Biosensors have globally been considered as biomedical diagnostic tools required in abundant areas including the development of diseases, detection of viruses, diagnosing ecological pollution, food monitoring, and a wide range of other diagnostic and therapeutic biomedical research. Recently, the broadly emerging and promising technique of plasmonic resonance has proven to provide label-free and highly sensitive real-time analysis when used in biosensing applications.
- biosensors
- plasmonics
- surface plasmon resonance
- lab-on-chip
1. Introduction
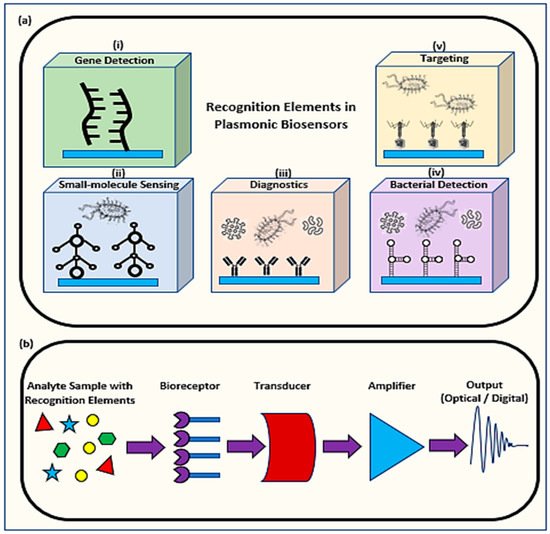
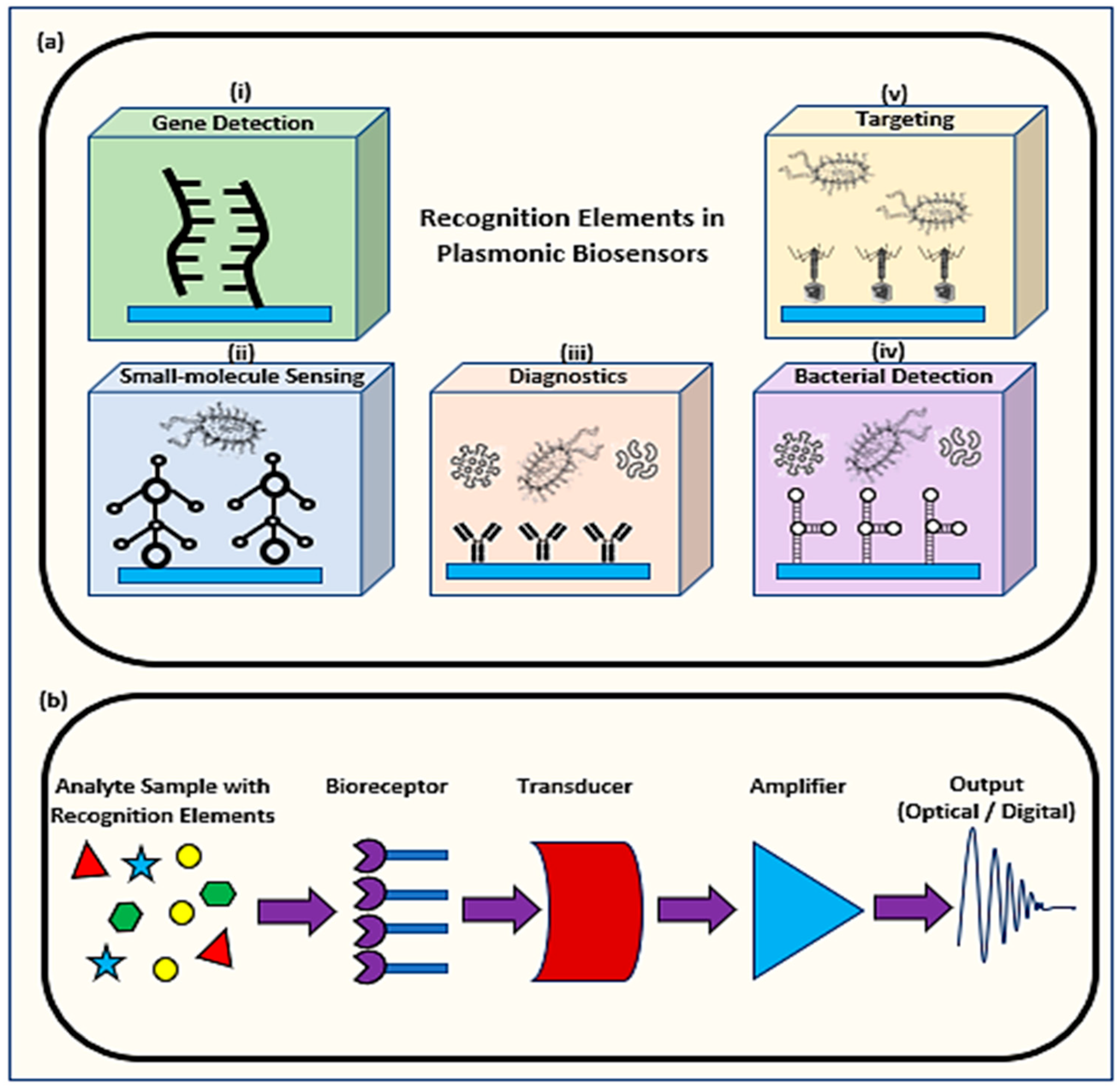
1.1. Mechanism of Plasmonic Biosensors
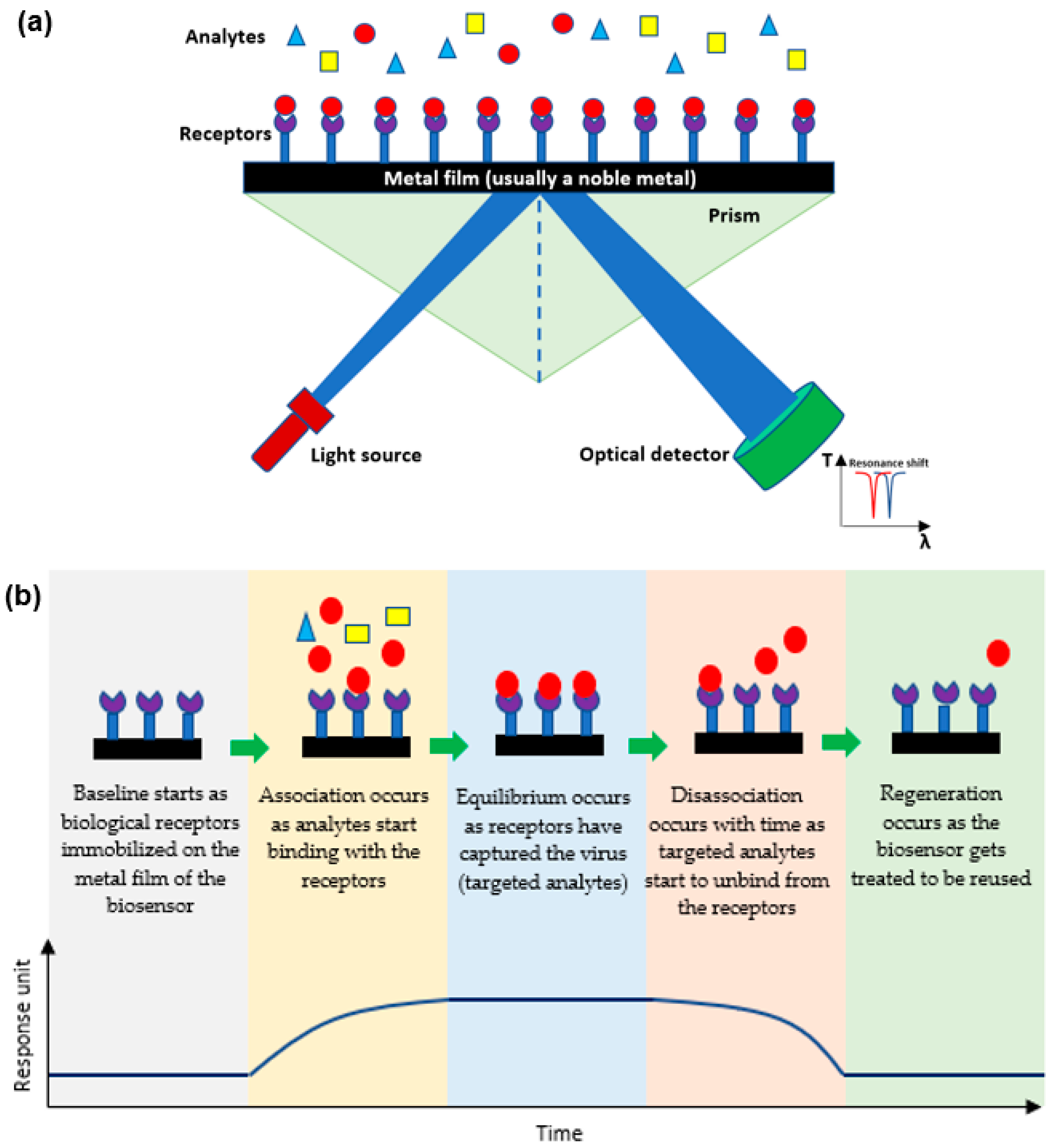
Plasmonic optical biosensors are classified into two types of platforms; one which uses a thin metal-based film and another which uses nanostructure-based inorganic plasmon resonance [21]. The most common plasmonic biosensor used is the surface plasmon resonance (SPR) which is a metal-based film sensor, mostly made of gold, used to characterize biomolecular interactions [22,23,24][22][23][24]. The angle at which the SPR is formed as light is focused on the metal film and reflected onto a detector. The output collected is due to energized plasmonic electrons formed from the collective refractive index (RI) of the oscillations of the electrons in the conduction band and the oscillations of the electric field formed by the light. The angle at which the SPR is measured relies on the RI of the material attached to the metal film. Hence, any alteration in the angle of the incidence or the RI of the material affects the resonance measured while detecting the analytes [25,26][25][26]. Decreasing the size results in a blueshift which holds a high frequency, while increasing the surrounding dielectric constant results in a red shift which holds a low frequency [27,28,29][27][28][29]. As shown in Figure 2a, the targeted analytes in a sample bind to the biological receptors restrained on the film to form a different RI which is usually known and indicates the presence of the targeted analyte [30]. This SPR biosensor can detect viruses and then be reused after applying proper chemical treatment practices as shown in Figure 2b [31,32][31][32].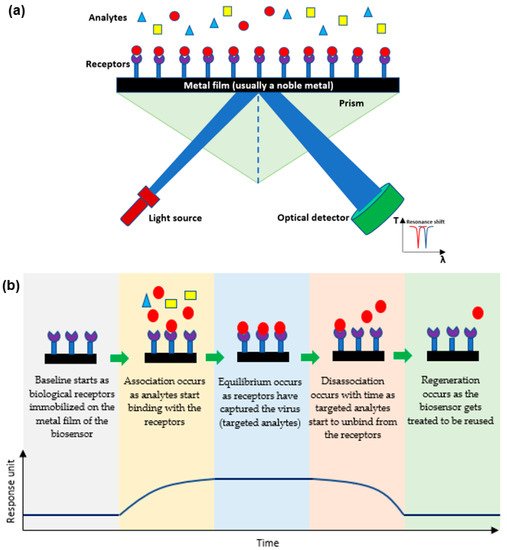
1.2. Determining the Efficiency of a Plasmonic Biosensor
To determine the efficiency of a biosensor, its limit of detection (LOD) and specificity(s) should be measured and attuned for its required application. The Clinical and Laboratory Standards Institute (CLSI) provides a guideline known as EP17 along with the protocols needed to determine the LOD [36] (p. 2).2. Applications of Plasmonic Optical Biosensors
2.1. The Use of Plasmonic Biosensors for Viral Detection
In 2019, an unanticipated disease known as the coronavirus (SARS-CoV-2) was discovered in Wuhan, China, causing a global pandemic. In less than two years, this novel virus affected millions and resulted in the death of more than four million people worldwide as per the WHO [40][37]. Furthermore, the coronavirus has significantly affected the world’s economy and resulted in a noticeable change in the social lifestyle of people to avoid getting infected with the fast-spreading, contagious virus. Hence, immediate detection methods of the virus with accurate, reliable, and instant results were needed to limit the virus from outspreading any further. Infectious viruses have been detected over the years directly by targeting the virus itself or indirectly by targeting the secondary responses of the virus [41][38]. Targeting a virus directly involves targeting the entire virion, the antigens of the virus, or the single or double-stranded RNA or DNA of the virus. Indirect detection methods include serological testing for precise antibodies released due to a primary response to an antigen (IgM) or a secondary response due to previous exposure to the same type of antigen (IgG) [42][39]. Some of the most common methods in detecting infectious viruses include immunofluorescence assays, hemagglutination assays, viral plaque assay, viral flow cytometry, enzyme linked immunosorbent assay (ELISA) [43][40], chest computed tomography (CT) [44][41], and nucleic acid amplification test (NAAT) including polymerase chain reaction (PCR) and real-time quantitative PCR (RT-qPCR) [45,46,47,48][42][43][44][45]. Although those methods have shown successful outcomes, they have also displayed substantial limitations that deferred their usage in future viral detection as shown in Table 1.|
Current Methods for Virus Detection |
Advantages |
Limitations |
Refs. |
|---|---|---|---|
|
Immunofluorescence Assays |
Numerous, simultaneous samples can be analyzed and stored for some time. |
Fluorescent molecules bound to primary antibody is limited. Low sensitivity may result in false negatives. |
2.3. The Use of Plasmonic Biosensors for Food Analysis
Diseases and malnutrition occurring due to the quality of products made food safety of great priority to diminish the health risks [118][58]. Conventional methods used to ensure food safety like PCR, ELISA, high-performance liquid chromatography (HPLC), and liquid chromatography-mass spectrometry (LCMS) are accurate but costly, time-consuming, and laborious [124][59]. Hence, using automated optical biosensors is an optimum solution for analyzing food by using a highly sensitive and selective low-cost analytical tool [125][60]. SPR among different types of optical biosensors has undergone great development to enable the detection of various pathogens found in food. Plasmonic biosensors were further upgraded for better analysis via coupling of various methods (Raman, fluorescence, and luminescence) to have less LOD and higher sensitivity. Hence, as shown in Table 2, SPR biosensor is considered much more efficient than traditional methods in monitoring and analyzing food.|
Component |
Other Methods |
SPR |
Refs. |
||
|---|---|---|---|---|---|
|
Heavy metals |
Atomic Absorption Spectroscopy
Inductively Coupled Plasma Mass Spectrometry
X-ray Fluorescence Spectrometry
|
|
|||
|
Hemagglutination Assays |
|||||
|
Food Allergens |
Low-cost instruments. Results within hours. |
ELISA Has standardization as it is recognized in labs worldwide. |
Little specificity. Requires trained personnel. Analysis needed by qualified individuals. Difficult inter-laboratory comparison of results due to the several controlled variables. |
| |
[ |
Viral Plaque Assay |
||||
|
Citrinin (Mycotoxin) |
Available in most labs. Rapid results. |
HPLC and LC-MS
Absence of standardization. |
Involves costly repeat runs for accurate results. |
| |
|
Viral Flow Cytometry |
|||||
|
Pesticides | Rapid results. Numerous cells analyzed instantly. |
LC-MS/MS
Requires highly trained personnel. |
Requires ongoing maintenance by service engineers. |
| |
|
ELISA |
Accurate/fast results. | ||||
|
β-Lactoglobulin |
Very sensitive process. Easily automated. |
ELISA and LC-MS
Expensive preparation method. Requires trained personnel. |
| ||
|
CT |
Combined assessment. Short acquisition time. |
Expensive preparation method. Requires trained personnel. Exposure to gamma rays. |
|||
|
NAAT |
Very sensitive process. Accurate and reliable |
Requires trained personnel. Expensive detection kits. Time-consuming (2–3 days). False-positive cases. |
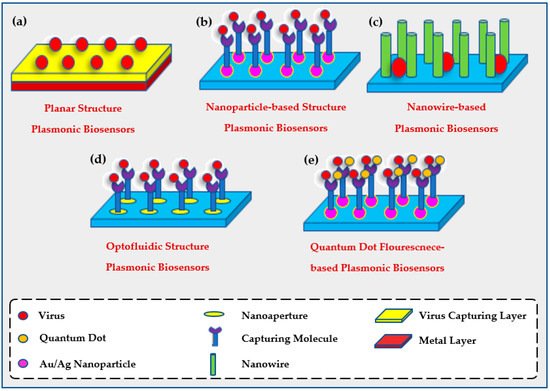
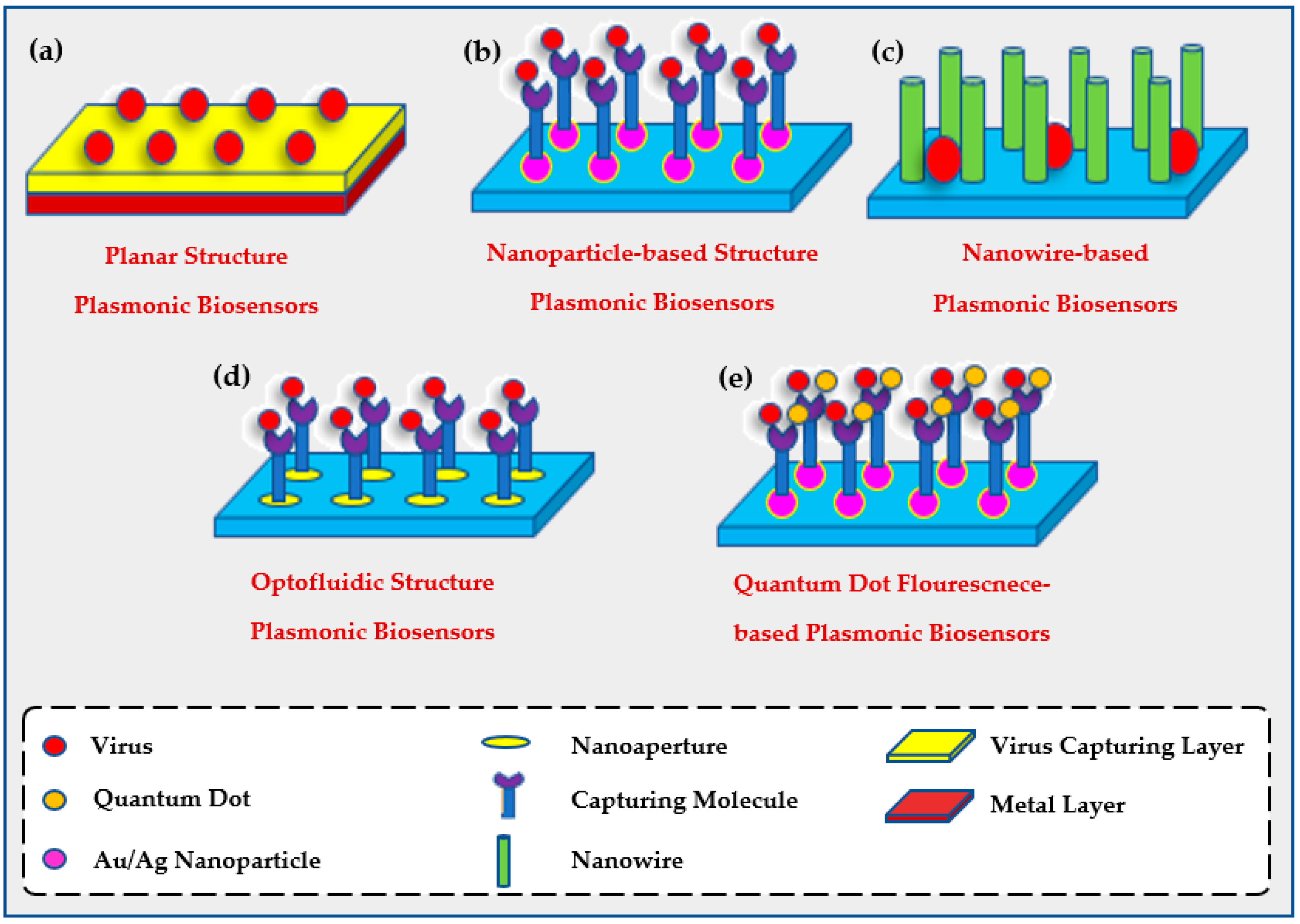
2.2. The Use of Plasmonic Biosensors for Environmental Evaluation
Plasmonic biosensors are great candidates when analyzing environmental contaminants. The amount of pollution increasing at a fast pace requires fast, highly specific, and cost-effective analytical tools that can be used for monitoring pollutants in our environment. Providentially, great initiatives have been made for controlling environmental pollution and several scientific researches were conducted and are still in progress to satisfy the concern of society regarding the environment and the pollution overtaking it [96,97,98,99][51][52][53][54]. Biosensors are great analytical techniques that can use a biological mechanism to detect analytes in the environment using chemical sensors for environmental evaluation [97,98,99][52][53][54][ | |||
, | |||
|
Tetrodotoxin (Fish toxin) |
LC-MS/MS, ELISA, and HPLC
|
|
3. Introducing Metamaterials to Plasmonic Biosensors
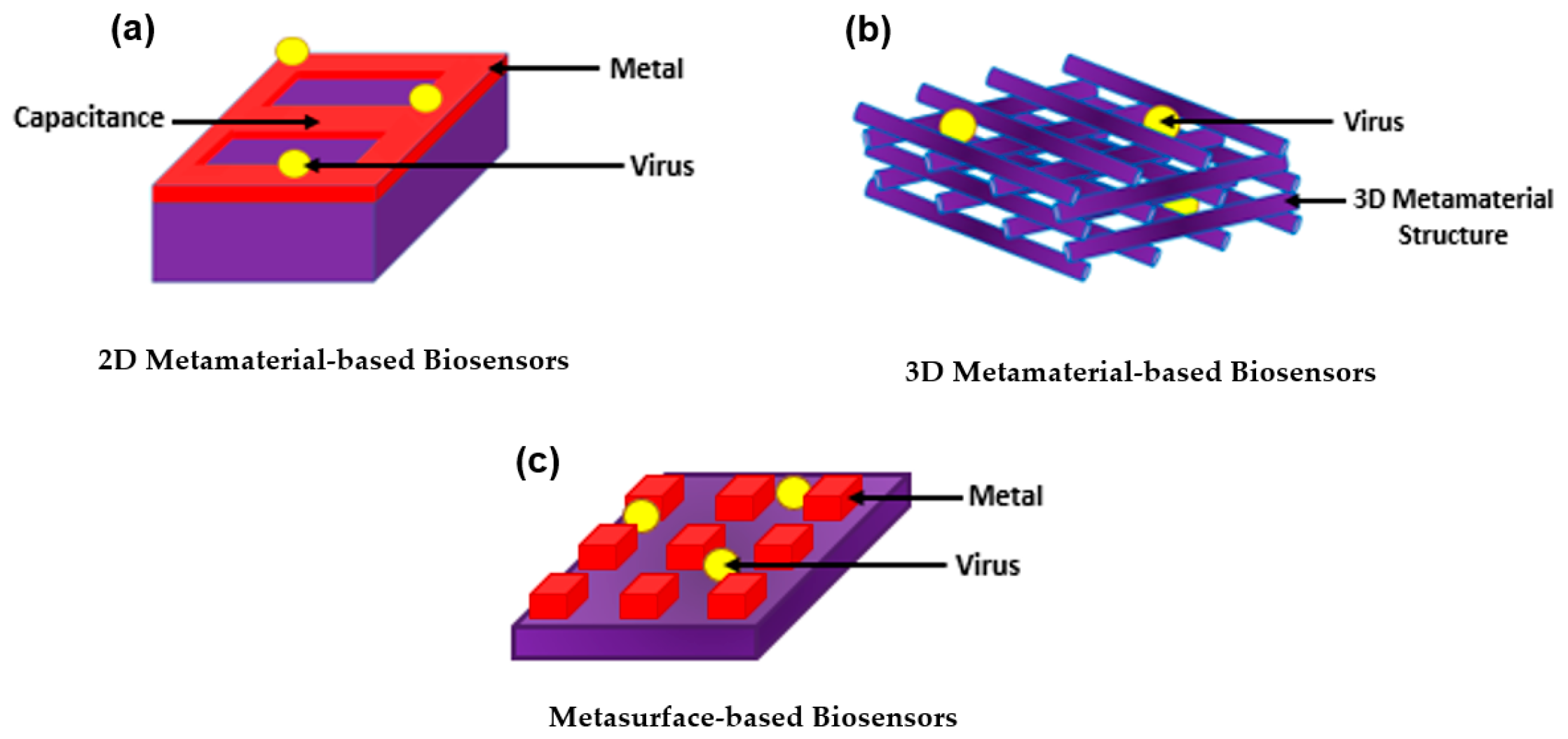
With all the exquisite properties of plasmonic biosensors, the sensitivity has been further improved by introducing metamaterials. Metamaterial-based biosensors provide different geometric structures, each having its own sensing properties, which expands and improves the use of conventional plasmonic biosensors [133][68]. In the 1960s, a Russian physic named Victor Veselago initiated a theoretical concept for materials with simultaneous negative permittivity and permeability where light propagates in an opposite direction to that of the flow of energy, giving an uncommon refraction of light. Materials that follow this concept are known as left-handed materials [134][69]. In 1999, a theory was made by Pendry et al. declaring that microstructures having extremely small nonmagnetic conducting sheets in comparison to the wavelength of radiation reveal a magnetic permeability that is highly effective and can be further modified to display changing magnetic permeability together with the imaginary component [135][70]. This substance was named in the same year by Rodger Walser as “metamaterials” which he defined as “macroscopic composites having a synthetic, three-dimensional, periodic cellular architecture designed to produce an optimized combination, not available in nature, of two or more responses to specific excitation” [136][71]. The following year, Smith et al. confirmed the use of left-handed metamaterial using a microwave regime by experimenting with interspaced nonmagnetic conductive split-ring resonators with continuous wires [137][72]. Since then, metamaterials have been used, manipulated, and geometrically enhanced to have tuned properties that can be used in different applications including sensors, biological imaging, and spectroscopy [138,139,140,141,142,143,144][73][74][75][76][77][78][79]. The use of metamaterials was further explored as biosensors as they were categorized based on their structure into three main groups; 2D metamaterial-based biosensors, 3D metamaterial-based biosensors, and meta-surface-based biosensors as shown in Figure 4. The breakthrough of metamaterials with their improved sensitivity allowed the successful detection of several viruses including HIV, Zika virus, avian influenza virus, CPMV, and PRD1 [145,146,147,148,149][80][81][82][83][84]. Even further, metamaterials became a tool for novelty in label-free point-of-care viral detection.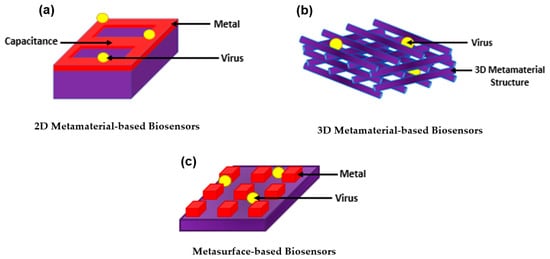
4. Lab-on-a-Chip (LoC) for Plasmonic Biosensors
Molecular and serological testing is critical for diagnosing patients. Hence, the need for having faster and more reliable diagnostic tools for immediate disease analysis and prevention of the spreading of pathogens and diseases is always pursued and high in demand [152][85]. LoC is considered the best device used for Point-of-Care testing (POCT) as it is based on biosensors that are designed for their application and can be upgraded with the most recent advances using microfluidics [153,154,155][86][87][88]. In this section, plasmonic biosensors are focused on due to their various LoC applications. The main target of the plasmonic-based LoC devices is to use planar technology to make an integrated photonic circuit that has good sensing capabilities [156,157,158,159,160][89][90][91][92][93]. This technology has a few unique proficiencies which integrates many different sensors on the same chip to detect different pathogens. In addition, the utilization of planar technology supports the mass production need and provides cost-effective solutions. Hence, silicon-based photonics are known to be a major technology platform for such applications [161,162,163][94][95][96]. Hence, plasmonics have been recently introduced at a wide range for such applications due to their greater sensitivity and improved selectivity [113,164][97][98]. The integration of plasmonic materials such as gold, silver, and aluminum along with silicon photonics standard technology has been a challenge with two folds; the first one is technology-related where metals may cause contamination for the conventional standard silicon photonics technology, while the second is the ability to efficiently couple the light from dielectric waveguides with modal field ranges from few microns, in case of optical fiber, to few hundreds of nanometers, for silicon waveguide, to a plasmonic mode with tight surface confinement and modal field in the range of few nanometers only. The impact of the former challenge has been reduced over time by optimizing the fabrication technology and using materials that have both plasmonic effects and compatibility of the silicon technology such as TiN and ITO [165,166,167][99][100][101]. Good coupling can be achieved between a silicon waveguide and the plasmonic slot mode over a wide range of wavelengths using an orthogonal coupling scheme similar to Otto configuration [163][96]. In 2015, the ability of doped silicon to support plasmonic mode in the mid-infrared wavelength range was introduced for the first time [164][98]. The main mechanism was to control the doping level of the silicon to achieve a plasmonic wavelength within the mid-infrared range by doping for silicon. For instance, a doping ranging from 10−19 to 5 × 1020 cm−3 can achieve a plasmonic behavior starting from ~10 to ~3 microns, respectively. Other III-VI semiconductors have been recently utilized for such applications [167][101]. The III-V materials have superior advantages over the silicon such that the on-chip detector can be fabricated using a compatible material from the same material group. The MIR range also has the advantage of providing unique absorption peaks for sensing gases or liquids for biomedical applications. This added advantage can help increase the selectivity of the proposed sensing system. Hence, a plasmonic biosensor made only from a dielectric can be apprehended in the MIR using the planar fabrication technology and yet with high sensitivity and selectivity at the same time [103,115,168,169][102][103][104][105].References
- Villalonga, A.; Pérez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72.
- Jadhav, D.A.; Chendake, A.D.; Ghosal, D.; Mathuriya, A.S.; Kumar, S.S.; Pandit, S. Advanced microbial fuel cell for biosensor applications to detect quality parameters of pollutants. In Bioremediation, Nutrients, and Other Valuable Product Recovery; Singh, L., Mahapatra, D.M., Thakur, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–139.
- Bollella, P.; Katz, E. Biosensors—Recent Advances and Future Challenges. Sensors 2020, 20, 6645.
- Nguyen, H.H.; Lee, S.; Lee, U.; Fermin, C.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121.
- Mauriz, E.; Lechuga, L.M. Plasmonic Biosensors for Single-Molecule Biomedical Analysis. Biosensors 2021, 11, 123.
- Solhi, E.; Hasanzadeh, M. Critical role of biosensing on the efficient monitoring of cancer proteins/biomarkers using label-free aptamer based bioassay. Biomed. Pharmacother. 2020, 132, 110849.
- Sezgintürk, M.K. Introduction to commercial biosensors. In Commercial Biosensors and Their Applications; Sezgintürk, M.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–28.
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159.
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406.
- Wu, K.; Saha, R.; Su, D.; Krishna, V.D.; Liu, J.; Cheeran, M.C.-J.; Wang, J.-P. Magnetic-Nanosensor-Based Virus and Pathogen Detection Strategies before and during COVID-19. ACS Appl. Nano Mater. 2020, 3, 9560–9580.
- Zhao, V.X.T.; Wong, T.I.; Zheng, X.T.; Tan, Y.N.; Zhou, X. Colorimetric biosensors for point-of-care virus detections. Mater. Sci. Energy Technol. 2020, 3, 237–249.
- Kaya, S.I.; Karadurmus, L.; Ozcelikay, G.; Bakirhan, N.K.; Ozkan, S.A. Electrochemical virus detections with nanobiosensors. Nanosens. Smart Cities 2020, 303–326.
- Antiochia, R. Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease. Biosensors 2021, 11, 110.
- Maddali, H.; Miles, C.E.; Kohn, J.; O’Carroll, D.M. Optical Biosensors for Virus Detection: Prospects for SARS-CoV-2/COVID-19. Chembiochem 2021, 22, 1176–1189.
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging applications of label-free optical biosensors. Nanophotonics 2017, 6, 627–645.
- Liu, Y.; Zhang, X. Microfluidics-Based Plasmonic Biosensing System Based on Patterned Plasmonic Nanostructure Arrays. Micromachines 2021, 12, 826.
- Liu, L.; Ye, K.; Jia, Z.; Xue, T.; Nie, A.; Xiang, J.; Mu, C.; Wang, B.; Wen, F.; Zhai, K.; et al. High-sensitivity and versatile plasmonic biosensor based on grain boundaries in polycrystalline 1L WS2 films. Biosens. Bioelectron. 2021, 194, 113596.
- Li, X.; Soler, M.; Özdemir, C.I.; Belushkin, A.; Yesilköy, F.; Altug, H. Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab Chip 2017, 17, 2208–2217.
- Armstrong, R.E.; Horáček, M.; Zijlstra, P. Plasmonic Assemblies for Real-Time Single-Molecule Biosensing. Small 2020, 16, 2003934.
- Minopoli, A.; Della Ventura, B.; Lenyk, B.; Gentile, F.; Tanner, J.A.; Offenhäusser, A.; Mayer, D.; Velotta, R. Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nat. Commun. 2020, 11, 6134.
- Mondal, B.; Zeng, S. Recent Advances in Surface Plasmon Resonance for Biosensing Applications and Future Prospects. 2020. Available online: https://hal-unilim.archives-ouvertes.fr/hal-02915657 (accessed on 18 March 2022).
- Duan, Q.; Liu, Y.; Chang, S.; Chen, H.; Chen, J. Surface Plasmonic Sensors: Sensing Mechanism and Recent Applications. Sensors 2021, 21, 5262.
- Hill, R.T. Plasmonic Biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 152–168.
- Miyazaki, C.; Shimizu, F.; Ferreira, M. Surface Plasmon Resonance (SPR) for Sensors and Biosensors. In Nanocharacterization Techniques; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 183–200.
- Chlebus, R.; Chylek, J.; Ciprian, D.; Hlubina, P. Surface Plasmon Resonance Based Measurement of the Dielectric Function of a Thin Metal Film. Sensors 2018, 18, 3693.
- Kravets, V.G.; Wu, F.; Yu, T.; Grigorenko, A.N. Metal-Dielectric-Graphene Hybrid Heterostructures with Enhanced Surface Plasmon Resonance Sensitivity Based on Amplitude and Phase Measurements. Plasmonics 2022.
- Zakaria, R.; Zainuddin, N.A.M.; Leong, T.C.; Rosli, R.; Rusdi, M.F.; Harun, S.W.; Sadegh Amiri, I. Investigation of Surface Plasmon Resonance (SPR) in MoS2- and WS2-Protected Titanium Side-Polished Optical Fiber as a Humidity Sensor. Micromachines 2019, 10, 465.
- Derkachova, A.; Kolwas, K.; Demchenko, I. Dielectric Function for Gold in Plasmonics Applications: Size Dependence of Plasmon Resonance Frequencies and Damping Rates for Nanospheres. Plasmonics 2016, 11, 941–951.
- Raza, S.; Stenger, N.; Kadkhodazadeh, S.; Fischer, S.V.; Kostesha, N.; Jauho, A.-P.; Burrows, A.; Wubs, M.; Mortensen, N.A. Blueshift of the surface plasmon resonance in silver nanoparticles studied with EELS. Nanophotonics 2013, 2, 131–138.
- Zhu, X.; Gao, T. Spectrometry. In Nano-Inspired Biosensors for Protein Assay with Clinical Applications; Li, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–264.
- Hassan, M.M.; Sium, F.S.; Islam, F.; Choudhury, S.M. A review on plasmonic and metamaterial based biosensing platforms for virus detection. Sens. Bio-Sens. Res. 2021, 33, 100429.
- Zhou, X.; Chen, K.; Li, L.; Peng, W.; Yu, Q. Angle modulated surface plasmon resonance spectrometer for refractive index sensing with enhanced detection resolution. Opt. Commun. 2017, 382, 610–614.
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward Flexible Surface-Enhanced Raman Scattering (SERS) Sensors for Point-of-Care Diagnostics. Adv. Sci. 2019, 6, 1900925.
- Park, S.-G.; Mun, C.; Xiao, X.; Braun, A.; Kim, S.; Giannini, V.; Maier, S.A.; Kim, D.-H. Surface Energy-Controlled SERS Substrates for Molecular Concentration at Plasmonic Nanogaps. Adv. Funct. Mater. 2017, 27, 1703376.
- Elsayed, M.; Gouda, A.; Ismail, Y.; Swillam, M. Silicon-Based SERS Substrates Fabricated by Electroless Etching. J. Lightwave Technol. 2017, 35, 3075–3081.
- EP17A2|Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures, 2nd ed. Available online: https://www.clsi.orghttps://clsi.org/standards/products/method-evaluation/documents/ep17/ (accessed on 18 March 2022).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 22 July 2021).
- Louten, J. Detection and Diagnosis of Viral Infections. Essent. Hum. Virol. 2016, 111–132.
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Laboratory Diagnosis of Virus Diseases. Fenner White’s Med. Virol. 2017, 135–154.
- Zhao, X.; Lin, C.-W.; Wang, J.; Oh, D.H. Advances in Rapid Detection Methods for Foodborne Pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312.
- Bağcı, U.; Bray, M.; Caban, J.; Yao, J.; Mollura, D.J. Computer-Assisted Detection of Infectious Lung Diseases: A Review. Comput. Med. Imaging Graph. 2012, 36, 72–84.
- Kumar, P. Methods for Rapid Virus Identification and Quantification. Mater. Methods 2013, 3, 13070.
- Yamamoto, K.; Ohmagari, N. Microbiological Testing for Coronavirus Disease 2019. JMA J. 2021, 4, 67–75.
- Bruce, E.A.; Mills, M.G.; Sampoleo, R.; Perchetti, G.A.; Huang, M.-L.; Despres, H.W.; Schmidt, M.M.; Roychoudhury, P.; Shirley, D.J.; Jerome, K.R.; et al. Predicting infectivity: Comparing four PCR-based assays to detect culturable SARS-CoV-2 in clinical samples. EMBO Mol. Med. 2022, 14, e15290.
- Ahmed, W.; Simpson, S.L.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Blackall, L.L.; Bofill-Mas, S.; Bosch, A.; Brandão, J.; Choi, P.M.; et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2022, 805, 149877.
- Toya, M.; Iida, Y.; Sugimoto, A. Imaging of Mitotic Spindle Dynamics in Caenorhabditis elegans Embryos. In Methods in Cell Biology; Cassimeris, L., Tran, P., Eds.; Microtubules: In vivo; Academic Press: Cambridge, MA, USA, 2010; Volume 97, pp. 359–372.
- Noah, D.L.; Hill, H.; Hines, D.; White, E.L.; Wolff, M.C. Qualification of the Hemagglutination Inhibition Assay in Support of Pandemic Influenza Vaccine Licensure. Clin. Vaccine Immunol. 2009, 16, 558–566.
- Mendoza, E.J.; Manguiat, K.; Wood, H.; Drebot, M. Two Detailed Plaque Assay Protocols for the Quantification of Infectious SARS-CoV-2. Curr. Protoc. Microbiol. 2020, 57, ecpmc105.
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973.
- Tymm, C.; Zhou, J.; Tadimety, A.; Burklund, A.; Zhang, J.X.J. Scalable COVID-19 Detection Enabled by Lab-on-Chip Biosensors. Cell. Mol. Bioeng. 2020, 13, 313–329.
- Rodriguez-Mozaz, S.; Marco, M.-P.; de Alda, M.J.L.; Barceló, D. Biosensors for environmental applications: Future development trends. Pure Appl. Chem. 2004, 76, 723–752.
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Marco, M.-P.; Barceló, D. Biosensors for environmental monitoring A global perspective. Talanta 2005, 65, 291–297.
- Rogers, K.R. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231.
- Rogers, K.R.; Gerlach, C.L. Peer Reviewed: Environmental Biosensors: A Status Report. Environ. Sci. Technol. 1996, 30, 486A–491A.
- Ju, H.; Kandimalla, V.B. Biosensors for pesticides. In Electrochemical Sensors, Biosensors and Their Biomedical Applications; Zhang, X., Ju, H., Wang, J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 31–56.
- Suryan, S.K. Biosensors: A Tool for Environmental Monitoring and Analysis. In Advances in Environmental Biotechnology; Kumar, R., Sharma, A.K., Ahluwalia, S.S., Eds.; Springer: Singapore, 2017; pp. 265–288.
- Gieva, E.; Nikolov, G.; Nikolova, B. Biosensors for Environmental Monitoring. In Proceedings of the Conference on Challenges in Higher Education & Research, Proceedings of the 12th Conference, Sozopol, Bulgaria, 17–19 November 2014; Volume 12.
- Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 30 December 2021).
- Ravindran, N.; Kumar, S.; Yashini, M.; Rajeshwari, S.; Mamathi, C.A.; Nirmal Thirunavookarasu, S.N.; Sunil, C.K. Recent advances in Surface Plasmon Resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–23.
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383.
- Wing Fen, Y.; Mahmood Mat Yunus, W. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314.
- Verma, R.; Gupta, B.D. Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chem. 2015, 166, 568–575.
- Ashley, J.; Shahbazi, M.-A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615.
- Atar, N.; Eren, T.; Yola, M.L. A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice. Food Chem. 2015, 184, 7–11.
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens. Bioelectron. 2019, 142, 111449.
- Çakır, O.; Baysal, Z. Pesticide analysis with molecularly imprinted nanofilms using surface plasmon resonance sensor and LC-MS/MS: Comparative study for environmental water samples. Sens. Actuators B Chem. 2019, 297, 126764.
- D’Aurelio, R.; Ashley, J.; Rodgers, T.L.; Trinh, L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Development of a NanoMIPs-SPR-Based Sensor for β-Lactoglobulin Detection. Chemosensors 2020, 8, 94.
- Ashley, J.; D’Aurelio, R.; Piekarska, M.; Temblay, J.; Pleasants, M.; Trinh, L.; Rodgers, T.L.; Tothill, I.E. Development of a β-Lactoglobulin Sensor Based on SPR for Milk Allergens Detection. Biosensors 2018, 8, 32.
- Yakes, B.J.; Deeds, J.; White, K.; Degrasse, S.L. Evaluation of surface plasmon resonance biosensors for detection of tetrodotoxin in food matrices and comparison to analytical methods. J. Agric. Food Chem. 2011, 59, 839–846.
- Hodnik, V.; Anderluh, G. Toxin detection by surface plasmon resonance. Sensors 2009, 9, 1339–1354.
- Chalyan, T.; Potrich, C.; Schreuder, E.; Falke, F.; Pasquardini, L.; Pederzolli, C.; Heideman, R.; Pavesi, L. AFM1 Detection in Milk by Fab’ Functionalized Si3N4 Asymmetric Mach-Zehnder Interferometric Biosensors. Toxins 2019, 11, 409.
- Smith, D.R.; Padilla, W.J.; Vier, D.C.; Nemat-Nasser, S.C.; Schultz, S. Composite Medium with Simultaneously Negative Permeability and Permittivity. Phys. Rev. Lett. 2000, 84, 4184–4187.
- Beruete, M.; Jáuregui-López, I. Terahertz Sensing Based on Metasurfaces. Adv. Opt. Mater. 2020, 8, 1900721.
- Chen, T.; Li, S.; Sun, H. Metamaterials application in sensing. Sensors 2012, 12, 2742–2765.
- Zhou, R.; Wang, C.; Xu, W.; Xie, L. Biological applications of terahertz technology based on nanomaterials and nanostructures. Nanoscale 2019, 11, 3445–3457.
- Ahmadivand, A.; Gerislioglu, B.; Tomitaka, A.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Extreme sensitive metasensor for targeted biomarkers identification using colloidal nanoparticles-integrated plasmonic unit cells. Biomed. Opt. Express 2018, 9, 373–386.
- Lee, D.-K.; Kang, J.-H.; Kwon, J.; Lee, J.-S.; Lee, S.; Woo, D.H.; Kim, J.H.; Song, C.-S.; Park, Q.-H.; Seo, M. Nano metamaterials for ultrasensitive Terahertz biosensing. Sci. Rep. 2017, 7, 8146.
- Ahmed, R.; Ozen, M.O.; Karaaslan, M.G.; Prator, C.A.; Thanh, C.; Kumar, S.; Torres, L.; Iyer, N.; Munter, S.; Southern, S.; et al. Tunable Fano-Resonant Metasurfaces on a Disposable Plastic-Template for Multimodal and Multiplex Biosensing. Adv. Mater. 2020, 32, e1907160.
- Park, S.J.; Cha, S.H.; Shin, G.A.; Ahn, Y.H. Sensing viruses using terahertz nano-gap metamaterials. Biomed. Opt. Express 2017, 8, 3551–3558.
- Sreekanth, K.V.; El Kabbash, M.; Alapan, Y.; Ilker, E.I.; Hinczewski, M.; Gurkan, U.A.; Strangi, G. Hyperbolic metamaterials-based plasmonic biosensor for fluid biopsy with single molecule sensitivity. EPJ Appl. Metamater. 2017, 4, 1.
- Aristov, A.I.; Manousidaki, M.; Danilov, A.; Terzaki, K.; Fotakis, C.; Farsari, M.; Kabashin, A.V. 3D plasmonic crystal metamaterials for ultra-sensitive biosensing. Sci. Rep. 2016, 6, 25380.
- Cheng, D.; He, X.; Huang, X.; Zhang, B.; Liu, G.; Shu, G.; Fang, C.; Wang, J.; Luo, Y. Terahertz biosensing metamaterial absorber for virus detection based on spoof surface plasmon polaritons. Int. J. RF Microw. Comput.-Aided Eng. 2018, 28, e21448.
- Ahmadivand, A.; Gerislioglu, B.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Rapid Detection of Infectious Envelope Proteins by Magnetoplasmonic Toroidal Metasensors. ACS Sens. 2017, 2, 1359–1368.
- Alam, M.M.; Malik, H.; Khan, M.I.; Pardy, T.; Kuusik, A.; Le Moullec, Y. A Survey on the Roles of Communication Technologies in IoT-Based Personalized Healthcare Applications. IEEE Access 2018, 6, 36611–36631.
- Kotb, R.; Ismail, Y.; Swillam, M. Integrated metal-insulator-metal plasmonic nano resonator: An analytical approach. Prog. Electromagn. Res. Lett. 2013, 43, 83–94.
- El-Zohary, S.E.; Azzazi, A.A.; Okamoto, H.; Okamoto, T.; Haraguchi, M.; Swillam, M.A. Resonance-based integrated plasmonic nanosensor for lab-on-chip applications. JNP 2013, 7, 073077.
- Swillam, M.; Helmy, A. Analysis and applications of 3D rectangular metallic waveguides. Opt. Express 2010, 18, 19831–19843.
- Sherif, S.M.; Zografopoulos, D.C.; Shahada, L.A.; Beccherelli, R.; Swillam, M. Integrated plasmonic refractometric sensor using Fano resonance. J. Phys. D Appl. Phys. 2017, 50, 055104.
- Gamal, R.; Ismail, Y.; Swillam, M.A. Optical biosensor based on a silicon nanowire ridge waveguide for lab on chip applications. J. Opt. 2015, 17, 045802.
- Elsayed, M.Y.; Sherif, S.M.; Aljaber, A.S.; Swillam, M.A. Integrated Lab-on-a-Chip Optical Biosensor Using Ultrathin Silicon Waveguide SOI MMI Device. Sensors 2020, 20, 4955.
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519.
- Zaki, A.O.; Kirah, K.; Swillam, M.A. Integrated optical sensor using hybrid plasmonics for lab on chip applications. J. Opt. 2016, 18, 085803.
- Khalifa, A.E.; Swillam, M.A. Plasmonic silicon solar cells using titanium nitride: A comparative study. JNP 2014, 8, 084098.
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative Plasmonic Materials: Beyond Gold and Silver. Adv. Mater. 2013, 25, 3264–3294.
- Naik, G.V.; Kim, J.; Boltasseva, A. Oxides and nitrides as alternative plasmonic materials in the optical range . Opt. Mater. Express OME 2011, 1, 1090–1099.
- Lau, B.; Swillam, M.A.; Helmy, A.S. Hybrid orthogonal junctions: Wideband plasmonic slot-silicon waveguide couplers. Opt. Express OE 2010, 18, 27048–27059.
- Ayoub, A.B.; Ji, D.; Gan, Q.; Swillam, M.A. Silicon plasmonic integrated interferometer sensor for lab on chip applications. Opt. Commun. 2018, 427, 319–325.
- Gamal, R.; Ismail, Y.; Swillam, M.A. Silicon Waveguides at the Mid-Infrared. J. Lightwave Technol. JLT 2015, 33, 3207–3214.
- Elsayed, M.Y.; Ismail, Y.; Swillam, M.A. Semiconductor plasmonic gas sensor using on-chip infrared spectroscopy. Appl. Phys. A 2017, 123, 113.
- Sherif, S.M.; Swillam, M.A. Metal-less silicon plasmonic mid-infrared gas sensor. JNP 2016, 10, 026025.
- Ayoub, A.B.; Swillam, M.A. Silicon Plasmonics On-Chip Mid-IR Gas Sensor. IEEE Photonics Technol. Lett. 2018, 30, 931–934.
- Gamal, R.; Shafaay, S.; Ismail, Y.; Swillam, M.A. Silicon plasmonics at midinfrared using silicon-insulator-silicon platform. JNP 2017, 11, 016006.
- El Shamy, R.S.; Khalil, D.; Swillam, M.A. Mid Infrared Optical Gas Sensor Using Plasmonic Mach-Zehnder Interferometer. Sci. Rep. 2020, 10, 1293.
- Schwarz, B.; Reininger, P.; Ristanić, D.; Detz, H.; Andrews, A.M.; Schrenk, W.; Strasser, G. Monolithically integrated mid-infrared lab-on-a-chip using plasmonics and quantum cascade structures. Nat. Commun. 2014, 5, 4085.
- Moon, G.; Choi, J.-R.; Lee, C.; Oh, Y.; Kim, K.H.; Kim, D. Machine learning-based design of meta-plasmonic biosensors with negative index metamaterials. Biosens. Bioelectron. 2020, 164, 112335.
