped with two compartments: first, the blood vessel compartment, which contains the endothelial cells; and secondly, the organ compartment, in which the cells of the investigated organ are introduced. These compartments are usually separated by a porous polymeric membrane able to provide cell communication between the two compartments of the device. Thus, the polymeric membranes play an essential role in the successful functioning of the OOC system, acting as interfaces with selective permeability for cell adhesion and cell separation. The culture medium included in OOCs system may be artificially produced or naturally derived from living organs. The natural medium mainly contains biological fluids such as blood, plasma, etc., and organ fragments. The artificial environment is fabricated by involving nutrients that mimic the physiological conditions, such as salts, oxygen and CO2 gasses, and blood substitutes. Thus, the cell-culture medium will provide a continuous supply of nutrients and the porous polymeric membrane will deliver the cells of interest that are cultured on the other side of the membrane. In addition to the nutrient-rich fluid perfusion and oxygen ventilation from the blood-vessel compartment, in the organ compartment, mechanical forces can be applied to the polymeric membrane to simulate the peristalsis respiratory motions from the in vivo organs. Despite the significant progress in material science, only a few materials can recapitulate the physiological conditions of living organ testing. First and foremost, the materials involved in the construction of the OOCs microdevices should ensure the necessary biocompatibility to provide a biologically safe and nontoxic environment that allows cell migration without generating any inflammatory response or exacerbated immunogenicity. Polymeric materials are desirable because of their great structural similarity to the ECM. Besides biocompatibility, an essential requirement for the polymeric membrane that separates the OOCs compartments is the similarity with the ECM in order to support the cell attachment and diffusion through its pores from one side of the interface to the other compartment. Proteins such as collagen, fibronectin, and vitronectin are ideal candidates as they are involved in biological processes and play an important role in the cell-adhesion mechanism. Moreover, it was shown that the surface roughness of the membrane could affect cell behavior. In the case of synthetic polymers such as PMMA layers, it was observed that the cell adhesion and spreading of vascular cells improved with higher surface roughness, but proliferation was not affected, and it was indicated that the cell adhesion is dependent on the protein adsorption [70][67]. In the case of PDMS, surface roughness and surface energy play a key role in the cell-attachment process. It was observed that higher surface energy promotes the formation of stronger cell–ligand bonding, leading to improved cell growth and proliferation. However, the cell-membrane interaction drastically decreases above critical surface energy and critical roughness ratio. Thus, the study on the cellular behavior of HeLa and MDA MB 231 cells on a rough PDMS surface revealed that the optimum conditions for cell adhesion, growth, and proliferation are obtained at moderate surface energy and intermediate roughness ratios [71]| 93 | |||||||
| ] | |||||||
| Blood-brain barrier [ | |||||||
| 97 | |||||||
| ] | Blood-brain barrier | [ | 94 | ] | |||
| Polytetrafluoroethylene (PTFE) | 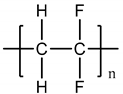 |
108° [98] | 108° [95] | 392 MPa [99] | 392 MPa [96] | Liver [100] | Liver [97] |
2.3. Polymers for Organ-on-Chips
Materials used to manufacture OOCs play a crucial role as they may directly interact with the biological fluids and cellular system, affecting the experiment results. Although several types of polymeric structures have been employed, only a few polymers exhibit the required specifications needed to fabricate the OOCs, and both synthetic polymers and biopolymers have been investigated. In the last few years, synthetic and natural polymers have been used for OOC applications as their performances depend on the polymer structure, porosity, transparency, flexibility, stiffness, etc. Synthetic polymers such as polydimethylsiloxane (PDMS) [101][98], polycarbonate (PC) [102][99], polyethylenetereftalate (PET) [103][100], aliphatic polyesters, polyurethanes [104][101], etc., have been employed for the preparation of porous-compartment-separation membranes due to their easily adjusting porosity, surface roughness, and mechanical properties. Exhibiting improved biocompatibility, natural polymers such as collagen, gelatin, or polysaccharides have attracted significant attention lately due to their faithful replication of the native tissues with channel interconnections that allow the perfusion of oxygen and nutrients to simulate the natural behaviors of cell differentiation, spreading, and adhesion along the separating membrane [105][102]. However, natural polymers show batch-to-batch composition variability and lower mechanical properties, thus affecting the reproducibility of the experiments. However, each employed biomaterial still exhibits weaknesses to achieve the optimal OOC performances. Polydimethylsiloxane (PDMS) is an elastomeric polymer and one of the most employed synthetic materials for organ-chip production, as it exhibits optical transparency, biocompatibility, gas-permeation properties, flexibility, and allows permanent microscopic observation of 3D cell structures for real-time assessment of tumor behavior and response to therapy [101,106,107][98][103][104]. Microfluidic systems made from PDMS offer a much closer physiological microenvironment to that of the living TME from a biomechanical point, exhibiting porosity and significantly lower stiffness. However, PDMS exhibits an important limitation in chemical screening due to its hydrophobicity, which leads to nonspecific absorption of small molecules such as drugs or pharmaceutical compounds [108,109][105][106]. Nevertheless, the limitations of PDMS materials have been overcome through surface modification treatments with another polymer that enhances the hydrophilicity of the PDMS-based materials to facilitate cellular adhesion and migration in microfluidic compartments. One of the most commonly employed surface-modification approaches consists of oxygen-plasma treatment by converting some hydrophobic methyl groups into hydroxyl functionalities via oxidation. It was reported that the hydrophilicity of the oxidized PDMS after plasma treatment can increase by almost 30° [110][107]. Moreover, the wettability behavior of PDMS materials can be improved by coating the polymer layers with other hydrophilic polymers. The proteins from ECM composition are generally used to achieve the natural moiety of PDMS to ensure cell attachment and proliferation. ECM proteins, such as collagen and fibronectin, create covalent bonding onto the PDMS surface and thus facilitate cell adhesion by modifying the surface roughness of the synthetic polymer [111][108]. Although the hydrophilicity and biocompatibility are significantly increased this way, dissociation processes during protein coating are associated with this approach [112][109]. Other biopolymers, such as gelatin [113][110], fibronectin [114][111], or hydrophilic synthetic polymers such as polydopamine [115,116][112][113] and polyethylene glycol [117][114] have been successfully employed for modifying the surface roughness of PDMS and managed to enhance the cellular adhesion for short-term cellular culture. Polycarbonate (PC) structures are commonly used polymeric materials for the OOCs fabrication and as porous membranes for Transwell® inserts. The PC structures are hydrophobic, exhibit high transparency, and are inert and stable under biological processes [118][115]. Similar to PDMS, the surface of PC is commonly modified by gas plasma treatment or protein coating [119][116]. However, PC structures exhibit higher rigidity than PDMS. They are not suitable for stretchable membranes or soft substrates. Other thermoplastics are tested, such as polyolefins [103][100], styrene-ethylene butylene styrene (SEBS) [120][117], polyurethanes [121][118], and copolymers [122][119], but there is still a demand for novel materials in this domain. Polyolefins such as cyclic olefin polymers (COP) and cyclic olefin copolymers (COC) are thermoplastic materials that have also been employed for the manufacturing of microfluidic devices, being less gas-permeable than PDMS. COP and COC are lipophobic materials and do not exhibit unspecific adsorption of small molecules, allowing their use in drug-development and diffusion experiments [122][119]. Moreover, they exhibit optical transparency, good chemical resistance to polar solvents, thermal resistance, and reproductible mass production. Polymethylmethacrylate (PMMA) is another frequently used material as a substrate [123,124][120][121]. Kang and coworkers [125][122] demonstrated that PMMA chemically attached to porous orbitally etched polyethylene terephthalate (PTFE) membranes is impermeable to small hydrophobic compounds and more consistent results concerning the anticancer vincristine drug cytotoxicity on human lung adenocarcinoma cells cultured in PDMS-based chip. Thus, PMMA shows promising features for tumor chip microdevices fabrication as the small molecules cannot infiltrate the material. Aliphatic polyesters such as polylactic acid (PLA) and poly (ε-caprolactone) (PCL) have been used to mimic the in vitro blood–brain barrier, functioning as membranes in OOCs microdevices. Both polymers are biodegradable and hydrophobic. Although their surface can be chemically modified to enhance the wettability and cell proliferation, these polymers have been sparsely employed as they tend to degrade under biological processes releasing acidic degradation products that could affect the cellular medium [123][120]. To fulfill the requirements of mechanical actuatable OOCs, the employed materials need to be optically transparent, flexible, easily moldable, and nonabsorptive to drugs and cell nutrients. Moreover, it is necessary to find sustainable alternative materials. At present, the manufacturing process of OOCs and experimental validation remain high-priced so that less-expensive and reusable materials should be employed. Gelatin is an animal protein obtained by collagen hydrolysis, and is very much employed in drug-delivery and tissue-engineering applications [126][123] due to its ability in drug transportation and cell-growth promotion [127][124]. Gelatin exhibits biocompatibility and flexibility, and forms complexes with proteins, growth-factor nucleotides, and biopolymers, and can be shaped in colorless gels [128][125]. Additionally, photo-crosslinked gelatin methacrylate (GelMa) is highly used in tissue engineering and shows interest in OOC formation [129,130][126][127]. Collagen-based ECM gel (known as Matrigel) is a commercial product that contains ECM hydrogel made of tumor-derived basement membrane proteins employed for cell culture [131][128]. Matrigel gives structural and signal-transduction functions. Tumor cells exhibit aggressive behavior in Matrigel medium, and it is frequently used to assess the malignancy of cancer cells and observe the mechanism of tumor growth [132][129]. Bacterial cellulose paper has also attracted interest for tumor-chip applications as it has good biocompatibility, and it is a naturally derived polymer that is densely vascularized through nanofibers [133,134][130][131]. Recent advances in bioprinting are multiplying the paths for creating complex perfused systems, and the discovery of new materials that accomplish the full requirements of native living cells is still under extensive development.References
- Faria, J.; Ahmed, S.; Gerritsen, K.G.; Mihaila, S.M.; Masereeuw, R. Kidney-Based in Vitro Models for Drug-Induced Toxicity Testing. Arch. Toxicol. 2019, 93, 3397–3418.
- Zanetti, F. Kidney-on-a-Chip. In Organ-on-a-Chip; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–253.
- Tiong, H.Y.; Huang, P.; Xiong, S.; Li, Y.; Vathsala, A.; Zink, D. Drug-Induced Nephrotoxicity: Clinical Impact and Preclinical in Vitro Models. Mol. Pharm. 2014, 11, 1933–1948.
- Day, C.-P.; Merlino, G.; Van Dyke, T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015, 163, 39–53.
- Berlin, J.A.; Glasser, S.C.; Ellenberg, S.S. Adverse Event Detection in Drug Development: Recommendations and Obligations beyond Phase 3. Am. J. Public Health 2008, 98, 1366–1371.
- Sun, W.; Luo, Z.; Lee, J.; Kim, H.; Lee, K.; Tebon, P.; Feng, Y.; Dokmeci, M.R.; Sengupta, S.; Khademhosseini, A. Organ-on-a-chip for Cancer and Immune Organs Modeling. Adv. Healthc. Mater. 2019, 8, 1801363.
- Taylor, D. The Pharmaceutical Industry and the Future of Drug Development. In Pharmaceuticals in the Environment; Royal Society of Chemistry: London, UK, 2015; pp. 1–33.
- Soo, J.Y.-C.; Jansen, J.; Masereeuw, R.; Little, M.H. Advances in Predictive in Vitro Models of Drug-Induced Nephrotoxicity. Nat. Rev. Nephrol. 2018, 14, 378–393.
- Akhtar, A. The Flaws and Human Harms of Animal Experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419.
- Bracken, M.B. Why Animal Studies Are Often Poor Predictors of Human Reactions to Exposure. J. R. Soc. Med. 2009, 102, 120–122.
- Baumans, V. Use of Animals in Experimental Research: An Ethical Dilemma? Gene Ther. 2004, 11, S64–S66.
- Festing, S.; Wilkinson, R. The Ethics of Animal Research: Talking Point on the Use of Animals in Scientific Research. EMBO Rep. 2007, 8, 526–530.
- European Parliament. Available online: Https://Www.Europarl.Europa.Eu/Plenary/En/Vod.Html?Mode=chapter&vodLanguage=EN&vodId=6ea360e5-0dd3-Decd-2a72-642c028c0a34&date=20210708# (accessed on 25 February 2022).
- Trujillo-de Santiago, G.; Flores-Garza, B.G.; Tavares-Negrete, J.A.; Lara-Mayorga, I.M.; González-Gamboa, I.; Zhang, Y.S.; Rojas-Martínez, A.; Ortiz-López, R.; Álvarez, M.M. The Tumor-on-Chip: Recent Advances in the Development of Microfluidic Systems to Recapitulate the Physiology of Solid Tumors. Materials 2019, 12, 2945.
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an in Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065.
- Przekwas, A.; Somayaji, M.R. Computational Pharmacokinetic Modeling of Organ-on-Chip Devices and Microphysiological Systems. Organ-A-Chip 2020, 311–361.
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling Cancer in Microfluidic Human Organs-on-Chips. Nat. Rev. Cancer 2019, 19, 65–81.
- Liu, X.; Fang, J.; Huang, S.; Wu, X.; Xie, X.; Wang, J.; Liu, F.; Zhang, M.; Peng, Z.; Hu, N. Tumor-on-a-Chip: From Bioinspired Design to Biomedical Application. Microsyst. Nanoeng. 2021, 7, 50.
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-Chips: Into the next Decade. Nat. Rev. Drug Discov. 2021, 20, 345–361.
- Ingber, D.E. Developmentally Inspired Human ‘Organs on Chips. Development 2018, 145, dev156125.
- Brill-Karniely, Y.; Dror, D.; Duanis-Assaf, T.; Goldstein, Y.; Schwob, O.; Millo, T.; Orehov, N.; Stern, T.; Jaber, M.; Loyfer, N. Triangular Correlation (TrC) between Cancer Aggressiveness, Cell Uptake Capability, and Cell Deformability. Sci. Adv. 2020, 6, eaax2861.
- Rahmanuddin, S.; Korn, R.; Cridebring, D.; Borazanci, E.; Brase, J.; Boswell, W.; Jamil, A.; Cai, W.; Sabir, A.; Motarjem, P. Role of 3D Volumetric and Perfusion Imaging for Detecting Early Changes in Pancreatic Adenocarcinoma. Front. Oncol. 2021, 11, 678617.
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat. Rev. Cancer 2014, 14, 430–439.
- Ehsan, S.M.; Welch-Reardon, K.M.; Waterman, M.L.; Hughesbce, C.C.; George, S.C. A three-dimensional in vitro model of tumor cell intravasation. Integr. Biol (Camb). 2014, 6, 603–610.
- Chen, Y.-A.; King, A.D.; Shih, H.-C.; Peng, C.-C.; Wu, C.-Y.; Liao, W.-H.; Tung, Y.-C. Generation of Oxygen Gradients in Microfluidic Devices for Cell Culture Using Spatially Confined Chemical Reactions. Lab Chip 2011, 11, 3626–3633.
- Shih, H.-C.; Lee, T.-A.; Wu, H.-M.; Ko, P.-L.; Liao, W.-H.; Tung, Y.-C. Microfluidic Collective Cell Migration Assay for Study of Endothelial Cell Proliferation and Migration under Combinations of Oxygen Gradients, Tensions, and Drug Treatments. Sci. Rep. 2019, 9, 8234.
- Whiteside, T. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912.
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772.
- Barrile, R.; van der Meer, A.D.; Park, H.; Fraser, J.P.; Simic, D.; Teng, F.; Conegliano, D.; Nguyen, J.; Jain, A.; Zhou, M. Organ-on-chip Recapitulates Thrombosis Induced by an Anti-CD154 Monoclonal Antibody: Translational Potential of Advanced Microengineered Systems. Clin. Pharmacol. Ther. 2018, 104, 1240–1248.
- Ferrari, E.; Nebuloni, F.; Rasponi, M.; Occhetta, P. Photo and Soft Lithography for Organ-on-Chip Applications. In Organ-on-a-Chip; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–19.
- Puryear, J.R., III; Yoon, J.-K.; Kim, Y. Advanced Fabrication Techniques of Microengineered Physiological Systems. Micromachines 2020, 11, 730.
- Sticker, D.; Rothbauer, M.; Lechner, S.; Hehenberger, M.-T.; Ertl, P. Multi-Layered, Membrane-Integrated Microfluidics Based on Replica Molding of a Thiol–Ene Epoxy Thermoset for Organ-on-a-Chip Applications. Lab Chip 2015, 15, 4542–4554.
- Tajeddin, A.; Mustafaoglu, N. Design and Fabrication of Organ-on-Chips: Promises and Challenges. Micromachines 2021, 12, 1443.
- Shrestha, J.; Ghadiri, M.; Shanmugavel, M.; Razavi Bazaz, S.; Vasilescu, S.; Ding, L. A Rapidly Prototyped Lung-on-a-Chip Model Using 3D-Printed Molds. Organs-A-Chip 2019, 1, 100001.
- Yang, Q.; Lian, Q.; Xu, F. Perspective: Fabrication of Integrated Organ-on-a-Chip via Bioprinting. Biomicrofluidics 2017, 11, 031301.
- Kasi, D.G.; de Graaf, M.N.; Motreuil-Ragot, P.A.; Frimat, J.-P.; Ferrari, M.D.; Sarro, P.M.; Mastrangeli, M.; van den Maagdenberg, A.M.; Mummery, C.L.; Orlova, V.V. Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography. Micromachines 2022, 13, 49.
- Christoffersson, J.; Mandenius, C.-F. Fabrication of a Microfluidic Cell Culture Device Using Photolithographic and Soft Lithographic Techniques. In Cell-Based Assays Using iPSCs for Drug Development and Testing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 227–233.
- Ferreira, D.A.; Rothbauer, M.; Conde, J.P.; Ertl, P.; Oliveira, C.; Granja, P.L. A Fast Alternative to Soft Lithography for the Fabrication of Organ-on-a-Chip Elastomeric-Based Devices and Microactuators. Adv. Sci. 2021, 8, 2003273.
- Huh, D.; Hamilton, G.; Ingber, D. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754.
- Chiadò, A.; Palmara, G.; Chiappone, A.; Tanzanu, C.; Pirri, C.F.; Roppolo, I.; Frascella, F. A Modular 3D Printed Lab-on-a-Chip for Early Cancer Detection. Lab Chip 2020, 20, 665–674.
- Samadian, H.; Jafari, S.; Sepand, M.; Alaei, L.; Malvajerd, S.S.; Jaymand, M.; Ghobadinezhad, F.; Jahanshahi, F.; Hamblin, M.; Derakhshankhah, H. 3D Bioprinting Technology to Mimic the Tumor Microenvironment: Tumor-on-a-Chip Concept. Mater. Today Adv. 2021, 12, 100160.
- Lantada, A.D.; Pfleging, W.; Besser, H.; Guttmann, M.; Wissmann, M.; Plewa, K.; Smyrek, P.; Piotter, V.; García-Ruíz, J.P. Research on the Methods for the Mass Production of Multi-Scale Organs-on-Chips. Polymers 2018, 10, 1238.
- Gröger, M.; Dinger, J.; Kiehntopf, M.; Peters, F.T.; Rauen, U.; Mosig, A.S. Preservation of Cell Structure, Metabolism, and Biotransformation Activity of Liver-on-chip Organ Models by Hypothermic Storage. Adv. Healthc. Mater. 2018, 7, 1700616.
- Shaegh, S.A.M.; Pourmand, A.; Nabavinia, M.; Avci, H.; Tamayol, A.; Mostafalu, P.; Ghavifekr, H.B.; Aghdam, E.N.; Dokmeci, M.R.; Khademhosseini, A. Rapid Prototyping of Whole-Thermoplastic Microfluidics with Built-in Microvalves Using Laser Ablation and Thermal Fusion Bonding. Sens. Actuators B Chem. 2018, 255, 100–109.
- Thakare, K.; Jerpseth, L.; Pei, Z.; Elwany, A.; Quek, F.; Qin, H. Bioprinting of Organ-on-Chip Systems: A Literature Review from a Manufacturing Perspective. J. Manuf. Mater. Process. 2021, 5, 91.
- Cao, X.; Ashfaq, R.; Cheng, F.; Maharjan, S.; Li, J.; Ying, G.; Hassan, S.; Xiao, H.; Yue, K.; Zhang, Y.S. A Tumor-on-a-chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv. Funct. Mater. 2019, 29, 1807173.
- O’Cearbhaill, E. 3D Bioprinting Chips Away at Glioblastomal Resistance. Sci. Transl. Med. 2019, 11, eaax1724.
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195.
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3D Bioprinting in Tissue Engineering: Advantages, Deficiencies, Improvements, and Future Perspectives. J. Mater. Chem. B 2021, 9, 5385–5413.
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284.
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-viscosity Bioink. Adv. Mater. 2016, 28, 677–684.
- Keenan, T.M.; Folch, A. Biomolecular Gradients in Cell Culture Systems. Lab Chip 2008, 8, 34–57.
- Nagy, J.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why Are Tumour Blood Vessels Abnormal and Why Is It Important to Know? Br. J. Cancer 2009, 100, 865–869.
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.-Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D Bioprinted Vascularized Tumour for Drug Testing. Int. J. Mol. Sci. 2020, 21, 2993.
- Hwang, D.G.; Choi, Y.; Jang, J. 3D Bioprinting-Based Vascularized Tissue Models Mimicking Tissue-Specific Architecture and Pathophysiology for in Vitro Studies. Front. Bioeng. Biotechnol. 2021, 9, 685507.
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D Bioprinting for Reconstituting the Cancer Microenvironment. NPJ Precis. Oncol. 2020, 4, 18.
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.-H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.-W. Development of a Functional Airway-on-a-Chip by 3D Cell Printing. Biofabrication 2018, 11, 015002.
- Kim, W.; Kim, G. Intestinal Villi Model with Blood Capillaries Fabricated Using Collagen-Based Bioink and Dual-Cell-Printing Process. ACS Appl. Mater. Interfaces 2018, 10, 41185–41196.
- Ali, M.; Pr, A.K.; Yoo, J.J.; Zahran, F.; Atala, A.; Lee, S.J. A Photo-crosslinkable Kidney ECM-derived Bioink Accelerates Renal Tissue Formation. Adv. Healthc. Mater. 2019, 8, 1800992.
- Fransen, M.F.; Addario, G.; Bouten, C.V.; Halary, F.; Moroni, L.; Mota, C. Bioprinting of Kidney in Vitro Models: Cells, Biomaterials, and Manufacturing Techniques. Essays Biochem. 2021, 65, 587–602.
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290.
- Sarkar, J.; Kamble, S.C.; Kashikar, N.C. Polymeric Bioinks for 3D Hepatic Printing. Chemistry 2021, 3, 164–181.
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V. Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-on-a-Chip. Biomaterials 2016, 110, 45–59.
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H. Instrumented Cardiac Microphysiological Devices via Multimaterial Three-Dimensional Printing. Nat. Mater. 2017, 16, 303–308.
- Burkholder-Wenger, A.C.; Golzar, H.; Wu, Y.; Tang, X.S. Development of a Hybrid Nanoink for 3D Bioprinting of Heterogeneous Tumor Models. ACS Biomater. Sci. Eng. 2022, 8, 777–785.
- Wu, Y.; Wenger, A.; Golzar, H.; Tang, X.S. 3D Bioprinting of Bicellular Liver Lobule-Mimetic Structures via Microextrusion of Cellulose Nanocrystal-Incorporated Shear-Thinning Bioink. Sci. Rep. 2020, 10, 20648.
- Lampin, M.; Warocquier-Clérout, R.; Legris, C.; Degrange, M.; Sigot-Luizard, M. Correlation between Substratum Roughness and Wettability, Cell Adhesion, and Cell Migration. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1997, 36, 99–108.
- Majhy, B.; Priyadarshini, P.; Sen, A. Effect of Surface Energy and Roughness on Cell Adhesion and Growth–Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476.
- Chen, L.; Yan, C.; Zheng, Z. Functional Polymer Surfaces for Controlling Cell Behaviors. Mater. Today 2018, 21, 38–59.
- Patel, S.; Thakar, R.G.; Wong, J.; McLeod, S.D.; Li, S. Control of Cell Adhesion on Poly (Methyl Methacrylate). Biomaterials 2006, 27, 2890–2897.
- Riau, A.K.; Mondal, D.; Yam, G.H.; Setiawan, M.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Surface Modification of PMMA to Improve Adhesion to Corneal Substitutes in a Synthetic Core–Skirt Keratoprosthesis. ACS Appl. Mater. Interfaces 2015, 7, 21690–21702.
- Jastrzebska, E.; Zuchowska, A.; Flis, S.; Sokolowska, P.; Bulka, M.; Dybko, A.; Brzozka, Z. Biological Characterization of the Modified Poly (Dimethylsiloxane) Surfaces Based on Cell Attachment and Toxicity Assays. Biomicrofluidics 2018, 12, 044105.
- Premnath, P.; Tavangar, A.; Tan, B.; Venkatakrishnan, K. Tuning Cell Adhesion by Direct Nanostructuring Silicon into Cell Repulsive/Adhesive Patterns. Exp. Cell Res. 2015, 337, 44–52.
- Azadbakht, M.; Madaeni, S.S.; Sahebjamee, F. Biocompatibility of Polyethersulfone Membranes for Cell Culture Systems. Eng. Life Sci. 2011, 11, 629–635.
- Ruben, B.; Elisa, M.; Leandro, L.; Victor, M.; Gloria, G.; Marina, S.; Pandiyan, R.; Nadhira, L. Oxygen Plasma Treatments of Polydimethylsiloxane Surfaces: Effect of the Atomic Oxygen on Capillary Flow in the Microchannels. Micro Nano Lett. 2017, 12, 754–757.
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl. Vitro Toxicol. 2016, 2, 82–96.
- Kim, S.; Takayama, S. Organ-on-a-Chip and the Kidney. Kidney Res. Clin. Pract. 2015, 34, 165–169.
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676.
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Geiser, T. Second-Generation Lung-on-a-Chip with an Array of Stretchable Alveoli Made with a Biological Membrane. Commun. Biol. 2021, 4, 168.
- Ogończyk, D.; Jankowski, P.; Garstecki, P. A Method for Simultaneous Polishing and Hydrophobization of Polycarbonate for Microfluidic Applications. Polymers 2020, 12, 2490.
- He, Y.; Guo, Y.; He, R.; Jin, T.; Chen, F.; Fu, Q.; Zhou, N.; Shen, J. Towards High Molecular Weight Poly (Bisphenol a Carbonate) with Excellent Thermal Stability and Mechanical Properties by Solid-State Polymerization. Chin. J. Polym. Sci. 2015, 33, 1176–1185.
- Aazmi, A.; Zhou, H.; Li, Y.; Yu, M.; Xu, X.; Wu, Y.; Ma, L.; Zhang, B.; Yang, H. Engineered Vasculature for Organ-on-a-Chip Systems. Engineering 2021, 9, 131–147.
- Ma, Y.; Pan, J.-Z.; Zhao, S.-P.; Lou, Q.; Zhu, Y.; Fang, Q. Microdroplet Chain Array for Cell Migration Assays. Lab Chip 2016, 16, 4658–4665.
- Gotoh, K.; Yasukawa, A.; Kobayashi, Y. Wettability Characteristics of Poly (Ethylene Terephthalate) Films Treated by Atmospheric Pressure Plasma and Ultraviolet Excimer Light. Polym. J. 2011, 43, 545–551.
- Bin, Y.; Oishi, K.; Yoshida, K.; Matsuo, M. Mechanical Properties of Poly (Ethylene Terephthalate) Estimated in Terms of Orientation Distribution of Crystallites and Amorphous Chain Segments under Simultaneous Biaxially Stretching. Polym. J. 2004, 36, 888–898.
- Xiang, Y.; Wen, H.; Yu, Y.; Li, M.; Fu, X.; Huang, S. Gut-on-Chip: Recreating Human Intestine in Vitro. J. Tissue Eng. 2020, 11, 2041731420965318.
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.-Y.; Ingber, D.E. Human Kidney Proximal Tubule-on-a-Chip for Drug Transport and Nephrotoxicity Assessment. Integr. Biol. 2013, 5, 1119–1129.
- Yu, F.; Deng, R.; Hao Tong, W.; Huan, L.; Chan Way, N.; IslamBadhan, A.; Iliescu, C.; Yu, H. A Perfusion Incubator Liver Chip for 3D Cell Culture with Application on Chronic Hepatotoxicity Testing. Sci. Rep. 2017, 7, 14528.
- Laput, O.; Vasenina, I.; Salvadori, M.C.; Savkin, K.; Zuza, D.; Kurzina, I. Low-Temperature Plasma Treatment of Polylactic Acid and PLA/HA Composite Material. J. Mater. Sci. 2019, 54, 11726–11738.
- Ko, H.-S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical Properties and Bioactivity of Poly (Lactic Acid) Composites Containing Poly (Glycolic Acid) Fiber and Hydroxyapatite Particles. Nanomaterials 2021, 11, 249.
- Pensabene, V.; Costa, L.; Terekhov, A.Y.; Gnecco, J.S.; Wikswo, J.P.; Hofmeister, W.H. Ultrathin Polymer Membranes with Patterned, Micrometric Pores for Organs-on-Chips. ACS Appl. Mater. Interfaces 2016, 8, 22629–22636.
- Janvikul, W.; Uppanan, P.; Thavornyutikarn, B.; Kosorn, W.; Kaewkong, P. Effects of Surface Topography, Hydrophilicity and Chemistry of Surface-Treated PCL Scaffolds on Chondrocyte Infiltration and ECM Production. Procedia Eng. 2013, 59, 158–165.
- Dziadek, M.; Menaszek, E.; Zagrajczuk, B.; Pawlik, J.; Cholewa-Kowalska, K. New Generation Poly (ε-Caprolactone)/Gel-Derived Bioactive Glass Composites for Bone Tissue Engineering: Part I. Material Properties. Mater. Sci. Eng. C 2015, 56, 9–21.
- Pensabene, V.; Crowder, S.W.; Balikov, D.A.; Lee, J.B.; Sung, H.-J. Optimization of Electrospun Fibrous Membranes for in Vitro Modeling of Blood-Brain Barrier; IEEE: Piscataway, NJ, USA, 2016; pp. 125–128.
- Wloch, J.; Terzyk, A.P.; Wisniewski, M.; Kowalczyk, P. Nanoscale Water Contact Angle on Polytetrafluoroethylene Surfaces Characterized by Molecular Dynamics-Atomic Force Microscopy Imaging. Langmuir 2018, 34, 4526–4534.
- Uehara, H.; Jounai, K.; Endo, R.; Okuyama, H.; Kanamoto, T.; Porter, R.S. High Modulus Films of Polytetrafluoroethylene Prepared by Two-Stage Drawing of Reactor Powder. Polym. J. 1997, 29, 198–200.
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y. Organ-on-a-chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv. Healthc. Mater. 2018, 7, 1700506.
- Grant, J.; Özkan, A.; Oh, C.; Mahajan, G.; Prantil-Baun, R.; Ingber, D.E. Predicting Drug Concentrations in PDMS Microfluidic Organ Chips. Lab Chip 2021, 21, 3509–3519.
- Wang, M.; Duan, B. Materials and Their Biomedical Applications. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–152.
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-Chip: Recent Breakthroughs and Future Prospects. Biomed. Eng. Online 2020, 19, 9.
- Osório, L.A.; Silva, E.; Mackay, R.E. A Review of Biomaterials and Scaffold Fabrication for Organ-on-a-Chip (OOAC) Systems. Bioengineering 2021, 8, 113.
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.-K. Biocompatible Polymers and Their Potential Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 3608–3619.
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958.
- Fujii, T. PDMS-Based Microfluidic Devices for Biomedical Applications. Microelectron. Eng. 2002, 61, 907–914.
- Carter, S.-S.D.; Atif, A.-R.; Kadekar, S.; Lanekoff, I.; Engqvist, H.; Varghese, O.P.; Tenje, M.; Mestres, G. PDMS Leaching and Its Implications for On-Chip Studies Focusing on Bone Regeneration Applications. Organs-Chip 2020, 2, 100004.
- Bunge, F.; den Driesche, S.V.; Vellekoop, M.J. Microfluidic Platform for the Long-Term on-Chip Cultivation of Mammalian Cells for Lab-on-a-Chip Applications. Sensors 2017, 17, 1603.
- Gezer, P.G.; Brodsky, S.; Hsiao, A.; Liu, G.L.; Kokini, J.L. Modification of the Hydrophilic/Hydrophobic Characteristic of Zein Film Surfaces by Contact with Oxygen Plasma Treated PDMS and Oleic Acid Content. Colloids Surf. B Biointerfaces 2015, 135, 433–440.
- Zhang, W.; Choi, D.S.; Nguyen, Y.H.; Chang, J.; Qin, L. Studying Cancer Stem Cell Dynamics on PDMS Surfaces for Microfluidics Device Design. Sci. Rep. 2013, 3, 2332.
- Chuah, Y.J.; Kuddannaya, S.; Lee, M.H.A.; Zhang, Y.; Kang, Y. The Effects of Poly (Dimethylsiloxane) Surface Silanization on the Mesenchymal Stem Cell Fate. Biomater. Sci. 2015, 3, 383–390.
- Pitingolo, G.; Riaud, A.; Nastruzzi, C.; Taly, V. Gelatin-Coated Microfluidic Channels for 3d Microtissue Formation: On-Chip Production and Characterization. Micromachines 2019, 10, 265.
- Steinfeld, B.; Scott, J.; Vilander, G.; Marx, L.; Quirk, M.; Lindberg, J.; Koerner, K. The Role of Lean Process Improvement in Implementation of Evidence-Based Practices in Behavioral Health Care. J. Behav. Health Serv. Res. 2015, 42, 504–518.
- Khetani, S.; Yong, K.W.; Ozhukil Kollath, V.; Eastick, E.; Azarmanesh, M.; Karan, K.; Sen, A.; Sanati-Nezhad, A. Engineering Shelf-Stable Coating for Microfluidic Organ-on-a-Chip Using Bioinspired Catecholamine Polymers. ACS Appl. Mater. Interfaces 2020, 12, 6910–6923.
- Park, S.E.; Georgescu, A.; Oh, J.M.; Kwon, K.W.; Huh, D. Polydopamine-Based Interfacial Engineering of Extracellular Matrix Hydrogels for the Construction and Long-Term Maintenance of Living Three-Dimensional Tissues. ACS Appl. Mater. Interfaces 2019, 11, 23919–23925.
- Mikhail, A.S.; Ranger, J.J.; Liu, L.; Longenecker, R.; Thompson, D.B.; Sheardown, H.D.; Brook, M.A. Rapid and Efficient Assembly of Functional Silicone Surfaces Protected by PEG: Cell Adhesion to Peptide-Modified PDMS. J. Biomater. Sci. Polym. Ed. 2010, 21, 821–842.
- Henry, O.Y.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-Chips with Integrated Electrodes for Trans-Epithelial Electrical Resistance (TEER) Measurements of Human Epithelial Barrier Function. Lab Chip 2017, 17, 2264–2271.
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic Blood–Brain Barrier Model Provides in Vivo-like Barrier Properties for Drug Permeability Screening. Biotechnol. Bioeng. 2017, 114, 184–194.
- Domansky, K.; Sliz, J.D.; Wen, N.; Hinojosa, C.; Thompson, G.; Fraser, J.P.; Hamkins-Indik, T.; Hamilton, G.A.; Levner, D.; Ingber, D.E. SEBS Elastomers for Fabrication of Microfluidic Devices with Reduced Drug Absorption by Injection Molding and Extrusion. Microfluid. Nanofluidics 2017, 21, 107.
- Domansky, K.; Leslie, D.C.; McKinney, J.; Fraser, J.P.; Sliz, J.D.; Hamkins-Indik, T.; Hamilton, G.A.; Bahinski, A.; Ingber, D.E. Clear Castable Polyurethane Elastomer for Fabrication of Microfluidic Devices. Lab Chip 2013, 13, 3956–3964.
- Ding, C.; Chen, X.; Kang, Q.; Yan, X. Biomedical Application of Functional Materials in Organ-on-a-Chip. Front. Bioeng. Biotechnol. 2020, 8, 823.
- Pasman, T.; Grijpma, D.; Stamatialis, D.; Poot, A. Flat and Microstructured Polymeric Membranes in Organs-on-Chips. J. R. Soc. Interface 2018, 15, 20180351.
- Busek, M.; Nøvik, S.; Aizenshtadt, A.; Amirola-Martinez, M.; Combriat, T.; Grünzner, S.; Krauss, S. Thermoplastic Elastomer (TPE)–Poly (Methyl Methacrylate)(PMMA) Hybrid Devices for Active Pumping PDMS-Free Organ-on-a-Chip Systems. Biosensors 2021, 11, 162.
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.-E.; Ahn, S.; Kang, J.H. Robust Chemical Bonding of PMMA Microfluidic Devices to Porous PETE Membranes for Reliable Cytotoxicity Testing of Drugs. Lab Chip 2019, 19, 3706–3713.
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584.
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin Carriers for Drug and Cell Delivery in Tissue Engineering. J. Control. Release 2014, 190, 210–218.
- Lee, H.; Cho, D.-W. One-Step Fabrication of an Organ-on-a-Chip with Spatial Heterogeneity Using a 3D Bioprinting Technology. Lab Chip 2016, 16, 2618–2625.
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable Gelatin and Hyaluronic Acid for Stereolithographic 3D Bioprinting of Tissue-engineered Cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2649–2657.
- Nawroth, J.C.; Scudder, L.L.; Halvorson, R.T.; Tresback, J.; Ferrier, J.P.; Sheehy, S.P.; Cho, A.; Kannan, S.; Sunyovszki, I.; Goss, J.A. Automated Fabrication of Photopatterned Gelatin Hydrogels for Organ-on-Chips Applications. Biofabrication 2018, 10, 025004.
- Yu, F.; Hunziker, W.; Choudhury, D. Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines 2019, 10, 165.
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From Discovery and ECM Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliv. Rev. 2014, 79, 3–18.
- Li, Y.; Wang, S.; Huang, R.; Huang, Z.; Hu, B.; Zheng, W.; Yang, G.; Jiang, X. Evaluation of the Effect of the Structure of Bacterial Cellulose on Full Thickness Skin Wound Repair on a Microfluidic Chip. Biomacromolecules 2015, 16, 780–789.
- Anton-Sales, I.; Beekmann, U.; Laromaine, A.; Roig, A.; Kralisch, D. Opportunities of Bacterial Cellulose to Treat Epithelial Tissues. Curr. Drug Targets 2019, 20, 808–822.
More
