Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Bianca Galateanu and Version 2 by Camila Xu.
Organ-on-chips (OOCs) are microfluidic devices used for creating physiological organ biomimetic systems. OOC technology brings numerous advantages in the current landscape of preclinical models, capable of recapitulating the multicellular assemblage, tissue–tissue interaction, and replicating numerous human pathologies.
- organ-on-chip
- tumor-on-chip
- polymeric microfluidic devices
1. Introduction
Regardless of the therapeutic area of new emerging therapies and novel agents entering the market, nephrotoxicity is a major challenge, as the kidney is the second target of drugs and chemicals after the liver. More than 25% of the adverse effects in today’s pharmacotherapy are caused by nephrotoxic effects [1]. Of these, 20% are reported during postmarket surveillance [2], as early stages of drug development fail to deliver relevant output with this respect [3]. Consequently, the poor correlation between the preclinical and clinical outcomes has led to the failure of most drugs before reaching the patient [4]. Preclinically approved drugs have been withdrawn a few times due to the side effects observed in the clinical trials [5][6][5,6]. Therefore, the pharmaceutical industry is under high pressure to speed up the drug-development process and to design new cures that are very effective in humans with reasonable costs [7].
The traditional in vivo tests on animal models are costly and often fail to accurately predict the efficiency and toxicity in humans due to the species’ different metabolic responses to specific agents and the variations in some genes’ expressions, such as cytochrome P450 genes [8]. Consequently, the poor similarity between the physiological environment in animals and human bodies, which may alter the results of drug efficiency in various diseases, is a major barrier for future use of in vivo tests on animals [9]. With respect to cancer research, animal models in particular lack predictability [10] since they do not recreate the exact human tumor microenvironment (TME) and may exhibit different cell biology and cancer behavior when tumorous cells interact locally with stromal cells. In addition, the ethics concerns of sacrificing animals are a significant barrier in testing many discovered drugs on animals [11][12][11,12]. In 2021, the European Parliament agreed with a large majority to ban experiments on animals, which have killed about 12 million animals in 2017, revealing the importance of finding alternatives for biological assays developed with other procedures than sacrificing animals [13].
The main difficulties in cancer research are forming an effective in vitro TME able to accurately recapitulate the local tissue in which the tumor is forming [14][16]. Conventional preclinical in vitro models for anticancer drug screening are generally classified in 2D cell cultures and 3D cell architectures and have been extensively exploited as simple and cost-efficient methods to simulate cancer propagation and drug response [15][17]. The 3D cancer models deliver a helpful substitute to animals, but they still do not consider the dynamic environment of the human tissues or organs. However, they do not reproduce the complex assemblage of the human 3D cells from living organs to properly elucidate the cancer cell migration and invasion, also taking into consideration the mechanical forces (such as hydrostatic pressure, fluid shear stress, breathing motions in lung) naturally occurring in human bodies. Nonetheless, neither of these systems is transporting a blood or nutrient-rich medium through an endothelium-lined vasculature, limiting the real prediction of tissue–tissue interactions and circulating immune cells during therapeutic drug dosage [16][17][18,19].
Recently, new devices known as organ-on-chips (OOCs), which are able to recapitulate the multicellular assemblage, tissue–tissue interactions, and to replicate human pathologies and the appropriate physical TME, have emerged as a practical cost-efficient solution for tumor-growth investigation and anticancer-drug screening by combining the microfluidic technology with 3D cell-culture procedure to simulate the entanglement of the cells as in their native environment [17][18][19][19,22,23]. OOCs are compact and easy-to-use microphysiological functional units that recapitulate the native function and the mechanical strain that the cells experience in the human bodies, allowing the development of a wide range of applications such as disease modeling or even the development of diagnostic devices. However, important features of the membranes involved in the fabrication of OOC compartments to allow cells’ structural support and nutrient transportation are often poorly investigated. Nowadays, both synthetic and natural polymers are explored for the manufacturing process of advanced OOC microdevices, being able to replicate various organ bionic pathophysiological models. Poly(dimethylsiloxane) is one of the most employed synthetic polymers used for lung, liver, heart, and multi-organ-on-chip (MOOC) membranes in microfluidic devices due to its extraordinary high transparency and flexibility. However, it is not a degradable material able to contribute to the formation of the natural extracellular matrix (ECM). Alternative biopolymers with higher biocompatibility, such as collagen-based materials containing cell-growth factors and hormones, have been used for OOC fabrication to simulate the physiological behavior of living organs. Despite significant advances, many polymeric materials still do not meet the mechanical properties of the in vivo organs and do not exhibit optimal cytocompatibility suitable for accurate pharmaceutical screening or dynamic simulation of cancer cell behavior.
2. Fabrication of Organ-on-Chip
2.1. Principle and Manufacture of Organ-on-Chips
Microfluidic technologies have rapidly developed in the past years in terms of fabrication methodology, materials involved, and complexity of the systems to faithfully respond to the medical requirements. OOCs are microfluidic cell-culture micromachines that can recapitulate an organ-level response to medical treatment and reconstruct physiological dynamics observed in native human tissues, for instance, physiological flow, biomechanical motions, nutrient transportation, and drug delivery [18][22], in a convenient manner, delivering a platform with a new opportunity for oncology research. As biology-inspired engineered microdevices [20][24], the OOCs for tumor investigation must enable a series of possibilities: (i) the introduction of pharmaceutical compounds or reagents as fluids with the similar dynamic flow as for biological fluids; (ii) the ability to perfuse these fluids around on the chip, and to combine and mix them; (iii) the introduction of other sensors or devices for monitoring the results, such as detectors for bioanalysis. By incorporating tumor organoids in microfluidic devices, “tumor-on-chip” (TOCs) models that allow the reconstruction of the TME are created. These chips enable a deeper understanding of the tumor mechanism in vivo, which runs to enhanced preclinical evaluation of drug efficiency. Human solid tumors are highly heterogeneous [14][16], owning a complex microenvironment with a dense ECM, abnormal vessels, various stromal cells, or different immune-type cells [18][22]. Additionally, the nearby stromal tissues of the tumor act as an active source (and reservoir) of different cytokines and growth factors that affect the tumor development and pharmacological feedback. Several studies have shown that the complex variety of the cellular microenvironment may impact in some respect the tumor behavior, including tumorigenesis, angiogenesis, tumor invasion, metastasis, and endurance to therapeutic products. Many compounds generated by tumorous or stromal cells determine the propagation dynamics of solid tumors. Moreover, the 3D nature and the size of the tumor proved to be an utmost concern in the proper understanding of tumor dynamics showing a direct connection between the size of a tumor and its aggressiveness and the ability of a drug to be delivered to it [21][22][23][25,26,27]. Consequently, cancer is a true suite of complex pathologies that share some common elements and presents few differences that seriously complicate the choice of satisfactory treatment. Unlike in vivo tumor-growth microenvironments, in vitro cancer models are typically investigated under atmospheric conditions not specific to the living organs. In an in vitro metastasis cell culture, migrated tumor cells have to be subjected to varying microenvironments and oxygen gradients to mimic the activity of in vivo intravasation [24][28]. Tumor chips combine micromachining and cell biology to manipulate the external conditions and precisely mimic physiological environments, such as dynamic mechanical stress, fluid shear, oxygen, and drug concentration gradients and cell patterning to reflect the full picture of tumor formation and growth mechanism [25][26][29,30]. As TOCs resulted from the need to investigate appropriately cancerous cells in their specific TME, the fabrication technology is the same as for conventional OOCs, whereas the healthy organ cells were replaced by diseased cells. The microdevices are named chips since they were originally engineered using micromanufacturing techniques used in computer-microchip production [27][20]. Microfluidic systems can be employed to form tumor chips with a single-line channel by engaging cells from a single source, or more complex organ chips that associate two or more tissue categories that can be interposed right through a porous ECM-coated membrane or an ECM gel that fills one or more micronetworks [28][31]. The viability of the cells can be preserved over prolonged time intervals (up to several months) by perfusing the culture medium either across the endothelium-lined vascular microchannels, parenchymal microchannels, or both. More importantly, the culture medium can be substituted by blood for several hours through endothelialized vascular channels [29][32]. There are two steps required to be taken into consideration for the proper design of a tumor chip: (1) to comprehend the fundamentals essential for the physiological function of the aimed organ, and afterward to establish the key factors such as various cell types, structures, and the organ’s particular physiological microenvironment; (2) to construct a cell-culture device relying on the identified features. Different procedures have been embraced to build tumor-chip kits, among which the most extensively engaged are photolithography and soft lithography [30][31][33,34], replica molding [32][35], microcontact printing, and bioprinting techniques [33][34][35][36,37,38].2.1.1. Lithography
Lithography represents an etching process applied in microfabrication to project parts on a thin layer or the form of a substrate (also named a wafer) and is commonly divided into three categories: photolithography (or UV lithography), soft lithography, and replica molding. Photolithography employs light to transfer a geometric shape from a photomask to a photosensitive chemical photoresist on the wafer. In the first step, masks are required that correspond to the targeted constructions [18][31][22,34]. The manufacturing process continues with the deposition of a spin-coated photoresist layer on a wafer that can be corroded by chemical reagents, for instance, silicon, glass, or quartz, and the photoresist is subjected to UV photopolymerization. Afterward, the pattern is moved to the wafer and is etched to achieve a microfluidic chip with microflow channels. Although extensively employed, the lithography-manufacturing processes are costly due to the cleanroom requirements, the necessity of multiple masks, and time-consuming multiple processing steps. Recently, Kasi and coworkers proposed a rapid-prototyping organ-chip device using maskless photolithography [36][39]. The researcheuthors reported a simplified method that describes a rapid and cleanroom-free microfabrication compatible with soft lithography for fast-prototyping organ-chip devices in a maximum of 8 h. Soft lithography used for tumor-chip microfabrication involves in the first stage the preparation of a microchannel mold on a silicon substrate by photolithography [37][40], followed by the use of a liquid polymer (commonly polydimethylsiloxane, PDMS) to discharge the mold to acquire an optically transparent elastomeric stamp with microstructures. In the end, different complex 3D microchannels are achieved on various polymer wafers by transferring the pattern from the stamp. Ferreira et al. have recently developed a fast alternative to soft lithography to manufacture OOCs based on PDMS with integrated microactuators. The novel protocol decreases the complexity and number of steps, and is more time and cost-efficient compared to complex multilayered microfluidic devices [38][41]. Replica molding represents the technology in which a patterned silicon mold is employed, followed by the pouring step of a liquid polymer (usually PDMS) onto the mold for thermal crosslinking [39][42]. Later, the PDMS instrument is removed from the substrate and fixed on a clean, smooth wafer (for instance glass) to achieve a microfluidic chip with microfluidic networks. The microcontact-printing technology is very similar to the replica-molding technique [33][36]. It is differentiating only by the supplementary steps further used to manipulate the pattern of cultured cells by printing the PDMS stamp on the wafer with biomolecules (such as proteins) in a designed pattern so that the cells on the membrane can be modeled as well by adjusting the pattern of the printed proteins [40][41][43,44]. However, even though microfluidic devices have been successfully fabricated by lithography or related techniques, the procedures are still costly and time-consuming. Additionally, these procedures are able only to produce the microchip itself, while other components such as microtissues, mechanical stimuli, or result detectors need to be separately produced. Recent advances in lithography-based fabrication techniques have emerged in high-throughput technologies with a standardized format, being more user-friendly and affordable for large preclinical research studies. The OrganoPlate system (Mimetas, Leiden, The Netherlands) is a commercial compact microfluidic device that can process 96 independent cultures using a standard microtiter plate. The microdevice replaces the inner membrane with a gel-media boundary for transepithelial transportation showing good results for the investigation of fluidic diffusion, tumor-cell invasion, and aggregation and toxicity assays [2]. The main technologies used for the microfabrication of OOCs are shown in Figure 1.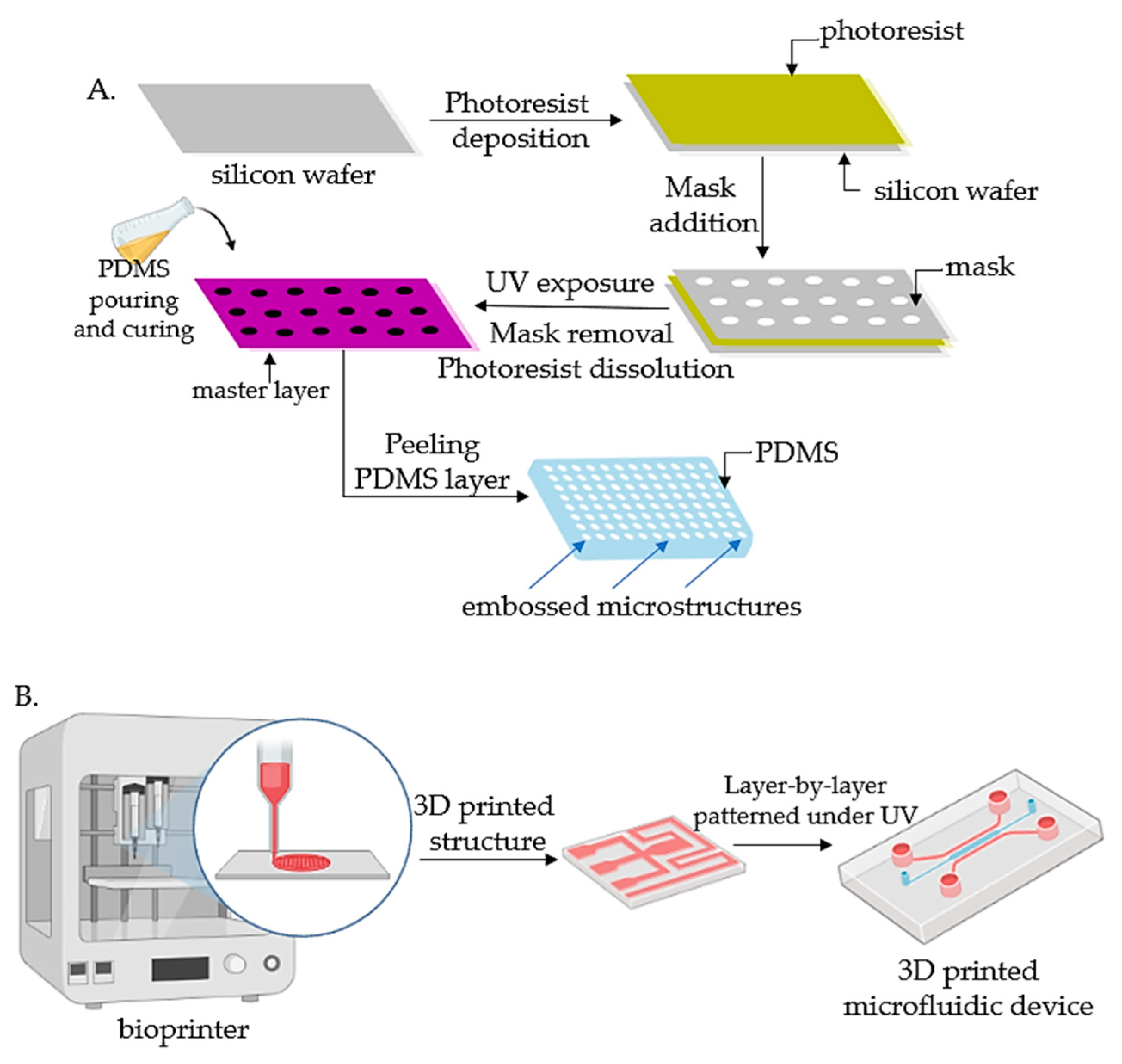
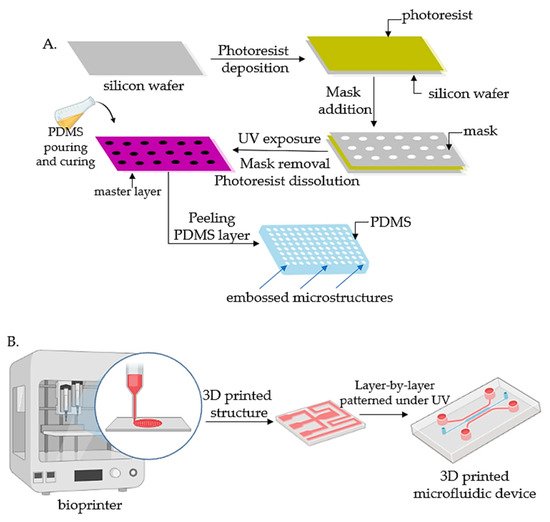
Figure 1. Fabrication technologies for OOCs: (A) The fabrication of micropatterned slabs of PDMS through photolithography; (B) Schematic 3D-printing process for the fabrication of microfluidic devices.
Injection molding allows fast replication of polymeric (micro)structures with great surface quality. Generally, the injection-molding process requires a few basic steps: (a) the material is melted and the molds are compressed together, and (b) the material is injected into the mold and cooled down prior to removal [31][34]. Although it has the advantage of large-scale production of OOCs, the fabrication process requires the careful adjustment of multiple parameters such as injection pressure, injection speed, melting temperature, etc. [42][45], to ensure high-quality production. Thermoplastic materials such as polystyrol [43][46] were used for the fabrication of liver-on-a-chip microdevices. One of the main disadvantages of this technique refers to the limited area of materials that can be used, as most of the polymers show thermal shrinkage during the fabrication [42][45]. However, the injection-molding process is reducing the time of fabrication and the final costs and therefore is predominantly used for the production of the commercially available elements of OOCs.
Laser-ablation methods, such as micromachining or computer numerical-control (CNC) micromachining, are surpassing the limitations of manual control during the fabrication of OOC microdevices. Laser micromachines use a laser to engrave the OOC device, and the process is applicable to a wide range of materials such as metals, glass or polymers. Shaegh et al. [44][47] designed a rapid prototyping method to produce microfluidic chips from thermoplastics with patterned microvalves combining laser ablation and thermal-fusing bonding. In this study, a CO2-assisted laser micromachine was used to pattern and cut PMMA layers covered with polyurethane film in order to generate a gas-actuated microvalve for microfluidic lab-on-a-chip applications. CNC micromachining is a fully automated manufacturing process in which the machines are operated via numerical control, wherein a computer software dictates the shape of the desired object. The laser-processing techniques have the advantage of rapidly creating precise and complex geometries of microdevices. Moreover, the laser-ablation techniques may be combined with the previously discussed methods for rapid prototyping or master models in order to achieve the desired microfluidic system, but require high technical knowledge for operating and expensive machinery to perform OOC fabrication [31][34].
2.1.2. Bioprinting
Bioprinting technology has emerged in the last years as a versatile tool based on layer-by-layer addition that can be easily used for tumor-chip manufacturing [45][46][47][48,49,50]. A significant advantage is related to a wider range of materials and cells that can be printed simultaneously onto a substrate of cell-compatible biomaterials to construct 3D composite blocks with good spatial resolution and reproducibility [48][49][51,52]. Bioprinting also comprises a large group of procedures that can be divided into fused-deposition modeling (FDM), stereolithography, inkjet printing, and laser-assisted bioprinting [50][53]. Bioprinting procedures are appealing due to numerous advantages that accommodate the tumor-chip fabrication. Bioprinting technology can reconstruct the diverse TME and the 3D nature of the tumor by using bioinks that form cell conglomerations that can include various cell types, such as tumor-related fibroblasts, immune cells, and endothelial cells, to create vascular systems [51][54]. In addition, layer-by-layer bioprinting enables the formation of the biomimetic microenvironment for a heterogenous supply of biologically important proteins and growth factors that are essential to managing cancer-cell signaling, growth, and invasion [52][55]. Furthermore, the bioprinting technique allows for the printing of cells quickly and precisely in microfluidic chips, pattern vasculature, and model biological barriers. Vascularization channels play a significant role as they are vital to preserve tissue activities or to differentiate tissue parts. The tumor vasculature is much distinct from the blood vessels of healthy tissues, showing alterations in heterogeneity, multidirectional blood flow, permeability, and unordered distribution all over the diseased cells [53][56]. Proper vascularization of specific cancer-type cells has constantly been questioned in the production of functional in vitro tumor-cell cultures. Bioprinting technology manages to overcome these difficulties, showing the ability to replicate the abnormalities encountered in tumor tissue by building miniaturized pathophysiological models and offering control of features at the same size scale of living cells [54][55][56][57,58,59]. The main polymers used as bioinks for OOCs by bioprinting are summarized in Table 1.Table 1.
The main polymers used as bioinks for OOCs by bioprinting.
[71].
Additionally, the hydrophilicity of the polymeric membrane represents an important factor in determining the protein adsorption and, therefore, cell adhesion. It was observed that the hydrophobicity of the polymeric membrane could cause the absorption of small nonspecific molecules such as drugs or biomolecules from the cellular media, misleading the experimental results [69][72]. For this reason, most synthetic polymers have been submitted to chemical or physicochemical surface modifications to increase the hydrophilicity of the materials by enhancing their wettability properties. PMMA structures have been modified with different hydrophilic functionalities such as aminated polyethyleneglycol [70][73] or submitted to oxygen plasma treatment for the activation of the PMMA surface [71][74] to control the cell adhesion. The cellular adhesion is strongly determined by the surface wettability and roughness of the polymer, while the cell attachment is influenced by the cell type [72][75]. Premnath and colleagues [73][76] developed a simple approach to laser-modify the surface of a silicon chip to adjust cell adhesion and proliferation, and showed that the cervical cancer cells’ behavior is modulated to migrate onto untreated sites. Transparent polyethersulfone (PES) membranes have proven to be with optimized morphology, and have showed improved adhesion and cell viability compared to the commercial hydrophilic polytetrafluoroethylene (PTFE) [74][77]. Moreover, the increase in hydrophilicity of the polymeric layers enhanced the cell adhesion as well as the cell-adhesive proteins. It is worth mentioning that hydrophilicity/hydrophobicity balance can also control the essential nutrient diffusion through the polymeric membrane from the OOCs compartments and generally, water contact-angle measurements are performed to determine the wettability properties of the polymers (Table 2).
| Bioink Composition | Bioprinting Method | OOC Model | References | |
|---|---|---|---|---|
| Collagen | extrusion | Lung, gut | [57][58] | [60,61] |
Table 2.
The main polymers employed in the fabrication of membranes in OOCs microdevices.
As the surface properties such as surface roughness and wettability influence the first stages of cell adhesion and migration, the mechanical properties of the polymeric membrane are modulated by the material’s stiffness and influence the later stages of cell growth. Investigations such as tensile strength, compressive stress, and wettability are generally achieved to determine if the polymer properties are suitable for the application. Thus, the mechanical performances of the interface membrane from the OOCs should be chosen accordingly to the native organ characteristics and to mimic the occurring physiological stimuli present in the replicated environment. Moreover, the porosity of the membrane layer represents an essential feature as it provides cell communication between the chip compartments and allows nutrient transportation to the cells. Pore size and shape also determine the space available for the cells to migrate, and small pores mainly reduce the cell adhesion, but increase the barrier function of cells.
| Polymer | Chemical Structure | Contact Angle with Water | Young’s Modulus | Application | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polydimethylsiloxane (PDMS) | 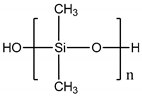 |
107° [75] | 107° [78] | Variable from kPa to MPa | Cardiovascular [76] Kidney [77] Liver [78] Lung [79] | Cardiovascular [79] Kidney [80] Liver [81] Lung [82] |
||||
| Gelatin | extrusion | kidney | ||||||||
| Poly (bisphenol-A- carbonate) (PC) | 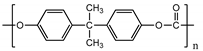 |
~85° [80] | ~85° [83] | [59][60] | [62,63] | |||||
| ~1.2 GPa | [ | 81 | ] | ~1.2 GPa [84] | Tumor vasculature [82] Colon and breast cancer [83] | Tumor vasculature [85] Colon and breast cancer [86] |
Methacrylate gelatin (GelMa) | extrusion | Vessel, liver | |
| Poly (ethylene terephthalate) (PET) | 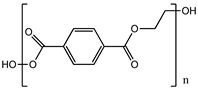 |
83° [84] | 83° [87] | [61][62] | [64,65] | |||||
| 4.7 GPa | [ | 85 | ] | 4.7 GPa [88] | Gut [86] Kidney [87] Liver [88] | Gut [89] Kidney [90] Liver [91] |
Alginate | extrusion | Heart | [63 |
| Polylactic acid (PLA) | 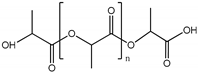 |
~75° [89] | ~75° [92] | ] | 3.1 GPa [90[64] | [66,67] | ||||
| ] | 3.1 GPa [ | 93 | ] | Endothelial barrier [91] | Endothelial barrier [94] | Cellulose | extrusion | tumor, liver | ||
| Poly (ε-caprolactone) (PCL) | 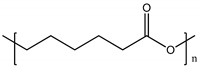 |
120° [92] | 120° [95] | [65][66] | [68,69] | |||||
| Polyethyleneglycol (PEG) Poly ε caprolactone (PCL) |
inkjet, extrusion | Colon tumor | [56][62] | [59,65] |
2.2. Material Requirements for the Fabrication of Organ-on-Chips
Many biomaterials and fabrication methods have been recently developed to meet the OOCs’ functions. While designing the OOC microdevice, the organ or tissue characteristics and behavior must be considered to accurately simulate the in vivo motions, cell proliferation, or drug responses. Considering the organ characteristics aimed to be mimicked, different types of OOCs can be created. However, although each type of OOCs may function differently, the main components remain the same. The OOC system is generally equipped with two compartments: first, the blood vessel compartment, which contains the endothelial cells; and secondly, the organ compartment, in which the cells of the investigated organ are introduced. These compartments are usually separated by a porous polymeric membrane able to provide cell communication between the two compartments of the device. Thus, the polymeric membranes play an essential role in the successful functioning of the OOC system, acting as interfaces with selective permeability for cell adhesion and cell separation. The culture medium included in OOCs system may be artificially produced or naturally derived from living organs. The natural medium mainly contains biological fluids such as blood, plasma, etc., and organ fragments. The artificial environment is fabricated by involving nutrients that mimic the physiological conditions, such as salts, oxygen and CO2 gasses, and blood substitutes. Thus, the cell-culture medium will provide a continuous supply of nutrients and the porous polymeric membrane will deliver the cells of interest that are cultured on the other side of the membrane. In addition to the nutrient-rich fluid perfusion and oxygen ventilation from the blood-vessel compartment, in the organ compartment, mechanical forces can be applied to the polymeric membrane to simulate the peristalsis respiratory motions from the in vivo organs. Despite the significant progress in material science, only a few materials can recapitulate the physiological conditions of living organ testing. First and foremost, the materials involved in the construction of the OOCs microdevices should ensure the necessary biocompatibility to provide a biologically safe and nontoxic environment that allows cell migration without generating any inflammatory response or exacerbated immunogenicity. Polymeric materials are desirable because of their great structural similarity to the ECM. Besides biocompatibility, an essential requirement for the polymeric membrane that separates the OOCs compartments is the similarity with the ECM in order to support the cell attachment and diffusion through its pores from one side of the interface to the other compartment. Proteins such as collagen, fibronectin, and vitronectin are ideal candidates as they are involved in biological processes and play an important role in the cell-adhesion mechanism. Moreover, it was shown that the surface roughness of the membrane could affect cell behavior. In the case of synthetic polymers such as PMMA layers, it was observed that the cell adhesion and spreading of vascular cells improved with higher surface roughness, but proliferation was not affected, and it was indicated that the cell adhesion is dependent on the protein adsorption [67][70]. In the case of PDMS, surface roughness and surface energy play a key role in the cell-attachment process. It was observed that higher surface energy promotes the formation of stronger cell–ligand bonding, leading to improved cell growth and proliferation. However, the cell-membrane interaction drastically decreases above critical surface energy and critical roughness ratio. Thus, the study on the cellular behavior of HeLa and MDA MB 231 cells on a rough PDMS surface revealed that the optimum conditions for cell adhesion, growth, and proliferation are obtained at moderate surface energy and intermediate roughness ratios [68]| ~400 MPa | |||||||
| [ | |||||||
| 93 | |||||||
| ] | |||||||
| ~400 MPa [ | |||||||
| 96 | ] | Blood-brain barrier | [ | 94] | Blood-brain barrier [97] | ||
| Polytetrafluoroethylene (PTFE) | 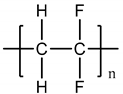 |
108° [95] | 108° [98] | 392 MPa [96] | 392 MPa [99] | Liver [97] | Liver [100] |
