Rare Diseases (RD) are defined by their prevalence in less than 5 in 10,000 of the general population. Considered individually, each RD may seem insignificant, but together they add up to more than 7000 different diseases. Research in RD is not attractive for pharmaceutical companies since it is unlikely to recover development costs for medicines aimed to small numbers of patients. Since most of these diseases are life threatening, this fact underscores the urgent need for treatments. Drug repurposing consists of identifying new uses for approved drugs outside the scope of the original medical indication. It is an alternative option in drug development and represents a viable and risk-managed strategy to develop for RDs. In 2008, the “off label” therapeutic benefits of propranolol were described in the benign tumor Infantile Hemangioma. Propranolol, initially prescribed for high blood pressure, irregular heart rate, essential tremor, and anxiety, has shown increasing evidence of its antiangiogenic, pro-apoptotic, vasoconstrictor and anti-inflammatory properties in different RDs, including vascular or oncological pathologies.
- beta-adrenergic receptor antagonist
- propranolol
- HIF
- apoptosis
- inflammation
- angiogenesis
1. Rare Diseases and Drug Repurposing Opportunities
2. Propranolol as a Repurposed Drug for Rare Diseases
Propranolol, a non-specific β1-and β2-adrenergic receptor (ADRB1-2) antagonist, initially prescribed for cardiac disorders, has, in the last 10–15 years, become a paradigmatic example of an extremely valuable drug, showing multiple off-target clinical therapeutical properties in cancer and RDs. Propranolol was initially proposed in 1964 as a β-receptor antagonist that showed suitable properties to fight against cardiac disorders [6], acting as a nonselective β1- and β2-adrenergic antagonist without partial agonist effects. Since then, the treatment of cardiac disorders has remained the main clinical prescription for propranolol [6]. Nevertheless, propranolol continued with the initial therapeutic indication until 2008 when Léauté-Labrèze et al., based on the induction of apoptosis in capillary endothelial cells (ECs) [7], showed its clinical antiangiogenic therapeutic properties in 11 cases of Infantile Hemangioma (IH) [8]. IH is a benign vascular tumor present in a 4–5% of neonates and normally affecting the face and limbs. When IHs do not spontaneously remit, surgery was the only treatment before the advent of propranolol. Several case reports and successful trials later, Hemangiol (an oral liquid presentation of propranolol), was designated for the IH by EMA in 2014 [9][10][11]. After the success of IH treatment with propranolol, its use and potential therapy has been expanded to other RDs such as Hereditary Hemorrhagic Telangiectasia (HHT), von Hippel-Lindau disease (VHL), soft tissue sarcoma, and Cerebral Cavernous Malformations (CCMs), [12][13][14][15]. More recently, in a murine in vivo model of Lafora, a neurological RD where propranolol shows a potential therapeutic effectiveness, modulating the microglia and astroglia inflammation, being proposed as a putative candidate for treatment [16]. Propranolol exhibits certain lipophilic properties which makes it able to cross the blood brain barrier [17]. It also displays pharmacodynamic characteristics such as vasoconstriction, inhibition of angiogenesis and induction of apoptosis, all of which are related to its therapeutic possibilities, as described below and depicted in Figure 1.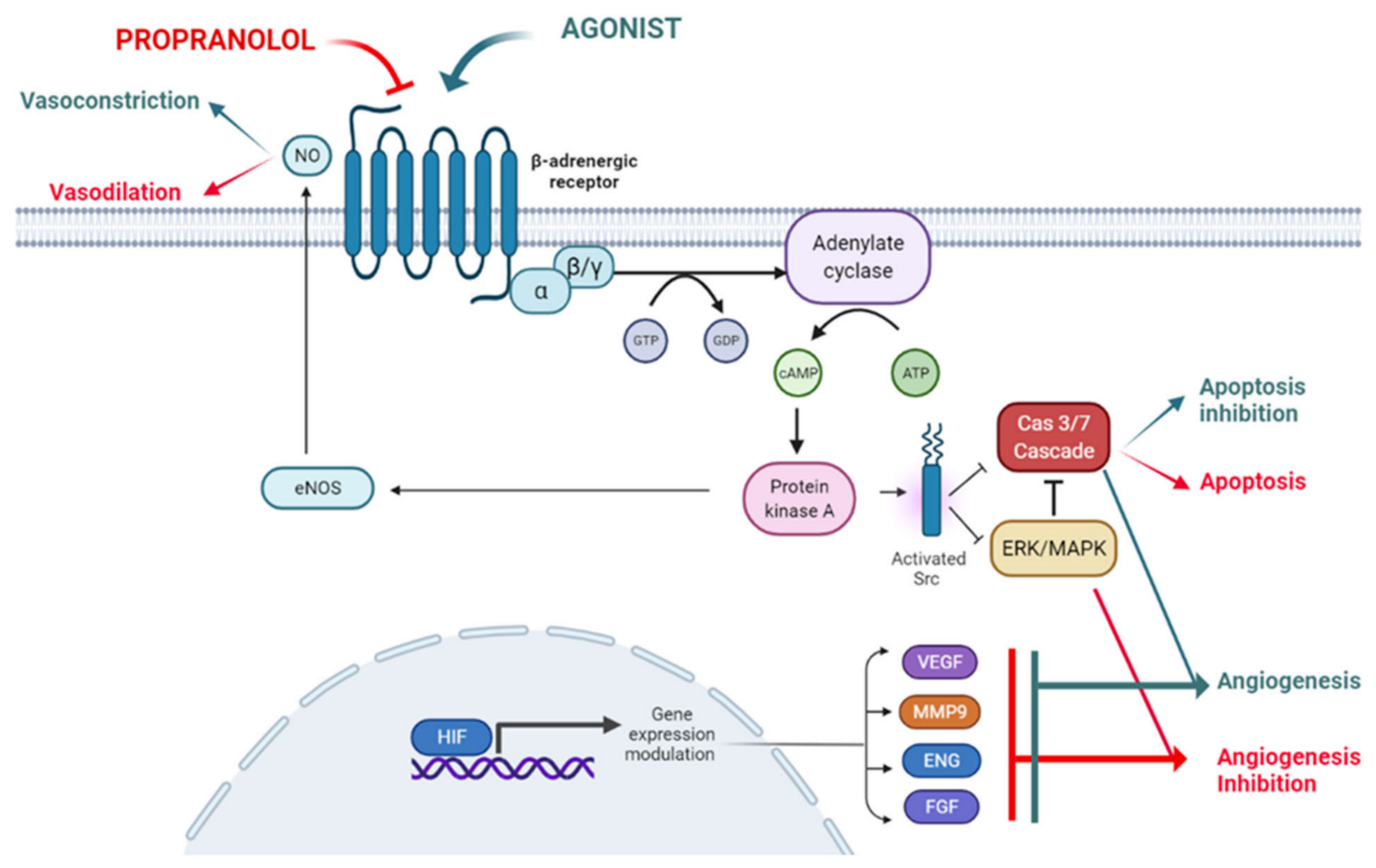
Hereditary hemorrhagic telangiectasia (HHT) with a prevalence of 1:5000 is a vascular dysplasia with defective angiogenesis leading to cutaneous telangiectases, epistaxis, anemia and arteriovenous malformations in internal organs. In this disease. either topical propranolol applied on the nose as cream, or in combination with polidocanol sclerotherapy, decreased epistaxis and hemoglobin decreased levels due to bleeding, were recovered.
Cerebral familiar Cavernomatosis (CCM) is another rare vascular inherited disease, with a prevalence of 1:10,000, characterized by the presence of multiple cavernous angiomas all over the brain parenchyma, which may bleed causing headaches, convulsions, and problems with speech, vision or balance. Case reports in literature referred an improvement in patients treated with propranolol. These observations led to the start of the 3 clinical trials currently underway. One of them run in Europe is very close to its end. After this clinical trial, It is very likely that propranolol could be designated as an orphan drug for CCM.
Angiosarcoma, a metastatic vascular tumor that mainly affects the skin, has two clinical trials underway with no final results yet. However interim results in the clinical trials demonstrate promising results.
Von Hippel Lindau (VHL) syndrome, is a rare tumoral disease where multiple tumors, hemangioblastomas (HBs), develop in the central nervous system including retina, clear cell renal carcinoma or neuroendocrine tumors. A clinical trial was conducted with 7 patients with retinal HBs who, after treatment with propranolol, experienced a halt in their growth and a complete disappearance of retinal exudates present in 2 of the patients.
Paragangliomas (PPGLs) are neuroendocrine tumors due mainly to mutations in Succinate Dehydrogenase genes (SDHs). In a case report, a patient with metastatic paraglanglioma, experienced a decrease in the number of metastasis present in bone, and a stability in the growth of tumors remaining, after being treated with temozolamide and propranolol,
3. Conclusions:
1. Propranolol has emerged as a candidate drug for repurposing in a growing number of Rare Diseases. Its safety profile and therapeutic experience help to use it in mono or combined therapies. In each case (each rare disease), the known side effects of bradycardia and hypotension should be considered if compatible with the rare disease and other concomitant medications.
2. Propranolol is involved in a number of physiological and molecular mechanisms that support its potential therapeutic value, including vasodilation, apoptosis of actively dividing cells or angiogenesis.
3. Propranolol is being used in monotherapy and in combinatorial therapy in different clinical trials for different types of Rare Diseases. A highlight would be Angiosarcoma and Cavernomatosis diseases, with 3 or more active trials at present. Future promising issues may be neuroinflammatory diseases, as Lafora disease.
