Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Michele Arienzo.
The factors that can influence the biological responses to engineered nanomaterials (ENMs) exposure are temperature, feeding, reproductive status and salinity. These influences have only been studied to a limited extent and there is a limited comprehensive understanding of EMPs’ impact on estuary environments and their risk assessment. Salinity is the major parameter that is responsible for the stress of estuarine organisms and influences the toxicological effects of ENMs.
- metal engineered nanoparticles
- estuaries
- sources
- distribution
- bioaccumulation
- bioavailability
- ecotoxicity
1. Bioavailability and Bioaccumulation of Engineered Nanomaterials (ENMs)
When ENMs are released from the source for example, i.e., WWTPswastewater treatment plants (WWTPs), they move in freshwaters towards estuaries and find different physico-chemical conditions throughout their trip. In freshwater, the dissolved metals from the ENMs form a complex with colloidal oxyhydroxides of Fe and Al and tend to sediment, as is the case for eexample of Ag+. In an estuary, nanoparticles aggregate, with a reduction of the surface area and bioavailability. The bioavailability of ENMs is influenced by the NOM’s surface coating, aggregation and disaggregation, and hence, so is the ENMs’ transport in surface waters and sedimentation. The stabilisation of ENMs via surface coating increases their residence time in the water column and their transport distance. In contrast, aggregation induces ENM settling to the sediments and accumulation by benthic organisms as final receptors [15][1].
In the sea environment, NPs are rarely nano-sized, as they aggregate with the salinity increase, with a consequent reduction in the surface area and dissolution. In salty water, the dissolved metal cations from ENM complexes with free Cl− ions, and hence toxicological effects, are limited to situations where nanoparticles are adsorbed or internalised in biota with the successive dissolution of the metal [15][1]. Bustamante and Miramand [120][2] measured high levels of cerium (10.85 µg/g) and silver (61.2 µg/g) accumulation in the digestive gland of Chlamys varia relative to other organs and tissues, such as the gonads, gills, muscle and soft tissues. Finally, disaggregation results in the formation of small aggregates that are resuspended and become mobile in the water column, carrying pollutants and nutrients with them [20][3].
Bioaccumulation in aquatic organisms depends on many properties of the nanoparticles, and many of them are interrelated. First, in terms of size, the smaller the particles are, the easier they are taken up, with a substantial difference between the smaller particles following the pinocytosis path, and the larger particles taking the phagocytosis route, and yet others that are capable to create their own membrane channels [121][4].
Bioaccumulation may occur via adsorption on the surface of a cell, organ and body; internalisation in cells; dissolution of ions; and a mechanistic nano effect. Since marine fish drink a consistent amount of water to compensate for water lost through osmosis, ENMs dissolve in the digestive tract, releasing free ions into the organisms [15][1]. The main targeted organs in biota are the gills, gut and intestine [15][1]. Some evidence of tissue bioaccumulation was reported for the estuary species sheephead minnow, Cyprinodon variegatus, exposed to Ag NPs. Another tested freshwater organism was the medaka fish, Oryzias melastigma, exposed to ZnO NPs. It was observed that non-oxidative stress occurred as mechanical injury and genotoxicity [49][5]. Many studiweres reported how ENMs, such as Ag NPs, can accumulate in biofilms [15,122[1][6][7],123], with direct uptake of Ag+ from the water columns or metals released from marine-discarded consumer products (CPs) and then transferred to bivalves via biofilm ingestion, for example. Thus, the uptake of ENMs depends on the species, as well the delivery method of metals, ions, NPs and CPs; the capping agents’ size; dissolution; and the manufacturing process of CPs [123][7].
An important factor regulating bioavailability and bioaccumulation is the rate of dissolution of ENMs, which varies with the nature of the metal oxide nanoparticle. For example, CeO2 NPs are insoluble [124][8], Cu2O NPs are soluble [125][9] and Ag NPs dissolve Ag+ and form peroxide radicals [21][10]. For the same nanoparticle, metal dissolution is regulated by the size, coating and medium. In freshwater, the physico-chemical characteristics are different than those in marine water and, hence, so are the bioaccumulation and toxicity. An interesting cexasmple is that of ZnO NPs, which dissolve high levels of Zn2+ up to levels of 3.2–4.8 mg/L in a marine system [49][5]; this cexasmple is particularly important on beaches, where bathers make large use of ZnO NPs in UV filter creams. Dissolution and, hence, bioaccumulation and potential toxicity, are also affected by the particle shape, with rods being more soluble than spheres and causing additional damage due to sharp edges, piercing cell membranes [126][11].
2. Toxicity of ENMs in Marine Biota
One of the main factors limiting the knowledge of ENMs’ toxicity in water environments is the lack of ENM quality standard regulations for freshwater and saltwater. The existing ones released by the US EPA deal with the presence of free metal ions and are based on the application of the biotic free ligand model (BLM) and the free ion active model (FIAM) [6][12], which have limited applications to evaluating ENM toxicity [127][13].
ENM toxicity is regulated by water quality parameters and physico-chemical and colloidal properties of ENM formulation. According to some authors, ENMs pose a relatively low risk for most environmental compartments [44][14]. Of the same opinion are Callaghan and MacCormack [6][12], who reported that ENMs generally cause mild acute toxicity to adult fish and crustaceans, with a sensitivity that may be significant for a limited number of species and life stages.
Other authors, such as Baker et al. [15][1] and Roma et al. [128][15], considered that once ENMs release their vehiculated metals, these ions can be extremely toxic for marine fauna and this depends on their speciation and physical and chemical forms. The metal present in ENMs and that in free ionic form differs depending on the charge. For instance, ionic silver exists as Ag+, while Ag NPs have a negative charge [129][16].It Authorwas attributed the toxicity of these ENMs to ion shedding [130][17], while others repaborut additional toxicity mechanisms [131][18]. Johari et al. [132][19] studcarried tout the toxicity of Ag NPs and ionic silver on marine microalgae Dunaliella salina, evidencing higher toxicity of soluble ionic silver than Ag NPs. In the same way, Oukarroum et al. [133][20] and Miao et al. [134][21] attributed the Ag NPs’ toxicity to the direct interaction of NPs on the marine microalgae cell’s surface and the consequent formation of cell aggregates, the release of Ag+, formation of ROS and lipid peroxidation injury. The induced aggregation of NPs on the algal cells might cause a reduction in accessibility of irradiation with consequent inhibition of growth and nutrient adsorption [135][22]. In this context, high salinity levels can cause a reduction in the toxicity of Ag NPs. In fact, an increased salinity may lead to variation in the silver bioavailability, with a dominance of aggregation processes of NPs forming large agglomerates with different surface area, charge and size parameters [136][23]. In estuary waters, Ag+ toxicity is related to the ionic strength and presence of ligands, such as Cl−, I− and Br−, with AgCl insoluble salt formation competing with the different halogenated complexes [137][24]. At higher salinity levels, it is likely that excessive Cl− could react with the AgCl precipitate and form soluble forms of AgClm−1 [132][19].
This means that ENMs behave differently from their ionic counterparts in terms of ecological risk [128][15]. The direct interaction of ENMs or their dissolved metals with the organism proteins may induce ion regulatory stress and/or developmental toxicity [6][12]. A deeper discussion on the ENMs’ biological effects is given in Section 3.2.
TheA literature provides only a few studiesfew are reporting on specific casexamples of direct comparison between the toxicity effects of ENMs and their metal ions [49[5][25][26][27],138,139,140], and the results are quite contrasting.
Some papers [106][28] report the toxicity of ENMs similar to that of the corresponding ions released. This was tested through an in vitro test based on neutral red retention time (NRRT) applied to the genus Mytilus and taking copper, chromium, cobalt, gold and titanium oxide/dioxides into consideration. Mussels are filter feeder organisms and, hence, represent the ideal selective sentinel for probing the environmental fate of ENMs since they can bioconcentrate significant levels of NPs [141,142][29][30]. The NRRT assay detects decreased lysosomal membrane stability in haemocytes from bivalves exposed to pollutants [105][31] and, hence, represents an indication of organism health status because animals exposed to pollutants have compromised lysosomal stability [105][31]. In some csituasestions, abnormal lysosomal accumulation of toxic pollutants leads to oxidative damage and cell death [143][32]. The test showed how copper, chromium and cobalt were toxic and matched with other studies reporting the cytotoxic effects of the former metal oxides. Cong et al. [144][33] focustudied ed on the embryotoxicity of both ZnO NPs and ionic Zn2+ to marine medaka Oryzias melastigma. They reported how the biological effect of ZnO NPs was significantly higher than those of aqueous Zn2+, attributing this pattern to particulate or aggregate forms with increased mortality of embryos, decreased percentage of total hatching success and increased malformation and edema of newly hatched larvae. The same wauthors concluded that the Zn speciation from ZnO NPs released in marine seawater may play an important role in the dissolution and toxicity processes [144][33].
Others studieswere reported that on more toxic effects from the ionic form. Some examples are the case of e zinc oxide for the crustaceous Tigriopus japonicus and Elasmopus rapax. Noor et al. [145][34] reported how Zn2+ caused greater metabolic damages than ZnO NPs, inhibiting the production of ATP in mitochondria and modifying the profile of free amino acids in the blue mussel Mytilus edulis. A similar trend was observed by Cong et al. [146][35], exposing the polichete Hediste diversicolor to Ag NPs and ionic Ag+, showing a genotoxicity effect, even though there was no bioaccumulation difference between the two forms. In the same way, the experiments conducted by Mouneyrac et al. [138][25] on the H. diversicolor and the bivalve Scrobicularia plana reported similar oxidative stress response, apoptosis and genotoxicity, but silver nanoparticles produced more elevated levels of DNA damage. Different toxicity effects were observed by McCarthy et al. [139][26] in the oyster Crassostrea virginica exposed to ionic silver, showing cellular damage in gills, and nanosilver particles producing hepatopancreas function damage. D’Agata et al. [140][27] found a greater accumulation of TiO2 NPs in Mytilus galloprovincialis and greater toxicity of titanium dioxide, compromising metallothionein gene expression and producing histological impairments. Gomes et al. [147][36] reported different mechanisms of toxicity from silver nanoparticles and the relative ionic counterpart, as well as different sets of proteins expressed in gills and digestive glands, for the same mussel. Other organisms, including anellids such as Polychaeta, feeding on sediments also ingest ENMs and are exposed to metal dissolution or particle clogging in the gills, where they can internalise the nanoparticles with sublethal effects not related to dissolution [15][1]. Garcia Alonso et al. [148][37] exposed the polychaete Nereis diversicolor to Ag NPs capped with citrate and found particle aggregation in association with the villi and in the plasma membranes and endocytotic pits. In these pits, Ag NPs were internalised with particle sequestration in the lysosomes. TheIt studywas also evidenced the association of Ag+ with metallothionein proteins and Ag NPs associated with organelles and metal-rich granules, suggesting a double route of uptake, namely, aqueous ions and particulate uptake. Similarly, another study[35] [146] with coelomocytes exposed to Ag NPs reported genotoxic effects that did not depend on Ag+ dissolution. Sub-lethal effects of TiO2 NPs exposed to Arenicola marina were attributed to the particle association in the gut epithelium [149][38]. Fabrega et al. [150][39] exposed the shrimp Corophium volutator to ZnO NPs and found high levels of Zn in the hepatopancreas in insoluble sulphur-rich sphaerites.
The higher toxicity of the ionic form relative to the bound metal in NPs was reported by Sendra et al. [151][40]. The authors tested the toxicological response of the unicellular microalgae Chlorella autotrophica, which possesses a typical cellulosic cell wall, and Dunaniella salina, which lacks a cell wall, to Ag and CeO2 NPs. Being at the bottom of the marine phytoplankton food web, these microalgae are sensitive to NPs, with effects on reproduction and metabolic functions [151][40]. Exposure of two algae to Ag and Ce in both NP and ionic forms affected the reproductive, structural and physiological mechanisms of both algae. For both species, treatments with silver were more toxic than those with cerium and, for both metals, the ionic form was more toxic than the NP. The absence of a cell wall in D. salina did not influence its sensitivity, where it was more tolerant than C. autotrophica. Authors attributed the higher tolerance to the production of extracellular polymeric substances and the elimination of the compounds to the external environment.
What emerges from the literature survey is s that an important role is also given by the kind of exposed organism. Hanna et al. [152][41] exposed the amphipod Leptocherius plumulosus to CuO NPs and ZnO NPs for 10 d. The authors highlighted that, while for ZnO NPs, the main drive of toxicity was found to be dissolution, for CuO NPs, toxicity did not depend on metal dissolution [152][41]. The polychaete worm Nereodes diversicolor exposed to CuO NPs generated the accumulation of Cu and production of ROS and highlighted a different uptake route between NP and ionic metals. Burić et al. [153][42] assayed the species-specific effects of low concentrations of 60 nm Ag NPs in the range of 1–100 µg/L on the embryonal development in Mediterranean urchins Arbacia lixula, Paracentrotus lividus and Sphaerechinus granularis, with times of exposure from 30 min to 24 h. The most sensitive species was A. lixula, with a negative influence on embryonal development and even arrested development. The greatest impact was observed for embryos exposed to NPs at 6 and 24 h post fertilisation. Similar effects were observed for the other tested organisms but at higher concentrations of first exposure. The results indicated that the toxic effects of Ag NP were species-specific and that the time of exposure of embryos was an important factor in the development of abnormalities. Thus, the sea urchin embryo development test could be used for nanoparticle toxicity testing [153][42].
While the toxicity potential of ENMs in an aquatic environment is still a matter of debate, it is rather clear that ENMs have a unique toxicity mechanism and pathway: being nano-sized, they can easily enter cells, organelles and nuclei and cause oxidative stress [154][43]. Metals vehiculated by NPs have different paths of penetration depending on the shape, structure, surface charge, chemical composition, solubility, aggregation, presence of functional groups and reactivity with other compounds of NPs [128][15]. All these features influence how nanoparticles enter the cell via endocytosis, which is the conventional cellular route of entrance of a particle ≤100 nm due to the presence of protein particle binding sites, production of oxygen reactive species and inflammatory action [155][44]. The interaction of ENMs with proteins elicits the response of the immunological system and localised and systematic inflammations negatively impact the hatching and embryo growth and integrity [6][12].
Figure 3 summarizes the different routes of toxicity of ENMs. Regarding the toxicity target, they can cause inflammation of respiratory and digestive epithelia, compromise cardiovascular activity and produce hyper responses of the immune system [6][12]. They cause multiple forms of damage, such as blockage of cell channels, hindering membrane functions and DNA integrity, oxidation of proteins and release of reactive oxygen species (ROS) [15][1]. The generation of ROS can be direct or because of the immune system’s response in impacted organisms. This yields tissue damage and/or epithelial remodelling, such as in the gills and gut. The oxidative stress can occur on the surface of the nanoparticles via radicals, transition metals and interaction of the nanoparticles with cellular parts, or via macrophages activation [156][45].
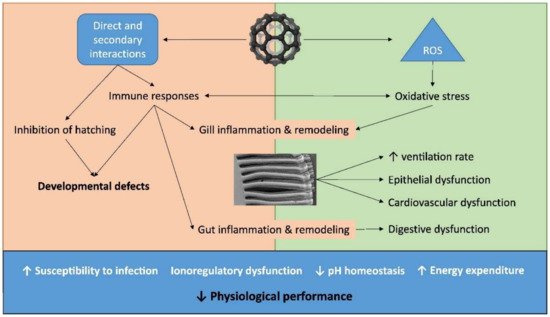
Figure 3. ENMs toxicity routes in an aquatic environment. Image used with the permission of Elsevier [6].
Since marine water and sediments of estuaries are the final sinks of ENMs, there are a plethora of organisms that are potentially exposed, including crustaceans, molluscs and algae [107][46]. However, as already seen, ENMs undergo an intense process of sedimentation and aggregation in seawater and, thus, benthic organisms are significantly exposed. Although the literaturet is quite sparse in terms of toxicity data on marine organisms, below is a summary of the most significant data found. Starting from the most primitive prokaryotic organisms, i.e.for example, bacteria organised in biofilms, it was reported that Ag NPs inhibit the growth of specific species, and the process increases the availability of the nanoparticles for biofilms grazing organisms as gastropods [15][1]. At a higher trophic level, i.for example., algae, ENMs may interfere with the adsorption of nutrients, as was the casexample observed by Peng et al. [157][47], who reported the inhibition growth of diatoms in the presence of ZnO NPs. Others [48] studiweres [158] considered the exposure of macroalgae to Ag NPs and found a toxicity relationship with the progressive dissolution of Ag+, with surface adsorption being the main bioaccumulation path. This might represent a risk for surface grazers. In the cexasmple of other microscopic marine organisms, such as rotifers, ingesting particles from the water column brought about the accumulation of smaller-sized NPs in the stomach and intestine, with evidence of toxicity from intracellular dissolution [159][49]. These mechanisms of bioaccumulation and adsorption internalisation cause ENMs to be available for macro-vertebrate filter feeders. In all cexasmples, ion dissolution is then responsible for toxicity, even though NOM complexation might attenuate toxicity. Wong et al. [48][50] reported how ZnO NPs were more toxic to the first stage of larvae of the copepod Tigriopus japonicus than ionic Zn2+, hypothesising mechanical inhibition of the nanoparticles besides Zn2+ dissolution.
Some marine organisms in coastal waters can be used as a way to monitor the presence of nanoparticles [160][51]. This is, for example, the case of TiO2 NPs, which were found to be toxic for fishes, invertebrates, bacteria [161,162,163][52][53][54] and phytoplankton [164][55]. Matranga and Corsi [107][46] proposed the use of model organisms and molecular approaches to evaluate the ecotoxicological impact of ENMs on the marine environment. Other organisms can be rather tolerant. The response of the marine organisms varies with the species, the particle’s physico-chemical properties, such as nature and size, photocatalytic activity, shape, ionic strength and pH of the medium, and UV irradiation. Important marine bioindicators of ENMs’ presence in estuary systems are microalgae, which are very important in the primary production of marine ecosystems and may transfer the metals along the marine food chain [102][56]. Barhoumi and Dewez [165][57] studcarried tout the hypersaline unicellular green algae Dunaliella salina as a pollution indicator of ENMs in a marine ecosystem. This was because the algae possess an elevated rate of bioaccumulation and sensitivity. Morelli et al. [166][58] exposed the green alga Dunaliella tertiolecta to TiO2 NPs and reported neither inhibition of growth nor alteration of cellular chlorophyll concentration. They observed a transient increase in extracellular ROS production and that the protein moiety of exopolymeric substances excreted by the algae significantly affected the biological activity of the NPs. Antizar-Ladsilso [167][59] studcarried tout the impact of Ag NPs on benthic prokaryotes in a heavily polluted Hooghly estuary in India and observed that microbial communities can be an indicator of sediment functioning. Authors found that exposure to NPs caused an increase in the number of bacteria in a consortium in sediments, highlighting that microbial communities may be resistant to the antimicrobial effects of commercial Ag-NPs. Key microorganisms, such as Pelobacter propionicus, disappeared, indicating that Ag-NPs have the potential to shape estuarine sediment bacterial community structure. Among the most used indicators of ENMs presence in a marine environment, there are the bivalve molluscs, which are filter feeders and able to capture particles from the water column [44][14]. They may internalise nano- and microparticles through endocytosis and, hence, used as indicators of ENMs [44][14]. Johnnson et al. [44][14] exposed eastern oysters Crassostrea virginica and hepatopancreas tissues to a range of TiO2-NP concentration (5–5000 mg/L) under natural sunlight. Authors reported a dose-dependent decrease in lysosomal stability at exposures as low as 50 mg/L. ENMs accumulation caused lysosomal destabilisation and the absence of oxidative stress. Particle size screening indicated a concentration effect over agglomeration, with small agglomerates and individual particles are more easily biologically taken up. Authors concluded that low TiO2-NPs levels might produce sublethal toxicity on oysters, especially under natural sunlight conditions.
2.1. Biological ENMs Effects
In a marine environment, the metals from ENMs can quite easily accumulate in organisms beyond the metabolic need and cause damage [168][60]. This depends on the metal speciation, method of exposure and specific organism characteristics [98][61]. Metals can bind to the surface of the organism or enter the cells [169][62] via endocytosis in the digestive system [170][63]. If the concentration of the metal is excessive, the excretion mechanisms are hampered [171][64]. When ENMs go through the membranes, they enter the lysosome and release a high amount of ions, generating ROS via Fenton-type reactions and increasing the antioxidant response [172][65]. Thus, marine organisms may respond to ENM exposure by altering their biological status, which can be measured through different enzymatic and non-enzymatic biomarkers. The former regard the upregulation of the phase II antioxidant enzymes, i.e., catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase and transferase. These biomarkers serve to scavenge or counter ROS, avoiding oxidative damage during chronic exposure [173][66]. Another enzymatic biomarker that can be measured is acetylcholinesterase, which regulates many physiological functions [174][67]. The excessive production of ROS becomes problematic for organisms [175][68] and ENMs and ROS can move to deeper tissues through blood flows [55][69]. Organisms can respond to the excess of ROS, producing antioxidant enzymes and non-enzymes, such as metallothioneins [128][15]. Dosages of such protein levels are used as biomarkers of ENM toxicity. Another often-used biomarker is the thiobarbituric acid reactive substances (TBRAS) assay, which measures the lipid peroxidation of polyunsaturated fatty acids caused by ROS [176][70]. Furthermore, the lysosomal membrane stability is a stress biomarker, and, in this case, the e neutral red activation retention assay is used to measure the increased lysosomal contents in the cytosol as a response to the increased ROS amount [128][15]. ROS can also cause DNA damage, including strand breaks, mutations, chromosomal aberration [177][71] and even cell death [178][72]. DNA strand breaks and mutagenicity endpoints are determined using chromosome aberrations and micronucleus assays [179][73].
2.2. Factors Influencing Marine ENM Effects
The factors that can influence the biological responses to ENM exposure are temperature, feeding, reproductive status and salinity [131,180,181,182][18][74][75][76]. These influences have only been studiedknown to a limited extent and there is a limited comprehensive understanding of EMPs’ impact on estuary environments and their risk assessment [183][77]. Salinity is the major parameter that is responsible for the stress of estuarine organisms and influences the toxicological effects of ENMs. Noor et al. [145][34] investigated the role of ZnO NPs on intermediate metabolite homeostasis in the blue mussel Mytilus edulis under different salinity conditions, finding that fluctuating salinity conditions were less stressful than low constant hypo-osmotic stress. A period of 24 h was found to be sufficient to restore the metabolic functions of this euryhaline species. The biological effects measured through different biomarkers decreased as the salinity stress increased.
A similar studyone was carried out by Wu et al. [131][18], who investigated the interactive effects of salinity (normal 15, low 5 and fluctuating, 5–15‰) and of ZnO NPs and ionic Zn2+ on the immune system of Mytilus edulis from a brackish area of the Baltic Sea. Exposure to ZnO NPs caused hemocyte mortality, phagocytosis and lysosomal volume increases. At a salinity of 15‰, ZnO NPs suppressed the mRNA expression of the Toll-like receptors involved in pathogen recognition. With fluctuating salinity, ZnO NPs increased the expression of multiple immune-related genes in hemocytes, whereas low salinity had immune suppressive effects that overshadowed those of ZnO NPs. This variable immune system response was attributed not to aggregation or solubility of ZnO NPs but to the interactive toxic effects of nanoparticles and the physiological effects of the osmotic stress.
2.3. Measurements of ENMs in Aquatic System
As already stated, the main difficulty in evaluating ENM toxicity arises from the objective difficulty in measuring nanoparticle levels in a water environment. Nano-sized metal particles in an aquatic environment are difficult to measure, for i.e.example, in terms of concentration, particle size distribution, surface area and electromagnetic behaviour [184][78]. Cell membranes having pores larger than the size of the nanoparticles trap them with difficulty, impairing detection [185][79]. Manufacturing membranes of the dimension of nanomaterials can, in some cases, resolve this problem, allowing for multiple studies of ENMs release measurements and modelling ENMs’ release and life cycles [128][15].
A stochastic approach was developed by Dumont et al. [186][80], who estimated an average level of silver nanoparticles of 0.002 ng/L and zinc oxide of 1.5 ng/L for a representative portion (~50%) of the European rivers. In ~10% of the rivers, the model estimated higher concentrations of 0.18 ng/L and 150 ng/L, respectively. One of the major problems of these models is that they consider only the initial form of the ENMs, ignoring the modification throughout the lifespan of the nanoparticle [5][81]. Another common problem of model approaches is that they too often consider riverine scenarios with few estimations for estuarine environments: Gottschalk et al. [47][82] estimated levels of TiO2 at 0.004–0.099 pg/kg in seawater and 4.3–120 pg/g in sediments, ZnO at 0.006–0.4 pg/L in seawater and 6–220 pg/kg in sediment, CuO at 0.02–0.07 pg/L in seawater and 25–83 pg/kg in sediment, Ag at 0–0.06 in seawater and 0–0.7 pg/kg in sediment, and CeO2 at 0.03–2 in seawater and 0.04–2 pg/kg in sediment.
