Artemisia vulgaris L. (common mugwort) is a species with great importance in the history of medicine and was called the “mother of herbs” in the Middle Ages. It is a common herbaceous plant that exhibits high morphological and phytochemical variability depending on the location it occurs. This species is well known almost all over the world. Its herb—Artemisiae vulgaris herba—is used as a raw material due to the presence of volatile oil, flavonoids, and sesquiterpene lactones and their associated biological activities. The European Pharmacopoeia has listed this species as a potential homeopathic raw material. Moreover, this species has been used in traditional Chinese, Hindu, and European medicine to regulate the functioning of the gastrointestinal system and treat various gynecological diseases. The general aim of this review was to analyze the progress of phytochemical and pharmacological as well as professional scientific studies focusing on A. vulgaris. Thus far, numerous authors have confirmed the beneficial properties of A. vulgaris herb extracts, including their antioxidant, hepatoprotective, antispasmolytic, antinociceptive, estrogenic, cytotoxic, antibacterial, and antifungal effects. In addition, several works have reviewed the use of this species in the production of cosmetics and its role as a valuable spice in the food industry.
- medical history
- traditional medicine
- chemical composition
- biological activity
- safety of use
1. Introduction
In 2015, the awarding of the Nobel Prize in Medicine for the discovery of artemisinin, a compound of plant origin found in Artemisia annua (annual mugwort), inspired the researchers to study the phytochemical and pharmacological properties of other species of the genus Artemisia. Recently this species has been taken under consideration to be active toward the virus SARS-CoV-2 and disease COVID-19[1][2] [1,2].
Artemisia vulgaris L. (common mugwort) is one of the best-known species of this genus, which has a widespread distribution in the natural habitats worldwide (Europe, Asia, North and South America, and Africa). For many centuries, this species has been mainly used for treating gynecological ailments and gastrointestinal diseases[3][4][5][6][7] [3–7]. Recently, researches have proved that this species exhibits antioxidant, hypolipidemic, hepatoprotective, antispasmolytic, analgesic, estrogenic, cytotoxic, antibacterial, antifungal, hypotensive, and broncholytic effects[8][9][10][11][12][13][14][15][16][17] [8–17].
The different applications of this plant species have been possible due to its rich chemical composition, which especially includes essential oils, flavonoids, phenolic acids, coumarins, and other group of metabolites.
The presence of essential oil in A. vulgaris contributes to the significance of this species as a culinary spice in the food industry in various regions of the world. Currently, this species is also increasingly used in the production of cosmetics in Europe as well as in Asia and North America[14][18][19][20] [14,18–20].
The latest review on A. vulgaris presented by Brazilian–Iran and Malaysian teams highlighted the value of this plant species from the South American–Asian point of view[21] [21].
The present review provides classical information about the importance of A. vulgaris in therapeutics and food industry from the European point of view and additionally discusses the possible new applications of this plant species in phytotherapy as a hepatoprotective, broncholytic, anthelmintic, and cytotoxic agent and in cosmetics industry as a raw material in Europe, East Asia (especially in Korea), and North America.
2. Distribution and taxonomic and genetic consideration of Artemisia
The Artemisia genus belongs to subtribe Artemisiinae of tribe Anthemidae from Asteraceae family and comprises more than 500 species. These species are distributed all over the world, especially in the moderate climate zones of Europe, East Asia, Americas, North Africa, and Australia[22][23][24] [22–24].
The plants of this genus are annual, biennial, and perennial herbs or small shrubs and halfshrubs. From the chemotaxonomic point of view, these plants are rich in essential oils and bitter substances (particularly sesquiterpenoid lactones). They are also a good source of flavonoids, coumarins, and phenolic acids[25] [25].
Several representatives of this genus are found in Asia—about 150 species in China and about 174 in the ex-USSR. Many species are characteristic of Japan and Iran flora, (about 50 and 35, respectively). The number of Artemisia sp. occurring in Europe is estimated at 57[26] [26].
In Poland, 10 different species, including A. vulgaris and Artemisia absinthium, are found in the natural habitats[27] [27]. Some of them (e.g. Artemisia petrosa Baung and Artemisia pontica L.) are distributed only on small areas in the country and are on the list of endangered plant species[27][28] [27,28].
One of the most popular species worldwide is A. vulgaris (common mugwort). It is known by various synonymous Latin names. This can be attributed to the broad distribution of this species on all continents and the differences in its chemical and genetic composition, which is characteristic of the plants of different origins.
According to the data available on the official website “The Plant List” (created by Global Strategy for Plant Conservation and World Flora Online), A. vulgaris has as many as 107 synonymous Latin names, including Absinthium spicatum (Wulfen ex Jacq.) Baumg[3][29] [3,29], Artemisia affinis Hassk.[3][29] [3,29], Artemisia opulenta Pamp.[3][29][30] [3,29,30], Artemisia vulgaris subsp. vulgaris[31] [31], and Artemisia vulgaris var. indica (Willd.) Hassk.[3][29][31] [3,29,31] (Table 1). In addition, again due to its widespread dissemination, this species is known by various foreign-language names, including Carline Thistle, Chiu Ts’Ao, common mugwort, Chrysanthemum weed, Cingulum Sancti Johannis, common wormwood (English), Ajenjo, altamis, altamisa (Spanish), altamiza, amarella (Italian), armoise, Armoise citronnelle (French), beiai (Chinese), Beifußkraut (German), Nagadamani (Ayurvedic), moxa (Japan) and Polynesian snare (Russian)[3][4][29][30][31] [3,4,29–31].
Artemisia (Asteraceae, Anthemideae, Artemisiinae) is the largest genus of the tribe Anthemideae. It is also one of the broadest genera of the family Asteraceae, which comprises more than 500 species[32][33] [32,33]. The genus exhibits high morphological and phytochemical variability and ecological plasticity, with species occurring from sea level to huge mountains and from arid zones to wetlands[22] [22]. At present, the following five main groups are considered at the subgeneric level, mostly based on the floral characteristics: Artemisia, Absinthium (Mill.) Less., Dracunculus (Besser) Rydb., Seriphidium Besser, and Tridentatae (Rydb.) McArthur[33][34] [33,34].
3. Botanical characteristics
The species A. vulgaris shows high morphological variability depending on the place of occurrence[30] [30]. Comparative studies conducted between different populations revealed variability in the branching (presence or absence and extent), leaf shape, and root diameter of the plant[7] [7] .The high variability of A. vulgaris has been confirmed by the study conducted by Barney and DiTommaso on two populations collected from different, geographically isolated areas of Ithaca (USA). The authors found that plants from one population had densely hairy stems and light green leaves, each with a few deep notches, whereas those collected from another part of the city had almost smooth stems and dark green leaves with many deep notches[35] [35].
Artemisia vulgaris is an herbaceous plant, which grows up to a length of 2.5 m and has a width of 75 cm. It is characterized by an intense aroma that is readily released when the leaves are crushed[30][36] [30,36], and a spicy taste[37] [37]. The plant has a thick main root and many small, fibrous lateral roots. The roots take on a light-brown color and measure up to 1 cm. They remain in the upper layer of the soil, at a depth of 7–18 cm, forming a vast, underground network[7][30] [7,30]. The stems of the plant are slightly wavy, straight, or branched, having a brown color at the lower end, and become woody with age, appearing green further up and purple at the top. Some of the stems are also hairy [7][38][7,38]. The leaves are 5–10 cm long. They are set densely, and alternately, primarily in the upper parts of the stem. The lower leaves with short petioles are divided into segments and take on a feathery shape, while the middle and upper ones are smaller and single or double pinnate. The dorsal side of the leaves has a dark green color, while the ventral side is whitish and tomentose[7][39] [7,39]. Small, almost bare, yellowish or brown-red flowers are embedded in small baskets that form heavily branched panicles with numerous lanceolate bracts at the top of the shoots[39] [39]. One basket may contain around 15–30 flowers with numerous stamens[7] [7]. Studies conducted in the eastern part of the USA showed that the inflorescences contain 52% of ligulate flowers and 48% of tubular flowers[7] [7], of which 25–50% are female[40] [40].
After the flowering period, which lasts from July to September in Europe, A. vulgaris develops fruit—called achenes. The seeds of these fruits are brown, weighing 0.12–0.14 mg, and have a narrow base. They are oblong and ridged, covered with fine hairs on the apex[7][41] [7,41].
The species reproduces from seeds, which can produce up to 200,000 per year depending on the habitat[42] [42], but so far this has been observed only in the native places of occurrence, namely in Asia and Europe. Some biotypes of this plant do not produce reproductive seeds[5] [5]. The seeds of A. vulgaris spread through wind, beetles, and flies[40] [40]. The plant usually reproduces vegetatively with the help of its roots, which can survive in the ground during winter. In the northern USA, pieces of roots are often moved long distances by local floods[7] [7].
The leaves and roots of A. vulgaris exhibit strong allelopathic properties[35][43] [35,43]. Although this has been confirmed in fresh leaves, it was not possible to isolate the specific compound that is responsible for these properties. It is suspected that these are contributed by a mixture of compounds from the monoterpenoid group[35] [35].
4. Natural habitats and cultivation
Most sources indicate that A. vulgaris originated from Europe and Asia[7][41] [7,41], and from there, this species was brought to North America, probably at the beginning of the 16th century[7] [7].
At present, the plant is abundantly seen in many regions of the world, ranging from the Himalayas in Asia, through Europe, to the warm areas of North America[22][30] [22,30]. The only continent where A. vulgaris does not occur is Antarctica[7] [7].
This species is widely considered a weed[7][31] [7,31]. It can be found in many habitats, for example, on roadsides, along rivers, or in abandoned mines, thickets, tree nurseries, and arable or other fields, where it interferes with the growth of different plants[3][5][7] [3,5,7].
Individual populations of A. vulgaris are well adapted to live in a wide range of pH and various soil types, including sandy and loamy. Due to its extensive root system, this plant can quickly occupy large areas[7] [7]. Controlling the spread of A. vulgaris is very difficult because only a few effective ways can limit its growth[5][7] [5,7].
As a species with low requirements, A. vulgaris can easily colonize successive sites and displace native species. Thus, it can easily disturb the local ecosystems[7][30] [7,30].
This species is cultivated on an industrial scale in Italy, France, Brazil, and Japan, as well as in the mountainous regions of India and Sri Lanka[3] [3]. It is also possible to grow this plant in home gardens or can be obtained from natural habitats.
The aerial parts of the plant die each year[39] [39], and hence, they are harvested at the beginning of flowering. The parts are obtained by cutting the tops of shoots, while the woody stems are omitted. Then, these are dried in airy drying sheds under natural conditions[18] [18]. After drying, the herb has a spicy, bitter taste and a balsamic aroma.
The appropriate time to harvest the roots is at the beginning of winter[18] [18]. Drying is carried out at 40°C in drying sheds. The roots that are properly harvested and dried are brittle and have a light-brown color.
5. Status of the plant in official phytotherapy
The aerial parts of A. vulgaris—Artemisiae vulgaris herba—are usually used as a pharmaceutical raw material[31] [31], while the roots—Artemisiae vulgaris radix—harvested in early winter, are less frequently used[18] [18]. Both these raw materials do not have their monographs in the latest editions of pharmacopoeias; however, A. vulgaris herba had a monograph in the German Pharmacopoeia published in 1988[44] [44]. In the latest European Pharmacopoeia[45] [45] and in the French Pharmacopoeia[46] [46], the species is only mentioned as a homeopathic raw material. Although the plant has a monograph published by the EFSA[18] [18], no opinions regarding it have been issued by the European Scientific Cooperative on Phytotherapy (ESCOP) or European Medicines Agency (EMA).
6. Phytochemical characteristics
Various groups of compounds can be distinguished in A. vulgaris, including sesquiterpenoid lactones, flavonoids, coumarins, phenolic acids, sterols, polyacetylenes, carotenoids, vitamins, and cyanogenic glycosides. Essential oil is another important substance found in the plant. Due to the high intraspecific diversity and discrepancies in the chemical composition of the plant determined by using various test methods, it is difficult to indicate a distinct phytochemical profile for A. vulgaris [15]. A characteristic feature of this species is the presence of sesquiterpenoid lactones, including psilostachyin, psilostachyin C, and vulgarin (Figure 1), and also artemisinin was confired. In addition, the presence of flavonoids—derivatives of kaempferol and quercetin, and coumarin compounds, such as esculin, umbelliferone, and scopoletin, is a distinguishing attribute of the plant (Table 2).
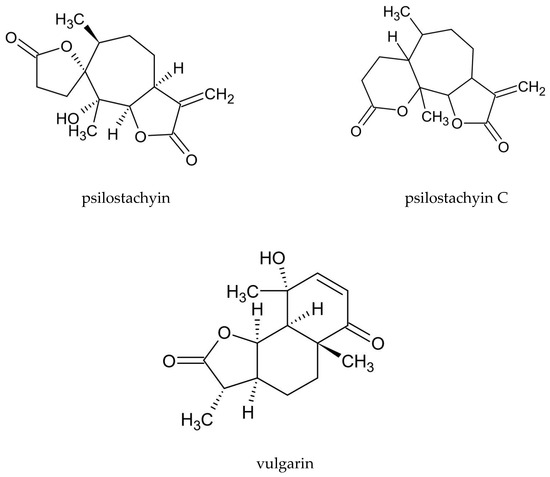
Figure 1. Chemical structure of sesquiterpenoid lactones characteristic of A. vulgaris.
Table 2. Chemical composition of A. vulgaris aerial parts.
|
Chemical group |
Compound |
Content |
References |
|
Sesquiterpenoid lactones |
1,2,3,4-diepoxy-11(13)-eudesmen-12,8-olide; yomogin |
na* |
[10] |
|
psilostachyin, psilostachyin C |
na |
||
|
vulgarin |
0.15% d.w. |
||
|
artemisinin |
0-2.3% dw. |
||
|
Flavonoids |
quercetin 3-galactoside, quercetin 3‑glucoside, kaempferol 3-glucoside, kaempferol 3-rhamnoside, |
~ 6 mg/kg d.w. ~ 11 mg/kg d.w. ~ 10 mg/kg d.w. ~ 5 mg/kg d.w. ~ 5 mg/kg d.w. ~ 5 mg/kg d.w. ~ 5 mg/kg d.w. |
[13] |
|
apigenin |
~ 6 mg/kg d.w. |
||
|
chrysoeriol |
~ 2.5 mg/kg d.w. |
[13] |
|
|
eriodictyol, |
~ 40 mg/kg d.w. ~ 5 mg/kg d.w. ~ 2.5 mg/kg d.w. |
||
|
eupafolin, homoeriodictyol |
~ 5 mg/kg d.w. ~ 10 mg/kg d.w. |
[13] |
|
|
hyperoside |
0.5 mg/g d.w. |
||
|
jaceosidin |
~ 3 mg/kg d.w. |
[13] |
|
|
quercetin |
~ 3 mg/kg d.w. |
||
|
luteolin |
~ 40 mg/kg d.w. |
||
|
rutoside |
~ 7-20 mg/kg d.w. |
||
|
tricine, |
~ 3 mg/kg d.w. |
[13] |
|
|
vitexin |
~ 4 mg/kg d.w. |
||
|
Coumarins |
esculin, esculetin, umbelliferone |
na |
|
|
Phenolic acids |
1,5-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid |
0.3% d.w. 0.2% d.w. |
[54] |
|
5-feruloylquinic acid quinic acid protocatechuic acid glucoside 3-O-caffeoylquinic acid 5-O-caffeoylquinic acid 4,5-O-di-caffeoylquinic acid |
0.37 mg/g d.w. 1.5 mg/g d.w. 3.2 mg/g d.w. 0.44 mg/g d.w. 2.8 mg/g d.w. 11 mg/g d.w. |
[52] |
|
|
caffeic acid |
na |
||
|
Sterols |
sitosterol, stigmasterol |
|
[3] |
|
Fatty acids |
na |
13.3 mg/g f.w. |
[39] |
|
Carotenoids |
(E)-β-ionone |
na |
|
|
Cyanogenic glycosides |
prunasin |
na |
|
|
Vitamins |
ascorbic acid |
na |
[30] |
|
Polyacetylenes |
na |
na |
|
|
Tannins |
na |
na |
[11] |
* na – no data available.
The presence of essential oil is an interesting characteristic of A. vulgaris. A major part of the oil extracted from the aerial parts is constituted by monoterpenoids (72%) and sesquiterpenoids (26%). Among the volatile compounds, the following are the most commonly identified: 1.8-cineol, sabinene, camphor, camphene, caryophyllene oxide, α-thujone, and β-thujone (Table 3 and Figure 2).
Table 3. Chemical composition of essential oil from A. vulgaris aerial parts.
|
Chemical groups / Compounds |
Estimated content (%) | References | ||||||
|
Monoterpenoids |
|
| ||||||
|
artemisia alcohol, artemisyl acetate, isobornyl acetate, (Z)-β-ocymen, terpinolene |
0-2.6 |
[55] |
||||||
|
artemisia ketone |
0-2.89 |
[55,57,58] |
||||||
|
borneol |
0.4-9.8 |
[3][15][17][24][55][57][58][59][60] |
[3,15,17,24,55,57–60] |
|||||
|
bornyl acetate |
0-6.29 |
[15–17,58,60] |
||||||
|
camphene |
1.8-9.1 |
[15–17,39,55,57,58] |
||||||
|
camphor |
0-47.7 |
[16][17][18][24][39][55][57][58][59][61][62] |
[16,17,62,18,24,39,55,57–59,61] |
|||||
|
carvone |
0-0.38 |
[58] |
||||||
|
trans-carveol, trans-pinocarveol |
0-0.77 |
[15,57,58] |
||||||
|
1,8-cineol |
2.6-17.6 |
[3][16][17][18][24][30][39][55][57][59][63] |
[3,16,63,17,18,24,30,39,55,57,59] |
|||||
|
cis-chrysanthenol, dehydrosabinaketone, methyleugenol, verbenyl acetate, p-cymene-8-ol, piperitone, p-mentha-1,4-dien-7-ol, sabinaketone, trans‑verbenol, cuminol |
0-7.0 |
[15] |
||||||
|
cymene |
0-1.14 |
[17,55,58] |
||||||
|
isoborneol |
0.3-8.2 |
[17,55,57] |
||||||
|
isobornyl 2-methylbutyrate, menthol |
0-5 |
[39] |
||||||
|
iso-3-thujanol |
0-1.4 |
[55,57,58] |
||||||
|
limonene |
0-0.46 |
[58] |
||||||
|
(E)-β-ocymen |
0.5-2.7 |
[55] |
||||||
|
3-thujanol |
|
[17,57] |
||||||
|
4-terpineol |
0-1.4 |
[3,15,16,24,55,57,59] |
||||||
|
cis-thujone |
0-12.9 |
[39,55] |
||||||
|
linalool |
0-0.4 |
[3,55,57,59] |
||||||
|
chrysanthenyl acetate |
0-23.6 |
[15,39,55] |
||||||
|
β-myrcene |
0.1-8.8 |
[16,55] |
||||||
|
sabinene |
0-0.67 |
[15–17,55,57,58] |
||||||
|
cis-sabinene hydrate, |
0-1.08 |
[55,57,58] |
||||||
|
trans-sabinene hydrate |
0-0.55 |
[15,55,57,58] |
||||||
|
santolina triene |
0-0.6 |
[15–17,55,57] |
||||||
|
α-thujone |
0-3.18 |
[15–17,24,39,57,64] |
||||||
|
β-thujone |
0-1.19 |
|
[17][16][24][39][57][62][64][65] |
[15–17,24,39,57,62,64,65] |
||||
|
α-fenchen |
|
[16,17,57] |
||||||
|
α-pinene |
0-0.9 |
[15,16,39,55,57,58] |
||||||
|
α-terpinene |
0-0.4 |
[55,57] |
||||||
|
α-terpineol |
0-1.6 |
[15,24,55] |
||||||
|
α-thujene |
0.2-4.1 |
[15,16,55] |
||||||
|
β-pinene |
0.1-12.9 |
[15,17,24,39,55,57] |
||||||
|
γ-terpinene |
0-0.54 |
[16,55,57,58] |
||||||
|
thymol |
0-0.39 |
[58] |
||||||
|
|
|
|||||||
|
Sesquiterpenoids |
|
| ||||||
|
aromadendrene |
0-0.2 |
[16,57] |
||||||
|
bicyclogermacrene |
0.9-2.2 |
[16,17,55,57] |
||||||
|
α-cadinol |
0-1.99 |
[3,17,59,60] |
||||||
|
caryophyllene |
0-37.45 |
[24,39,55,60] |
||||||
|
caryophyllene oxide |
1.52-5.5 |
[3][15][16][17][39][55][57][58][59] |
[3,15–17,39,55,57–59] |
|||||
|
trans-caryophyllene, trans-salvene |
2.5-12.2 |
[39,58] |
||||||
|
caryophylla-4(14),8(15)-diene-5-α-ol, (E)-nerolidol, humulene epoxide II, germacrene D-4-ol, ledol, farnesyl acetate, lanceol acetate, salvial-4(14)-en-1-one, silphiperfol-5-en-3-ol (Z)-β-farnesene, α-calacorene, β-chamigrene, β-longipinene |
0-0.5 |
[55,60] |
||||||
|
α-copaen |
0-1.0 |
[15–17,55,57] |
||||||
|
cubebene |
0-12 |
[3] |
||||||
|
davanone, silphiperfol-4,7(14)-diene, β-burbonen |
0-0.15 |
[55,57] |
||||||
|
β-elemene |
0-8 |
[17], [57] |
||||||
|
β-eudesmol |
0-8.95 |
[16,39,57,58] |
||||||
|
α-elemene, β-bisabolene |
0-8.8 |
[16,55] |
||||||
|
farnesene |
0-0.88 |
[15,58] |
||||||
|
germacrene D |
5.3-15.1 |
[15,39,55,57] |
||||||
|
α-humulene |
0.2-8.8 |
[16,55,57,58] |
||||||
|
epi-α-muurolol |
0.4-1.4 |
[15,55] |
||||||
|
spathulenol |
1-2.5 |
[15,17,39,55] |
||||||
|
7-α-silphiperfol-5-ene, epi-β-santalene, modhephene, petasitene, presilphiperfol-7-ene, silphin-1-ene, valeranone, humulene oxide, α-bisabololene, α-cedrene, |
0-0.5 |
[16] |
||||||
|
|
|
| ||||||
|
Diterpenoids |
|
| ||||||
|
phytol |
0-2.94 |
[55,60] |
||||||
|
γ-terpineol |
0-1.44 |
[58] |
||||||
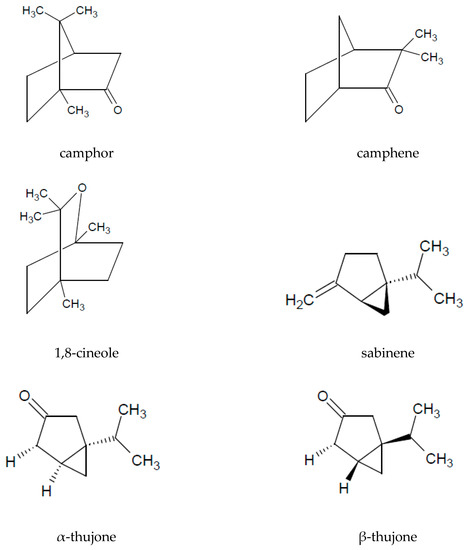
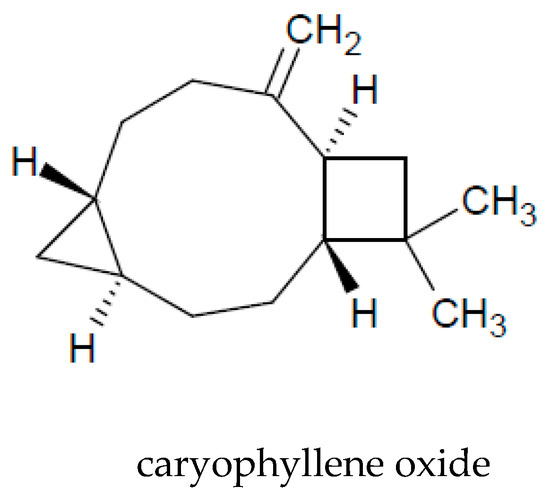
Figure 2. Chemical structure of volatile compounds characteristic of the essential oil of A. vulgaris herb.
Many experiments have revealed the significant differences in the quality and quantity of the components of the essential oils obtained from the A. vulgaris plants grown in different parts of the world.
Although the root of this species is sometimes used as raw material, only a few publications have described its chemical composition. The main compounds in the oil extracted from the root were neryl 2-methylbutanoate (13.2%), β-eudesmol (10.4%), and bornyl 3-methylbutanoate (8.45%). However, none of these substances was found in the oil obtained from the aerial parts. The underground part of the plant also contained some rare compounds belonging to, inter alia, the group of tricyclic sesquiterpenoids, such as (‑)-alpha-isocomene, (‑)-isocomene, silphin-1-ene, presilphiperfol-7-ene, and modhephene[16] [16] (Figure 3).
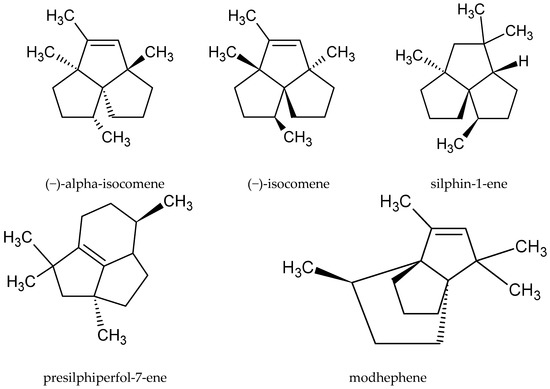
Figure 3. Chemical structure of tricyclic sesquiterpenoids characteristic of the root of A. vulgaris.
In the essential oil extracted from the aerial parts of A. vulgaris, no tricyclic sesquiterpenoids were detected. The dominant compounds that were identified in the study were 1,8-cineol (28.9%), sabinene (13.7%), β-thujone (13.5%), and β-caryophyllene oxide (6.5%), but these were absent in the underground parts of the plant. Based on these findings, the authors concluded that the differences in the chemical composition of the oils extracted from the root and aerial parts might indicate the coexistence of different biogenetic pathways in the plant species[16] [16].
7. History of medicinal use
Due to its widespread occurrence, A. vulgaris was well known in ancient Egypt, Greece, and Rome. According to ancient belief, its name is derived from the name of the Greek goddess Artemis, who is the patron of pregnant women and newly delivered mothers. Because of its beneficial effects on menstruation- and pregnancy-related ailments, A. vulgaris had great importance in the religious rites devoted to the goddesses Isis, Artemis, and Diana. The healing properties of this species were described in medical works in as early as the 1st century A.D. by Dioscorides in “Materia medica”[66] [66], by Pliny the Elder in “Naturalis Historia”[67] [67], and by Galen in “De simplicium medicamentorum facultatibus”[68] [68]. Furthermore, the mugwort plant was credited with warming and drying effects, and therefore, it was also recommended for the treatment of urological diseases, such as dysuria or nephrolithiasis. In medieval medicine, A. vulgaris, called “mater herbarum” (the mother of herbs), was used externally for treating wounds, against gout, and to remove leg fatigue, as well as in an attempt to treat fever[69] [69]. In addition, the plant gained popularity as a remedy for gastrointestinal ailments “resulting from cold”[70] [70], including stomach pain, diarrhea, and intestinal colic. It was believed to be effective against jaundice when served with wine and against goiter when applied as a poultice[71] [71]. During the Renaissance, thanks to, inter alia, the invention of printing by J. Gutenberg, the holistic medicine flourished in Europe, lasting until the 18th century. At that time, in addition to women’s diseases, the therapeutic spectrum of A. vulgaris was expanded to include spleen and liver diseases. Additional recommendations appeared “for enlarged and distended spleen”[72] [72], “against clogged liver”[73] [73], and “for cold lower abdomen”[74] [74]. During the development of modern medicine in the 19th century, epilepsy and neurosis were included among the indications for treatment with mugwort[75] [75]. In the 20th century, the scientific and laboratory analyses of the composition of A. vulgaris led to the declaration that due to its high allergic potential the herb is not suitable for medicinal use and can only be used in culinary, and that its place in the household is the kitchen, not the medicine cabinet[63] [63].
8. Applications in traditional medicine worldwide
In Asian medicine, A. Vvulgaris is often used for alleviating gastrointestinal discomfort and treating gynecological diseases[5][6] [5,6].
In China, A. vulgaris is traditionally used to treat cholera and leprosy. Other indications include hemorrhagic conditions, such as the presence of blood in sputum, stool, and vomit, and nosebleeds[18] [18]. The essential oil of this species is used as a popular herbal medicine called “Ai Hao,” and is prescribed for curing ulcers and diarrhea[7] [7].
The Traditional Chinese Medicine (TCM) also recommends thermopuncture consisting in burning “moxa”—dried and powdered leaves of A. vulgaris—directly on or close to the skin, or inhaling the resulting smoke, which allows the migration of “chi” energy[76] [76]. Indications for this treatment include tumors, although its effectiveness has not been confirmed so far[77] [77]. In 2009,[78] [78] investigated the harmfulness of compounds formed during the combustion of this plant species. The authors tested the smoke emitted by moxa for the presence of compounds commonly found in cigarettes, such as tar, nicotine, carbon monoxide, polycyclic aromatic hydrocarbons, ammonia, hydrogen cyanide, polyaromatic amines, volatile organic compounds, semi-volatile compounds, phenols, and carbonyls (e.g. formaldehyde and acetone), all of which are associated with the highest risk of cancer. Their results showed that even at concentrations higher by an order of magnitude than that achieved with the inhalation of normal smoke, only a few of the analytes exceeded the permissible standards. These included aromatic amines such as 1-aminonaphthalene, 2-aminonaphthalene, 3-aminobiphenyl, and 4-aminobiphenyl. Therefore, the authors concluded that burning moxa should not pose a health risk. Evidence of exceeded limits appeared only after the extrapolation of results for long-term use, which probably did not reflect the actual concentration of the tested compounds[78] [78]. In TCM, A. vulgaris is most often used in thermopuncture in the form of cotton buds impregnated with the plant extract, or as cigarettes filled with dried leaves and used to cauterize the skin.
The species is also considered a substitute for cannabis. When being smoked, it exhibits mild intoxicating properties and strong relaxing properties[18] [18]. In traditional Hindu medicine (Unani), many preparations based on A. vulgaris are used. For instance, “Arq-e-Afsanteen” is a preparation recommended for liver inflammation and obstruction. “Dava-ul-Luk” is used for treating enlarged liver or spleen and nephrolithiasis. The medication “Qurs-e-Gul” is indicated in chronic fever as a liver tonic, while decoctions from the plant are taken during dysmenorrhea[79] [79]. In addition, liquid extract or dried greens at a single dose of 0.5–2 g is used in Ayurvedic medicine[3] [3].
The species is also well known in the traditional medicine of South America, and often used against fever, malaria, and gastric disorders[80][81][82] [80–82].
In European folk medicine, after oral administration, the A. vulgaris herb stimulates the secretion of gastric juice. Hence, it is used against gastrointestinal catarrh, insufficient production of bile and digestive juices, flatulence, and poor appetite. The plant is also used as a relaxant for the gastrointestinal tract and bile ducts and for relieving colic[3] [3], while the observed laxative effect is utilized in the treatment of obesity. It is also used as infusions for external use for alleviating rheumatic and leg pains, as well as for preparing sitz baths for hemorrhoids. Other traditional applications of A. vulgaris include the treatment of nervous system disorders such as insomnia[31] [31], epilepsy, depression, and excessive stress exposure[61] [61]. Furthermore, it is recommended for relieving hypertension and inducing labor or miscarriage[83] [83].
9. Applications in modern phytotherapy and medicine
At present, A. vulgaris herb is not commonly used as a medication. However, due to its aroma and bitter taste, the herb is used for stimulating the secretion of digestive juices in the treatment of appetite loss, achlorhydria, gastritis, and flatulence[3][31] [3,31]. The roots of A. vulgaris are not rich in bitter components, and therefore, they do not induce gastric secretion.
The essential oil of A. vulgaris is used in insect repellents and fumigants. In addition, it exhibits antibacterial and antifungal properties[3][7][18] [3,7,18].
A monograph of the raw material A. vulgaris herba was prepared by German Commission in 1988. This document listed only the traditional, above-mentioned uses of the herb and emphasized that the effectiveness of preparations based on A. vulgaris had not been confirmed and hence they are not recommended for therapeutic uses[44] [44].
In one of the latest editions of European Pharmacopoeia[45] [45] and the French Pharmacopoeia[46] [46], A. vulgaris is listed as a homeopathic raw material. Its application includes the treatment of irregular menstrual cycles and menopausal symptoms[7] [7], and nervous disorders such as sleepwalking, seizures, epilepsy, and anxiety[84] [84]. The homeopathic medications are prepared using a fresh-root tincture, which is made of 65% ethanol. The tincture should contain a minimum of 0.01% (w/w) derivatives of hydroxycinnamic acid, quantified as the equivalents of chlorogenic acid[85] [85].
In allopathy, mugwort is used mainly in the form of infusions. These are prepared by pouring boiling water over one teaspoon (about 1.2 g) of dried herb and brewing the mixture covered for about 5 minutes. Another form of mugwort medication used in allopathy is tinctures, which are sold all over Europe[3] [3].
The applications of A. vulgaris documented in scientific works and the pharmacological activity profile of this species recommended in modern phytotherapy are presented in the below sections (Table 4).
Table 4. Pharmacological properties of A. vulgaris herb and root extract.
Activity* | Information | Compounds supposed to be responsible | References | ||||||||||||||
Antioxidant | Proved by different methods: DPPH, lipid peroxidation, protein glycation, xanthine oxidases, ABTS, hydroxyl, superoxide, nitric oxide, ferric reducing power activity and inhibition of lipid peroxidation by thiobarbituric acid reactive species assays. | Increasing the level of ascorbic acid and glutathione. | flavonoids, flavonols, phenolic acids |
[8,52,86–88] |
|||||||||||||
Hypolipemic | Normalized serum lipid profile, a significant increase in paraoxonase-1 activity and decrease in serum malondialdehyde, nitric oxide, tumor necrosis factor-α level and decrease in hydroxymethylglutaryl-CoA reductase activity. | Lowering total cholesterol, triglycerides, LDL, VLDL, and increasing HDL and atherogenicity indicator (aqueous extract of A. vulgaris roots)*. |
|
[9,89] |
|||||||||||||
Hepatoprotective | Prophylactic protective effect limiting inflammation, cellular oedema, apoptotic cell count, and hyperaemia of the hepatic parenchyma. |
|
[90] |
||||||||||||||
Antispasmolytic | Antagonism towards H1 histamine receptors. |
|
[10,11] |
||||||||||||||
Bronchodilatory | Anticholinergic and Ca2+ antagonist mechanisms. Histamine H1 antagonism in the ileum and trachea. | yomogin (sesquiterpene lactone), alkaloids, coumarins, flavonoids, saponins, sterols, tannins, terpenes |
[10,91] |
||||||||||||||
Analgesic | Mild peripheral anti-nociceptive effect, probably induced by rutoside, hydroxybenzoic acid derivatives, and caffeic acid and its derivatives. | na* |
[11] |
||||||||||||||
MAO inhibition | Inhibition of mouse brain monoamine oxidase (MAO) enzyme. | flavonoids: jaceosidine, eupafolin, luteolin, quercetin, apigenine; coumarins: aesculetin, esculetin-6-methylether, scopoletin |
[92] |
||||||||||||||
Antihypertensive | Inhibiting the hypertensive effect of noradrenaline. | Moxibustion showed lowering the blood pressure compared to antihypertensive drugs by stimulation of acupoint KI 1. | na |
| Moxibustion - a traditional Chinese method that uses the heat generated by burning herbal preparations containing A. vulgaris to stimulate acupuncture points |
[12] |
|
[93] |
|||||||||
Estrogenic | Antagonism towards the oestrogen receptor and activation of gene transcription. Induction of gene transcription by eriodictyol and apigenin. | Anti-implantation activity and estrogenic activity on female Wistar rats. | flavonoids |
[13,94] |
|||||||||||||
Cytotoxic | Inhibition of tumour cell growth in cancer cell lines: MCF7, HeLa, A7R5, 293T, HL-60 and SW-480. | phenolic compounds, flavonoids, essential oil |
[14,95,96] |
||||||||||||||
Antifungal and antibacterial | Inhibitory effect of the oil fraction on the development of Candida albicans. | Inhibitory effect of the oil fraction on the development of Escherichia coli, Salmonella enteritidis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus mutans, Candida albicans, and Aspergillus niger, probably associated with the presence of. | essential oils, 1,8-cineol, α-thujone, camphene |
[15,16,60,87,97–99] |
| ||||||||||||
Anti-inflammatory | Normalization of serum lipid profile, increase in paraoxonase-1 activity and decrease in serum malondialdehyde, nitric oxide and tumor necrosis factor-α level. Proved by lipoxygenase (LOX) inhibitory activity assay and “Cotton Pellet Granuloma method”. | na |
[88,89,100] |
||||||||||||||
Antialergenic | Decrease in skin sensitivity and eye sensitivity. | na |
[101] |
||||||||||||||
Antimalarial | Activity angainst Plasmodium yoelii and P. berghei. | na |
[102,103] |
||||||||||||||
Anthelmintic | Activity against Trichinella spiralis. | na |
[104] |
* all of the listed activities have been proved for extracts of A. vulgaris herb, except for hypolipidemic effects; **na – no data available.
10. Applications in cosmetology
Based on its profile of activity, the European database of cosmetic raw materials, CosIng (Cosmetic Ingredients database), recommends the use of A. vulgaris in eight forms, including skin care agents, humectants, skin protectants, and fragrances. An original form used in cosmetics is filtrates obtained as a result of fermentation by bacteria (Bacillus sp., Lactobacillus sp.) or fungi (Saccharomyces sp.).
During fermentation, Bacillus sp. produce valuable physiologically active substances such as peptides, viscous compounds (with polysaccharide structure), antioxidants, and fibrin.
A combination of A. vulgaris and Bacillus sp. has been shown to enhance the effects of fermentation and increase the antiaging and antiwrinkle effects by inhibiting the production of matrix metalloproteinase-1 and -9 enzymes (decomposed of collagen) and increasing cell regeneration and collagen synthesis[105] [105].
In cosmetics production, the extracts of herb and essential oil are used, which are the raw materials that do not contain pollen causing allergic reaction.
11. Applications in the food industry
The essential oil extracted from the aerial parts of A. vulgaris is useful in the food industry, as it exhibits antiseptic, antioxidant, and antimicrobial activities. In addition, the oil has larvicidal, nematicidal, and pesticidal effects[39] [39].
The species A. vulgaris is highly valued as a spice because of the aroma and bitter taste of the herb and the sweet and spicy taste of the roots. The leaves and buds collected just before flowering are used as a bitter additive for seasoning rice dishes and tea in Asia[14][18] [14,18]. The species is also used as an additive to meat, poultry, fish, and salads, in the production of vodkas and herbal wines[18] [18], and in the preparation of sweet and savory cakes (especially in Japan). Before the introduction of hops, A. vulgaris had been used to flavor beer[18] [18].
Despite its earlier popularity, A. vulgaris is relatively rarely used as a spice in Poland. Currently, there are attempts being made to make it more popular as a seasoning agent for, inter alia, mutton, liver, cabbage, spinach, and mushroom dishes, as well as soups.
In addition, A. vulgaris tincture is widely used in animal feed as a sensory (aromatic) additive[106] [106].
The EFSA reports that as an insecticide A. vulgaris is intended for use in arable fields, for protecting plants in orchards and vineyards, and for growing vegeTables. The plant targets insects such as Ae. aegypti (Egyptian mosquito), Musca domestica (housefly), and Tribolium castaneum (red flour beetle). There is increasing interest in using the plant for this purpose because it is less harmful to both humans and the environment compared to other preparations[18][39][107] [18,39,107].
12. Safety of use
The use of A. vulgaris herb extracts in therapeutic doses is not likely to cause side effects; however, the plant can cause allergies, as confirmed by the U.S. Food and Drug Administration (FDA). Its pollen contains allergenic glycoproteins that cause the type I (immediate) allergic reaction, related to IgE antibodies. In addition, anaphylactic shock has been observed in patients after swallowing the pollen[3][108] [3,108]. The species is also considered to be the main cause of hay fever and allergic asthma in northern Europe, North America, and part of Asia[18][109] [18,109].
If a person is allergic to any ingredient of A. vulgaris or any plant of the family Asteraceae, he/she should avoid contact with them. Cross-reactions of the plant with pollen from other plants as well as with food substances have also been observed—with birch, cabbage, grasses, hazelnuts, honey, pollen of the European olive and sweet pepper, and also with royal jelly, sunflower, kiwi, peach, mango, apple, celery, and carrot[18][110] [18,110].
In addition to anaphylactic shock, A. vulgaris pollen, as an allergen, can cause breathing difficulties, bronchospasm, airway hypersensitivity, asthma attack, seasonal rhinitis, and conjunctivitis. Allergic skin reactions, such as dermatitis and urticaria, may also occur[77][109][111][112] [77,109,111,112].
When consumed in large doses, A. vulgaris may cause miscarriage[44][77] [44,77], nausea, vomiting, and nervous system damage. Furthermore, cases of hypertension have been reported[18] [18].
The EFSA lists that the ingredients in the essential oil of A. vulgaris herb, such as α-thujone, β-thujone, camphor, and 1,8-cineol, have potentially adverse effects on human health, when taken with food or dietary supplements [113]; however, it emphasizes that most research has concerned only a more concentrated oil, and not a less concentrated extract[18] [18].
It should be remembered that A. vulgaris should be used with caution in patients with diabetes, as it can increase blood glucose levels. The species is also not recommended for the prevention of malaria and in vitro studies have not shown protozoicidal activity. In addition, it was reported that patients who used A. vulgaris as a prophylactic agent when traveling to eastern Africa were not prevented from developing malaria[18] [18].
The EFSA has also published a document describing the safety of using a hydroethanolic tincture of A. vulgaris having a dry matter content of approximately 1.7% as an animal feed additive. The ruling emphasizes that although the isolated phenolic compounds are not toxic, the safety profile of this tincture cannot be determined because as much as 74% of the dry matter fraction remains undetermined. It is also not known whether the tincture can cause irritation to the skin or eyes. The document is summarized with a statement declaring that no further research is needed because A. vulgaris and its extracts are commonly used as aromatic substances in food, where they perform similar functions as in feed[106] [106].
Scientists from four Japanese centers—Science University of Tokyo (Tokyo), The Institute of Physical and Chemical Research (RIKEN) (Wakō), Nagoya University School of Medicine (Nagoya), and Aichi Cancer Center Research Institute (Nagoya)—have isolated prunasin from A. vulgaris (Figure 4). They used 300 mg of acetone extract and obtained 25 mg of prunasin[56] [56]. Prunasin is a cyanogenic glycoside that releases toxic hydrogen cyanide (HCN) during enzymatic hydrolysis by β-3-glucosidases in the macerated plant tissue or under the influence of intestinal microflora. In the human body, HCN combines with cytochrome oxidases and prevents oxygen access to tissues, thereby leading to body hypoxia[114] [114]. However, further research is needed on the prunasin content in A. vulgaris and its effect on the human body.
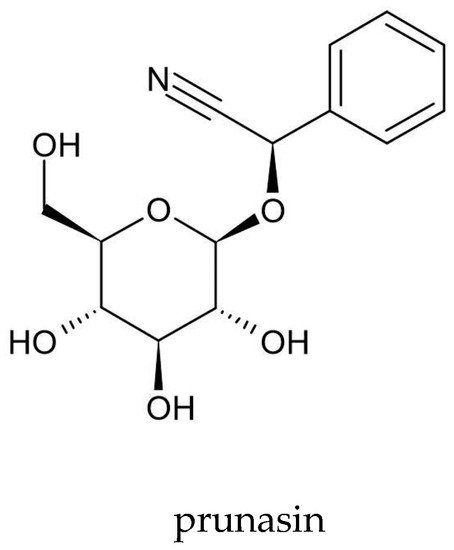
Figure 4.
Chemical structure of prunasin—a cyanogenic glycoside isolated from
A. vulgaris.
The publication entitled “Significance of Artemisia vulgaris L. (common mugwort) in the history of medicine and its possible contemporary applications substantiated by phytochemical and pharmacological studies” can be found in Molecules 2020, 25(19), 4415; https://doi.org/10.3390/molecules25194415
References
- Haq, F.U.; Roman, M.; Ahmad, K.; Rahman, S.U.; Shah, S.M.A.; Suleman, N.; Ullah, S.; Ahmad, I.; Ullah, W. Artemisia annua: Trials are needed for COVID-19. Phyther. Res. 2020, doi:10.1002/ptr.6733.
- Efferth, T.; Zacchino, S.; Georgiev, M.I.; Liu, L.; Wagner, H.; Panossian, A. Nobel Prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine Int. J. Phyther. & Phytopharm. 2015, 22, 1–4.
- Wichtl, M. Herbal drugs and phytopharmaceuticals: a handbook for practice on a scientific basis.; 3 edition.; Medpharm: Marburg, 2004;
- The Plant List Available online: http://www.theplantlist.org/ (accessed on Mar 12, 2020).
- Holm, L.; Doll, J.; Holm, E.; Pnacho, J.; Herberger, J. World Weeds: Natural Histories and Distribution; John Wiley and Sons: New York, 1997;
- Chevallier, A. The encyclopedia of medicinal plants: A practical reference guide to more than 500 key medicinal plants and their uses.; DK Publishing: New York, 1996;
- Barney, J.N.; DiTommaso, A. The biology of Canadian weeds. 118. Artemisia vulgaris L. Can. J. Plant Sci. 2003, 83, 205–215.
- Temraz, A.; El-Tantawy, W.H. Characterization of antioxidant activity of extract from Artemisia vulgaris. Pak. J. Pharm. Sci. 2008, 21, 321–326.
- Khan, K.A. A preclinical antihyperlipidemic evaluation of Artemisia vulgaris root in diet induced hyperlipidemic animal model. Int. J. Pharmacol. Res. 2015, 5, 110–114.
- Natividad, G.M.; Broadley, K.J.; Kariuki, B.; Kidd, E.J.; Ford, W.R.; Simons, C. Actions of Artemisia vulgaris extracts and isolated sesquiterpene lactones against receptors mediating contraction of guinea pig ileum and trachea. J. Ethnopharmacol. 2011, 137, 808–816, doi:10.1016/j.jep.2011.06.042.
- Pires, J.M.; Mendes, F.R.; Negri, G.; Duarte-almeida, J.M.; Carlini, E.A. Antinociceptive Peripheral Effect of Achillea millefolium L . and Artemisia vulgaris L .: Both Plants known popularly by Brand Names of Analgesic Drugs. Phyther. Res. 2009, 219, 212–219, doi:10.1002/ptr.
- Tigno, X.T.; de Guzman, F.; Flora, A.M.; V., T. Phytochemical analysis and hemodynamic actions of Artemisia vulgaris L. Clin. Hemorheol. Microcirc. 2000, 23, 167–175.
- Lee, S.J.; Chung, H.Y.; Maier, C.G.A.; Wood, A.R.; Dixon, R.A.; Mabry, T.J. Estrogenic Flavonoids from Artemisia vulgaris L. J. Agric. Food Chem. 1998, 46, 3325–3329, doi:10.1021/jf9801264.
- Erel, B.; Aydin, F.; Ballar, P. In vitro cytotoxic properties of six Artemisia L. species. Turkish J. Pharm. Sci. 2011, 8, 247–251.
- Obistioiu, D.; Cristina, R.T.; Schmerold, I.; Chizzola, R.; Stolze, K.; Nichita, I.; Chiurciu, V. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 2014, 8, 6, doi:10.1186/1752-153X-8-6.
- Blagojević, P.; Radulović, N.; Palić, R.; Stojanović, G. Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J. Agric. Food Chem. 2006, 54, 4780–4789, doi:10.1021/jf060123o.
- Govindaraj, S.; Ranjitha Kumari, B.D. Composition and Larvicidal Activity of Artemisia Vulgaris L. Stem Essential Oil against Aedes Aegypti. Jordan J. Biol. Sci. 2013, 6, 11–16.
- European Food Safety Authority Artemisia Vulgaris Basic Substance Application; 2013;
- European Commission Cosing CosIng - Cosmetic Database.
- Anwar, S.; Asif, N.; Naqvi, S.A.H.; Malik, S. Evaluation of multiple risk factors involved in the development of diabetic Retinopathy. Pakistan J. Med. Sci. 2019, 35, 156–160.
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B.; Shaharuddin, N.A.; Malik, S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018, 109, 403–415, doi:10.1016/j.foodres.2018.03.072.
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566, doi:10.3390/molecules17032542.
- Tan, R.X.; Zheng, W.F.; Tang, H.Q. Biologically Active Substances from the Genus Artemisia;
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109, doi:10.3109/13880209.2010.497815.
- Hegnauer, R. Chemotaxonomie der Pflanzen; Springer Basel AG, 1962;
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H. Flora Europaea: Volume 4 Plantaginaceae to Composite (and Rubiaceae) ; Cambridge University Press, 1976; Vol. 4;.
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie. Vol. I-III; Państwowe Wydawnictwo Naukowe, Warszawa, Poland, 1986;
- Zarzycki, K.; Kaźmierczakowa, R.; Mirek, Z. Polska Czerwona Księga Roślin. Paprotniki i rośliny kwiatowe.; Wyd. III.; Instytut Ochrony Przyrody PAN: Kraków, 2014;
- Missouri Botanical Garden Tropicos.org.
- Weston, L.A.; Barney, J.N.; DiTommaso, A. A review of the biology and ecology of three invasive perennials in New York State: Japanese knotweed (Polygonum cuspidatum), mugwort (Artemisia vulgaris) and pale swallow-wort (Vincetoxicum rossicum). Plant Soil 2005, 277, 53–69, doi:10.1007/s11104-005-3102-x.
- van Wyk, B.-E.; Wink, M. Medicinal plants of the world; Timber Press: Portland, 2004;
- Vallès, J.; McArthur, D.E. Artemisia Systematics and Phylogeny: Cytogenetic and Molecular Insights. In Shrubland ecosystem genetics and biodiversity: proceedings; McArthur, E.D., Fairbanks, Daniel, J., Eds.; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, USA, 2001; pp. 13–15.
- Pellicer, J.; Garcia, S.; Canela, M.Á.; Garnatje, T.; Korobkov, A.A.; Twibell, J.D.; Vallès, J. Genome size dynamics in Artemisia L. (Asteraceae): Following the track of polyploidy. Plant Biol. 2010, 12, 820–830, doi:10.1111/j.1438-8677.2009.00268.x.
- Tabur, S.; Civelek, Ş.; Öney, S.; Ergün, Ş.B.Y. Chromosome counts and karyomorphology of some species of Artemisia (Asteraceae) from Turkey. Turk. J. Botany 2012, 36, 235–246.
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265, doi:10.1007/s10886-005-1339-8.
- The Herb Society of America Artemesia: An Essential Guide 2014.
- Borzabad, R.K.; Sudarshana, M.S.; Niranjan, M.H. In vitro Plant Regeneration from Leaf Explants of Artemisia vulgaris L. – A Medicinal Herb. Mod. Appl. Sci. 2010, 4, 130–134, doi:10.5539/mas.v4n9p130.
- Uva, R.H.; Neal, J.C.; DiTomaso, J.M. Weeds of the Northeast; Cornell University Press: Ithaca, 1997;
- Anwar, F.; Ahmad, N.; Alkharfy, K.M.; Gilani, A.H. Mugwort (Artemisia vulgaris) oils; Preedy, V.R., Ed.; Academic Press: London, 2016; ISBN 9780124166448.
- Garnock-Jones, P.J. Floret specialization, seed production and gender in Artemisia vulgaris L. (Asteraceae, Anthemideae). Bot. J. Linn. Soc. 1986, 92, 285–302.
- Gleason, H.A.; Cronquist, A. Manual of Vascular Plants of Northeastern United States and Adjacent Canada; 2nd ed.; The New York Botanical Garden: New York, 1991;
- Pawłowski, F.; Kapeluszny, T.; Kolasa, A.; Lecyk, Z. Fertility of some species of ruderal weeds. Ann. Univ. Mariae Curie-Slodowska 1968, 22, 221–223.
- Hale, M. Allelopathic potential of Artemisia vulgaris rhizomes. Plant Physiol. 1982, 69, Supplement 126.
- und Medizinprodukte, B. für A. German Commission E monograph; Blaumenthal, M.T., Hall, R., Rister, B., Eds.; American Botanical Council: Austin, 1984;
- European Directorate for the Quality of Medicine European Pharmacopoeia 10.0; Strasbourg, 2010;
- Française Pharmacopée Pharmacopée française; Noculak, A., Ed.; 11ème édit.; Georg Olms Verlag,: Hildesheim, New York, 2020; Vol. 37;.
- Marco, J.A.; Sanz, J.F.; del Hierro, P. Two eudesmane acids from Artemisia vulgaris. Phytochemistry 1991, 30, 2403–2404, doi:10.1016/0031-9422(91)83661-4.
- Geissmax, T.A.; Ellestad, A. Vulgarin, a Sesquiterpene Lactone from Artemisia vulgaris L. J. Org. Chem. 1961, 27, 1855–1859.
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H. Assessment of Artemisinin Contents in Selected Artemisia Species from Tajikistan (Central Asia). Medicines 2019, 6, 23, doi:10.3390/medicines6010023.
- Nganthoi, M.; Sanatombi, K. Artemisinin content and DNA profiling of Artemisias pecies of Manipur. South African J. Bot. 2019, 125, 9–15, doi:10.1016/j.sajb.2019.06.027.
- Lee, K.H.; Jung, M.Y.; Kim, S.Y. Effects of Ascorbic Acid on the Light-Induced Riboflavin Degradation and Color Changes in Milks. J. Agric. Food Chem. 1998, 46, 407–410.
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 2020, 5, 192–200, doi:10.1016/j.jff.2014.05.019.
- Wallnofer, B.; Hofner, O.; Greger, H. Polyacetylenes from the Artemisia ‘Vulgares’ group. Phytochemistry 1989, 28, 2687–2691.
- Carnat, A.; Heitz, A.; Fraisse, D.; Carnat, A.P.; Lamaison, J.L. Major dicaffeoylquinic acids from Artemisia vulgaris. Fitoterapia 2000, 71, 587–589, doi:10.1016/S0367-326X(00)00163-5.
- Judžentien, A.; Buzelyte, J. Chemical composition of essential oils of Artemisia vulgaris L. ( mugwort ) from North Lithuania. Chemija 2006, 17, 12–15.
- Mizushina, Y.; Takahashi, N.; Ogawa, A.; Tsurugaya, K.; Koshino, H.; Takemura, M.; Yoshida, S.; Matsukage, A.; Sugawara, F.; Sakaguchi, K. The cyanogenic glucoside, prunasin (D-mandelonitrile-β-D-glucoside), is a novel inhibitor of DNA polymerase β. J. Biochem. 1999, 126, 430–436, doi:10.1093/oxfordjournals.jbchem.a022468.
- Govindaraj, S.; Kumari, B.D.R.; Cioni, P.L.; Flamini, G. Mass propagation and essential oil analysis of Artemisia vulgaris. J. Biosci. Bioeng. 2008, 105, 176–183, doi:10.1263/jbb.105.176.
- Madhav, K.; Kunal, M.; Zafar, H.; Ujjwal, B.; Gaurav, N. Antioxidant analysis of essential oils and methanolic extracts of Artemisia vulgaris. Int. J. Agric. Sci. 2018, 10, 5710–5713.
- Pickenhagen, W.; Willhalm, B. New Irregular Monoterpenes in Artemisia vulgaris. 1981, 64, 1424–1430.
- Malik, S.; de Mesquita, L.; Silva, C.; de Mesquita, J.; de Sá Rocha, E.; Bose, J.; Abiri, R.; de Maria Silva Figueiredo, P.; Costa-Júnior, L. Chemical Profile and Biological Activities of Essential Oil from Artemisia vulgaris L. Cultivated in Brazil. Pharmaceuticals 2019, 12, 49, doi:10.3390/ph12020049.
- Koul, B.; Taak, P. The Artemisia Genus: A Review on Traditional Uses, Phytochemical Constituents, Pharmacological Properties and Germplasm Conservation. J. Glycomics Lipidomics 2017, 7, 142, doi:10.4172/2153-0637.1000142.
- European Food Safety Authority Botanical Summary Report.; 2020;
- Duke, J.A.; Bogenshutz-Godwin, M.J.; DuCellier, M.J.; Duke, P.A. Handbook of Medicinal Herbs; 2nd editio.; CRC Press: Boca Raton, 2002;
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107, doi:10.1016/j.yrtph.2012.11.002.
- European Union Reference Laboratory for Feed Additives Evaluation Report on the Analytical Methods submitted in connection with the Application for Authorisation of a Feed Additive according to Regulation (EC) No 1831/2003; 2020;
- Berendes, J. Des Pedanios Dioskurides aus Anazarbos Arzneimittellehre in fünf Büchern. Übersetzt und mit Erklärungen versehen.; Band III.; Ferdinand Enke: Stuttgart, 1902;
- König, R.; Winkler, G.; Plinius Secundus d. Ä., C. Naturkunde Lateinisch-deutsch. 37 Bücher (und Register) in 32 Bänden; Artemis & Winkler: München, 1977;
- Karl Gottlob Kühn Claudi Galeni Opera Omnia, Bd. 11, Leipzig 1826; Olms-Verlag Hildesheim, 1965;
- Stoll, U. Das „Lorscher Arzneibuch“ (Codex Bambergis Medicinalis 1); Franz Steiner Verlag: Stuttgart, 1902;
- Wölfel, H. Das Arzneidrogenbuch Circa Instans in einer Fassung des XIII. Jahrhunderts aus der Universitätsbibliothek Erlangen: Text und Kommentar als Beitrag zur Pflanzen- und Drogenkunde des Mittelalters; Mathematisch-naturwissenschaftliche Dissertation: Berlin 1939 (A. Preilipper, Hamburg 1939), 1939;
- Choulant (Hrsg.), J.L. Macer Floridus ‚De viribus herbarum‘ una cum Walafridi Strabonis, Othonis Cremonensis et Ioannis Folcz’’ carminibus similis argumenti secundum codices manuscriptos et veteres editione srecensuit, supplevit et adnotatione criticains truxit Ludovicus Choulan’; Leopold Voss: Leipzig, 1832;
- Wonnecke von Kaub, J.; Schöffer, P. Gart der Gesundheit, Mainz 1485, Kap. 1; Konrad Kölbl: München, 1996;
- Lonitzer, A.; Uffenbach, P. Kräuter-Buch und Künstliche Conterfeyungen der Bäumen, Stauden, Hecken, Kräuter; Verlag Bartholomae: Ulm, 1703;
- Brunfels, O. Contrafayt Kreüterbuch (mit naturgetreuen Abb. Hans Weidnitz); 2 Teile.; Basel, 2018;
- Madaus, G. Lehrbuch der Biologischen Heilmittel; Band I.; Georg Olms Verlag,: Hildesheim, New York, 1976;
- Tusaie, K.R.; Fitzpatric, J.J. Advanced Practice Psychiatric Nursing; Second Edi.; Springer Publishing Company: New York, 2017;
- Ulbricht, C.E. Natural Standard, Herb and supplement guide, An Evidence-Based Reference; Mosby Elsevier: Missouri, 2010;
- Wheeler, J.; Coppock, B.; Chen, C. Does the burning of moxa (Artemisia vulgaris) in traditional Chinese medicine constitute a health hazard? Acupunct. Med. 2009, 27, 16–20, doi:10.1136/aim.2009.000422.
- Khare, C.P. Indian Herbal Remedies; Springer: Berlin, 2004;
- Rodrigues, E. Plants and animals utilized as medicines in the Jaú National Park (JNP), Brazilian Amazon. Phyther. Res. 2006, 20, 378–391, doi:10.1002/ptr.1866.
- de Albuquerque, U.P.; Monteiro, J.M.; Ramos, M.A.; de Amorim, E.L.C. Medicinal and magic plants from a public market in northeastern Brazil. J. Ethnopharmacol. 2007, 110, 76–91, doi:10.1016/j.jep.2006.09.010.
- Milliken, W. Malaria and antimalarial plants in Roraima, Brazil. Trop. Doct. 1997, 27 Suppl 1, 20–5, doi:10.1177/00494755970270S108.
- Quisumbing, E. Medicinal Plants of the Philippines; Bureau of Printing: Manila, 1978;
- Lockie, A. Encyclopedia of Homeopathy; DK Publishing: New York, 2006; ISBN 9780756618711.
- Agence Nationale de sécurité du Médicamen Mugwort for Homoeopathic Preparations Artemisia Vulgaris 2004, 3–5.
- Oyedemi, S.; Coopoosamy, R. Preliminary Studies on the Antibacterial and Antioxidative Potentials of Hydroalcoholic Extract from the Whole Parts of Artemisia vulgaris L. . Int. J. Pharmacol. 2015, 2, 1–9.
- Baykan Erel, Ş.; Reznicek, G.; Şenol, S.G.; Karabay Yavaşoğulu, N.Ü.; Konyalioğlu, S. Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turk J Biol 2012, 75, 75–84, doi:10.3906/biy-0912-27.
- Ben Nasr, S.; Aazza, S.; Mnif, W.; Miguel, M. In-vitro antioxidant and anti-inflamatory activities of Pituranthos chloranthus and Artemisia vulgaris from Tunisia. Int. J. Appl. Pharm. Sci. Res. 2020, 11, 605–614, doi:10.13040/IJPSR.0975-8232.11(2).605-14.
- El-Tantawy, W.H. Biochemical effects, hypolipidemic and anti-inflammatory activities of Artemisia vulgaris extract in hypercholesterolemic rats. J. Clin. Biochem. Nutr. 2015, 57, 33–38, doi:10.3164/jcbn.14-141.
- Gilani, A.H.; Yaeesh, S.; Jamal, Q.; Ghayur, M.N. Hepatoprotective activity of aqueous-methanol extract of Artemisia vulgaris. Phyther. Res. 2005, 19, 170–172, doi:10.1002/ptr.1632.
- Khan, A.U.; Gilani, A.H. Antispasmodic and bronchodilator activities of Artemisia vulgaris are mediated through dual blockade of muscarinic receptors and calcium influx. J. Ethnopharmacol. 2009, 126, 480–486, doi:10.1016/j.jep.2009.09.010.
- Lee, S.-J.; Chung, H.-Y.; Lee, I.-K.; Oh, S.-U.; Yoo, I.-D. Phenolics with Inhibitory Activity on Mouse Brain Monoamine Oxidase (MAO) from Whole Parts of Artemisia vulgaris L (Mugwort). Food Sci. Biotechnol. 2000, 9, 179–182.
- Yang, X.; Xiong, X.; Yang, G.; Wang, J. Effectiveness of Stimulation of Acupoint KI 1 by Artemisia vulgaris (Moxa) for the Treatment of Essential Hypertension: A Systematic Review of Randomized Controlled Trials. 2014, doi:10.1155/2014/187484.
- Shaik, A.; Kanhere, R.S.; Cuddapah, R.; Nelson, K.S.; Vara, P.R.; Sibyala, S. Antifertility activity of Artemisia vulgaris leaves on female Wistar rats. Chin. J. Nat. Med. 2014, 12, 180–185, doi:10.1016/S1875-5364(14)60030-3.
- Jakovljević, M.R.; Grujičić, D.; Vukajlović, J.T.; Marković, A.; Milutinović, M.; Stanković, M.; Vuković, N.; Vukić, M.; Milošević-Djordjević, O. In vitro study of genotoxic and cytotoxic activities of methanol extracts of Artemisia vulgaris L. and Artemisia alba Turra. South African J. Bot. 2020, 132, 117–126, doi:10.1016/j.sajb.2020.04.016.
- Saleh, A.M.; Aljada, A.; Rizvi, S.A.A.; Nasr, A.; Alaskar, A.S.; Williams, J.D. In vitro cytotoxicity of Artemisia vulgaris L. essential oil is mediated by a mitochondria-dependent apoptosis in HL-60 leukemic cell line. BMC Complement. Altern. Med. 2014, 14, 226, doi:10.1186/1472-6882-14-226.
- Raj Singh, B.; Singh, V.; Karan Singh, R.; Toppo, S.; Haque, N.; Ebibeni, N. Antimicrobial effect of Artemisia vulgaris essential oil . Nat. Prod. An Indian J. 2011, 5, 1–7.
- Hiremath, S.K.; Kolume, D.G.; Muddapur, U.M. Antimicrobial activity of Artemisia vulgaris Linn. (Damanaka). Int. J. Res. Ayurveda Pharm. 2011, 2, 1674–1675.
- Singh, R.; Verma, P.; Singh, G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J. Intercult. Ethnopharmacol. 2012, 1, 101, doi:10.5455/jice.20120525014326.
- Afsar, S.K.; Rajesh Kumar, K.; Venu Gopal, J.; Raveesha, P. Assessment of anti-inflammatory activity of Artemisia vulgaris leaves by cotton pellet granuloma method in Wistar albino rats. J. Pharm. Res. 2013, 7, 463–467, doi:10.1016/j.jopr.2013.04.056.
- Olsen, O.T.; Frolund, L.; Heinig, J.; Jacobsen, L.; Svendsen, U.G. A double-blind, randomized study investigating the efficacy and specificity of immunotherapy with Artemisia vulgaris or Phleum pratense/Betula verrucosa. Allergol. Immunopathol. (Madr). 1995, 23, 73–78.
- Kodippili, K.; Ratnasooriya, W.D.; Premakumara, S.; Udagama, P. V. An investigation of the antimalarial activity of Artemisia vulgaris leaf extract in a rodent malaria model. Int. J. Green Pharm. 2011, 5, 1–7, doi:10.22377/IJGP.V5I4.213.
- Bamunuarachchi, G.S.; Ratnasooriya, W.D.; Premakumara, S.; Udagama, P. V. Antimalarial properties of Artemisia vulgaris L. ethanolic leaf extract in a Plasmodium berghei murine malaria model. J. Vector Borne Dis. 2013, 50, 278–284.
- Caner, A.; Döşkaya, M.; Deǧirmenci, A.; Can, H.; Baykan, Ş.; Üner, A.; Başdemir, G.; Zeybek, U.; Gürüz, Y. Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella spiralis) in rats. Exp. Parasitol. 2008, 119, 173–179, doi:10.1016/j.exppara.2008.01.012.
- Seul Park, Y.; He Nam, G.; Jo Jo, K.; Won Kawk, H.; Yoo, J.-G.; Dong Jang, J.; Moon Kang, S.; Yong Kim, S.; Min Kim, Y. Adequacy of the Anti-aging and Anti-wrinkle Effects of the Artemisia vulgaris Fermented Solvent Fraction. Korean Soc. Biotechnol. Bioeng. J. 2019, 34, 199–206, doi:10.7841/ksbbj.2019.34.3.199.
- Bampidis, V.; Azimonti, G.; Bastos, M. de L.; Christensen, H.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; Pechová, A.; Petkova, M.; Ramos, F.; Sanz, Y.; Villa, R.E.; Woutersen, R.; Brantom, P.; Chesson, A.; Westendorf, J.; Gregoretti, L.; Manini, P.; Dusemund, B. Safety and efficacy of a tincture derived from Artemisia vulgaris L. (Mugwort tincture) when used as a sensory additive in feed for all animal species. EFSA J. 2019, 17, 5879, doi:10.2903/j.efsa.2019.5879.
- EFSA Supporting Publications; 2014; Vol. 11;.
- Food and Drug Administration Pollens - Weeds and Garden Plants 2011.
- Ipsen, H.; Formgren, H.; Løswenstein, H.; Ingemann, L. Immunochemical and Biological Characterization of a Mugwort (Artemisia vulgaris) Pollen Extract. Allergy 1985, 40, 289–294, doi:10.1111/j.1398-9995.1985.tb00234.x.
- Yong, W.L.; Soo, Y.C.; Eun, K.L.; Jung, H.S.; Park, J.W.; Hong, C.S. Cross-allergenicity of pollens from the Compositae family: Artemisia vulgaris, Dendranthema grandiflorum, and Taraxacum officinale. Ann. Allergy, Asthma Immunol. 2007, 99, 526–533, doi:10.1016/s1081-1206(10)60382-1.
- Wrangsjö, K.; Ros, A.M.; Wahlberg, J.E. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis 1990, 22, 148–154, doi:10.1111/j.1600-0536.1990.tb01550.x.
- Haw, S.; Cho, H.-R.; Lee, M.-H. Allergic contact dermatitis associated with mugwort ( Artemisia vulgaris ). Contact Dermatitis 2010, 62, 61–63, doi:10.1111/j.1600-0536.2009.01672.x.
- European Food Safety Authority Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 2012, 10, doi:10.2903/j.efsa.2012.2663.
- Journal, T.E. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on hydrocyanic acid in flavourings and other food ingredients with flavouring properties. EFSA J. 2004, 2, 1–28, doi:10.2903/j.efsa.2004.105.
