Fluorescein is a fluorescent organic dye used as a diagnostic tool in various fields of medicine. Although fluorescein is a compound with relatively low toxicity, after photoactivation, it releases potentially toxic molecules, such as singlet oxygen and, as have been recently demonstrated, also carbon monoxide. Since both of these molecules are biologically active, it is essential to explore potential biological effects of fluorescein photochemistry.
- fluorescein
- irradiation
- singlet oxygen
- carbon monoxide
- cytotoxicity
- metabolism
- proliferation
1. Introduction
Fluorescein is a fluorescent small-molecule organic dye that is commonly employed in cellular biology as a tracer. Its sodium salt is widely used in clinical medicine, particularly as a diagnostic tool in ophthalmology[1]. It has also been studied for new therapeutic applications in various fields, such as urology[2][3][4] and neurosurgery[5][6]. Having an important role in the diagnostics of ocular diseases, fluorescein has been included on the List of Essential Medicines, published by the World Health Organization[7]. During diagnostic procedures, it can be administered locally[8], orally[9], or intravenously[10] with subsequent irradiation of the area of interest using blue light[1][3][4] (≈490 nm[11][12]).
Although fluorescein is considered to be generally safe, it is a photoactive compound whose biological effects associated with this activity have been neglected to date. For example, fluorescein is known to photosensitize oxygen to form singlet oxygen (1O2)[13][14]. Furthermore, it has been recently demonstrated that a fluorescein analog, xanthene-9-carboxylic acid, releases carbon monoxide (CO) upon photoactivation by green light[15] via decarbonylation of the carboxyl group. Although fluorescein is structurally different, it was hypothesized that it may also undergo the photodecarbonylation reaction. This hypothesis has been recently confirmed, as Srankova et al.[16] had shown that CO is produced through photochemical degradation of fluorescein in a relatively high chemical yield of 40%.
Both 1O2 and CO are biologically active molecules that affect physiological processes in the human body[17][18][19]. Over the past three decades, they have also been thoroughly studied for their use in the treatment of various diseases. 1O2 is a very reactive molecule, the cytotoxic properties of which are utilized in medicine, e.g., in photodynamic therapy[20]. CO is an important gasotransmitter with anti-inflammatory[21], antiapoptotic[22], and antiproliferative properties[23]. Its anticancer action was also studied in outhe researcher's laboratory, showing a positive effect on the survival rate of mice xenotransplanted with pancreatic cancer[24]. Although 1O2 and CO are being investigated for their potential therapeutic applications, both exert cytotoxicity at higher concentrations, particularly when their transport to target sites is not strictly controlled. While 1O2 causes oxidative damage and cell death[25][26][27][28], the toxicity of CO is related to its high binding affinity to blood hemoglobin[29][30][31][32] or the heme moiety of extravascular hemoproteins[33][34] such as cytochrome c oxidase[35], affecting their oxygen carrier properties or enzymatic activities, respectively. In addition, CO can trigger oxidative stress[36] and lipoperoxidation[37].
2. Impact of Fluorescein Irradiation on Biological Systems
Cytotoxicity of fluorescein
Fluorescein is a relatively nontoxic compound (LD50 = 6.7 g/kg for rats[38]), and it is widely used in medicine for diagnostic purposes, as it exhibits strong fluorescence in aqueous media[1][2][4][6]. This dye is also used as a fluorescent label in target tissues[1][5]. Low toxicity was also observed for its stable photoproducts, created by exhaustive fluorescein diacetate (FDA) irradiation (FDA is used to study intracellular effects of fluorescein, as, unlike fluorescein, it is able to penetrate the cellular membrane where it is hydrolyzed to fluorescein [38][39]). However, simultaneous irradiation of cells treated with fluorescein led to a significant and time-dependent decrease in cell viability (Figure 1), suggesting that one or more photoproducts formed during irradiation, which were not present in the solutions upon exhaustive irradiation, were responsible for the observed cytotoxicity. This means that these species must be either volatile or short-lived (although reactive), pointing to CO and 1O2[16].
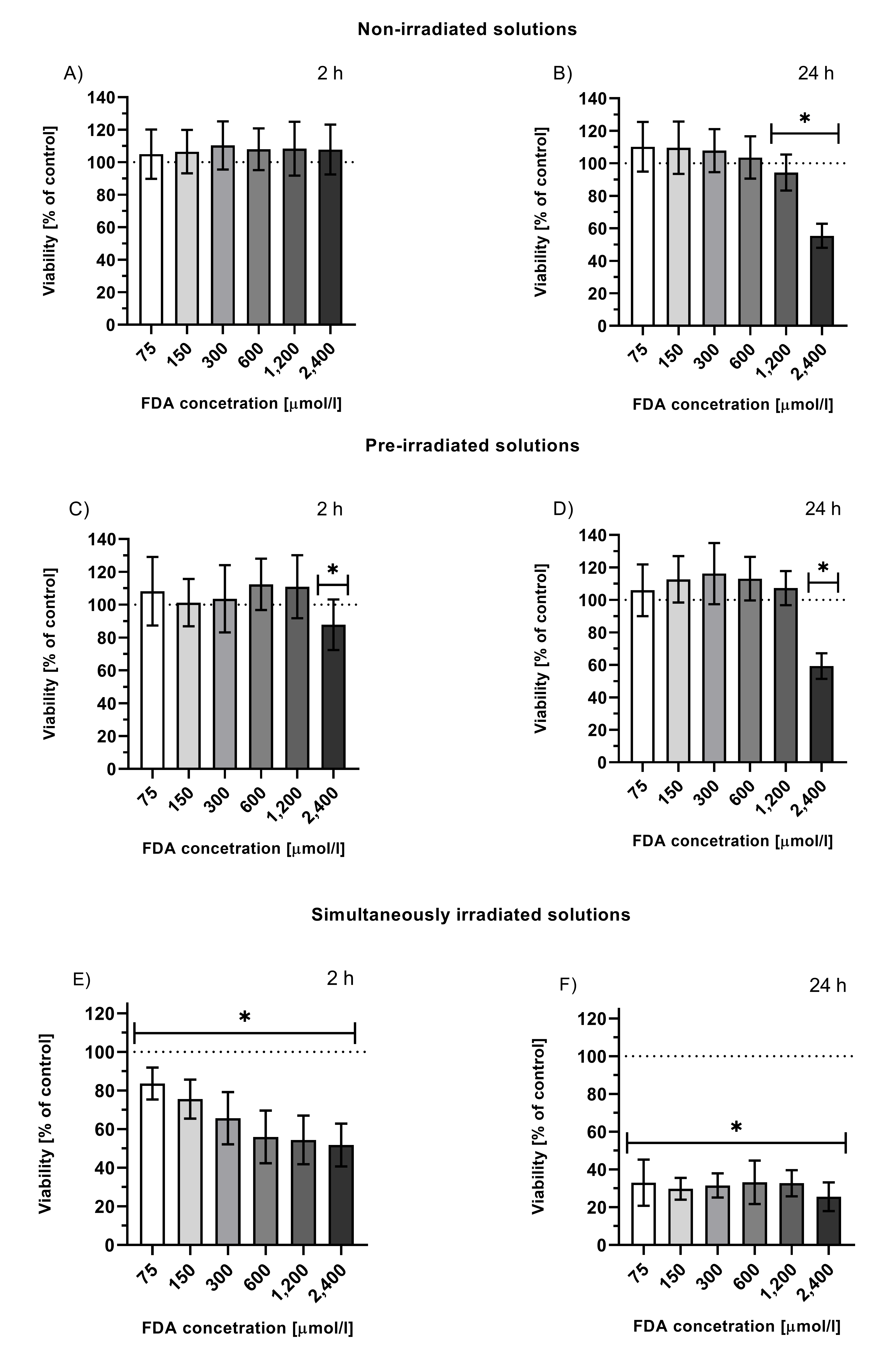
Figure 1. Viability of HepG2 cells treated with solutions of non-irradiated FDA solution (A, B), solution of fluorescein photoproducts (C, D; tir = 24 h, I = 160 mW/cm2 prior to the treatment, FDA concentration = initial concentration of FDA prior irradiation), or simultaneously irradiated solutions of FDA (E, F; irradiation throughout the entire incubation time 2 or 24 h, I = 160 mW/cm2, FDA concentration = FDA initial concentration prior irradiation); *p ≤ 0.05 vs. untreated control.
This data was, however, obtained upon long irradiation times. Therefore, to obtain more clinically relevant data, as diagnostic fluorescein angiography lasts only a few minutes, HepG2 cells treated with FDA or fluorescein were irradiated for 30 min, and analyzed immediately, or after further incubation for 2 or 24 h in the dark (Figure 3 of the article). Irradiation of cells treated with FDA showed a significant decrease in cell viability (≤ 80%) in the majority of the analyzed concentrations (19-300 μmol/l) for 2 and 24 h incubation intervals; whereas this effect was much weaker when cells were treated with fluorescein (Figure 3 of the article)[16].
Effect on Krebs Cycle
To elucidate the effects of fluorescein irradiation on cellular metabolism, the concentrations of Krebs cycle intermediates and its anaplerotic pathways were analyzed in HepG2 cells. HepG2 cells treated with FDA were irradiated directly to find the immediate impact of the photochemical transformation of fluorescein to photoproducts, including 1O2 and CO. This resulted in a significant decrease in the majority of metabolites, with the most significant changes in the concentrations of lactate, 2HG, 2OG, and citrate (Figure 5A of the article)[16]. This indicates that the above-mentioned biologically active by-products of fluorescein photoexcitation might affect the overall cellular energetic metabolism.
To test the hypothesis that the produced CO is responsible for decreased cell metabolism, HepG2 cells were incubated in an atmosphere containing 100 ppm of CO. A significant decrease in the concentrations of the Krebs cycle intermediates (2HG, glutamate, 2OG, and citrate) was observed following CO treatment for 2 h (Figure 5B of the article)[16], confirming the key role of this molecule in this process. These results correspond to those observed upon CO exposure that demonstrated the inhibition of respiration and glycolysis and a decrease in some Krebs cycle metabolites[3940]. On the other hand, some published data have proved that CO can promote oxidative phosphorylation[4041][4142], mitochondrial biogenesis[4243], and even an increase in cytochrome c oxidase activity[4344], suggesting that the effect of CO is concentration- and tissue-dependent and reflects the overall cell/tissue status or oxygen level. In our case, significant inhibition of the Krebs cycle was observed, which coincided with a relatively high FDA concentration and a long exposure to generated and simultaneously irradiated fluorescein.
Effect on Cell Cycle
Irradiation of FDA-treated cells also resulted in a significant increase in the G0 phase and a simultaneous decrease in G2/M, indicating reduced proliferation and thus the antiproliferative and anticancer potential of fluorescein. To assess the function of CO, an analogous experiment was performed under a CO atmosphere. No significant effect of CO was observed on cell cycle progression, indicating no involvement of the CO released during the photoreaction in this process (Figure 6 of the article)[16]. However, CO has been suggested to affect the cell cycle[4445][4546], showing that this effect might be both dose- and cell-dependent.
Comparison of Different Fluorescein Administration Modes
Comparing the effects of the two modes of fluorescein treatment (free fluorescein and cell-targeted fluorescein in the form of FDA) helped to assess the biological effects of 1O2 and CO when produced both intra- and extracellularly. Comparisons of the cell viability indicated that when administered as a free acid, fluorescein’s negative impact on viability is significantly smaller[16]. In this case, the 1O2 molecules released during the photoreaction do not necessarily reach the intracellular compartment because of the short half-life of 1O2 (τ1/2 = 3–4 μs[4647]). On the other hand, the long-lived CO (τ1/2 = 3–4 h) can freely pass through the plasma membrane and affect cellular processes when generated extracellularly, as shown by Lazarus et al.[4748], who studied the intra- vs. extracellular delivery of CO using two types of CO-releasing molecules (CORMs) differing in their cellular localization. They showed that extracellular CO production exhibited a lower toxic effect on cells, whereas anti-inflammatory cell signaling processes were similar to those of intracellular delivery.
Impact of O2 Concentration on Cytotoxicity of Fluorescein Irradiation
Experiments performed in a hypoxic chamber (a 9% O2 level was set according to the measured O2 level in rat livers[4849]) showed that hypoxia was associated with a significantly lower drop in the viability of cells treated with either fluorescein or FDA when compared with cells under normoxic conditions. Three different ways of how the O2 level may influence this parameter were proposed. A lower O2 level can result in: a lower yield of 1O2 (fewer O2 molecules available for sensitization); reduced efficiency of the fluorescein photoreaction (if 1O2 is responsible for its degradation) and thus less efficient CO release; or a different cellular metabolic status, any of which ultimately affects the cell’s survival[16].
3. Conclusion and Outlook
Irradiation of fluorescein results in the production of the biologically active molecules 1O2 and CO. These molecules might be responsible for the phototoxicity of fluorescein, which increases with increasing dosage, times of irradiation, and tissue oxygenation levels. Moreover, the fluorescein photoreaction products affect cell metabolism and proliferation. As it releases CO in substantial amounts, it might even be used therapeutically as a photoCORM to release CO into target tissues irradiated with light.
References
- Rosario Brancato; Francesco Bandello; Rosangela Lattanzio; Iris fluorescein angiography in clinical practice. Survey of Ophthalmology 1997, 42, 41-70, 10.1016/s0039-6257(97)84042-8.
- Philippe E. Zimmern; David Laub; Gary E. Leach; Fluorescein Angiography of the Bladder: Technique and Relevance to Bladder Cancer and Interstitial Cystitis Patients. Journal of Urology 1995, 154, 62-65, 10.1016/s0022-5347(01)67225-2.
- Geoffrey A. Sonn; Sha-Nita E. Jones; Tatum V. Tarin; Christine B. Du; Kathleen E. Mach; Kristin C. Jensen; Joseph C. Liao; Optical Biopsy of Human Bladder Neoplasia With In Vivo Confocal Laser Endomicroscopy. Journal of Urology 2009, 182, 1299-1305, 10.1016/j.juro.2009.06.039.
- Eugene Shkolyar; Mark A. Laurie; Kathleen E. Mach; Dharati R. Trivedi; Dimitar V. Zlatev; Timothy C. Chang; Thomas J. Metzner; John T. Leppert; Chia-Sui Kao; Joseph C. Liao; et al. Optical biopsy of penile cancer with in vivo confocal laser endomicroscopy. Urologic Oncology: Seminars and Original Investigations 2019, 37, 809.e1-809.e8, 10.1016/j.urolonc.2019.08.018.
- Francesco Acerbi; Morgan Broggi; Marica Eoli; Elena Anghileri; Claudio Cavallo; Carlo Boffano; Roberto Cordella; Lucia Cuppini; Bianca Pollo; Marco Schiariti; et al.Sergio VisintiniChiara OrsiEmanuele La CorteGiovanni BroggiPaolo Ferroli Is fluorescein-guided technique able to help in resection of high-grade gliomas?. Neurosurgical Focus 2014, 36, E5, 10.3171/2013.11.focus13487.
- Linda M. Wang; Matei A. Banu; Peter Canoll; Jeffrey N. Bruce; Rationale and Clinical Implications of Fluorescein-Guided Supramarginal Resection in Newly Diagnosed High-Grade Glioma. Frontiers in Oncology 2021, 11, -, 10.3389/fonc.2021.666734.
- 22nd Model List of Essential Medicines . World Health Organization. Retrieved 2022-4-21
- British National Formulary 81 . Web of Pharma. Retrieved 2022-4-21
- Tsutomu Hara; Mikino Inami; Takako Hara; Efficacy and safety of fluorescein angiography with orally administered sodium fluorescein. American Journal of Ophthalmology 1998, 126, 560-564, 10.1016/s0002-9394(98)00112-3.
- Harold R. Novotny; David L. Alvis; A Method of Photographing Fluorescence in Circulating Blood in the Human Retina. Circulation 1961, 24, 82-86, 10.1161/01.cir.24.1.82.
- Oliver R. Marmoy; Robert H. Henderson; Kuan Ooi; Recommended protocol for performing oral fundus fluorescein angiography (FFA) in children. Eye 2020, 36, 234-236, 10.1038/s41433-020-01328-6.
- Daniel X. Hammer; R. Daniel Ferguson; Ankit H. Patel; Vanessa Vazquez; Deeba Husain; Angiography with a multifunctional line scanning ophthalmoscope. Journal of Biomedical Optics 2012, 17, 0260081-02600811, 10.1117/1.jbo.17.2.026008.
- Yoshiharu Usui; DETERMINATION OF QUANTUM YIELD OF SINGLET OXYGEN FORMATION BY PHOTOSENSITIZATION. Chemistry Letters 1973, 2, 743-744, 10.1246/cl.1973.743.
- E. Gandin; Y. Lion; A. Van de Vorst; QUANTUM YIELD OF SINGLET OXYGEN PRODUCTION BY XANTHENE DERIVATIVES. Photochemistry and Photobiology 1983, 37, 271-278, 10.1111/j.1751-1097.1983.tb04472.x.
- Lovely Angel Panamparambil Antony; Tomáš Slanina; Peter Šebej; Tomáš Šolomek; Petr Klán; Fluorescein Analogue Xanthene-9-Carboxylic Acid: A Transition-Metal-Free CO Releasing Molecule Activated by Green Light. Organic Letters 2013, 15, 4552-4555, 10.1021/ol4021089.
- Mária Šranková; Aleš Dvořák; Marek Martínek; Peter Šebej; Petr Klán; Libor Vítek; Lucie Muchová; Antiproliferative and Cytotoxic Activities of Fluorescein—A Diagnostic Angiography Dye. International Journal of Molecular Sciences 2022, 23, 1504, 10.3390/ijms23031504.
- Stefan W. Ryter; Leo E. Otterbein; Carbon monoxide in biology and medicine. BioEssays 2004, 26, 270-280, 10.1002/bies.20005.
- Briviba, K.; Klotz, L.-O.; Sies, H.; Toxic and signaling effects of photochemically or chemically generated singlet oxygen in biological systems. Biol. Chem. 1997, 378,, 1259–1265.
- Devasagayam, T.; Kamat, J.P.; Biological significance of singlet oxygen. Indian J. Exp. Biol. 2002, 40, 680–692.
- Patrizia Agostinis; Kristian Berg; Keith A. Cengel Md; Thomas H. Foster; Albert W. Girotti; Sandra O. Gollnick; Stephen M. Hahn Md; Michael R. Hamblin; Asta Juzeniene; David Kessel; et al.Mladen KorbelikJohan MoanPawel MrozDominika Nowis MdJacques PietteBrian C. WilsonJakub Golab Photodynamic therapy of cancer: An update. CA: A Cancer Journal for Clinicians 2011, 61, 250-281, 10.3322/caac.20114.
- Leo E. Otterbein; Fritz H. Bach; Jawed Alam; Miguel Soares; Hong Tao Lu; Mark Allen Wysk; Roger J. Davis; Richard A. Flavell; Augustine M. K. Choi; Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Medicine 2000, 6, 422-428, 10.1038/74680.
- Sophie Brouard; Leo E. Otterbein; Josef Anrather; Edda Tobiasch; Fritz H. Bach; Augustine M.K. Choi; Miguel Soares; Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. Journal of Experimental Medicine 2000, 192, 1015-1026, 10.1084/jem.192.7.1015.
- Toshisuke Morita; S. Alex Mitsialis; Hideo Koike; Yuxiang Liu; Stella Kourembanas; Carbon Monoxide Controls the Proliferation of Hypoxic Vascular Smooth Muscle Cells. Journal of Biological Chemistry 1997, 272, 32804-32809, 10.1074/jbc.272.52.32804.
- Libor Vítek; Helena Gbelcová; Lucie Muchová; Kateřina Váňová; Jaroslav Zelenka; Renata Koníčková; Jakub Šuk; Marie Zadinova; Zdeněk Knejzlík; Shakil Ahmad; et al.Takeshi FujisawaAsif AhmedTomáš Ruml Antiproliferative effects of carbon monoxide on pancreatic cancer. Digestive and Liver Disease 2014, 46, 369-375, 10.1016/j.dld.2013.12.007.
- M L Agarwal; M E Clay; E J Harvey; H H Evans; A R Antunez; N L Oleinick; Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells.. Cancer Research 1991, 51, 5993-6.
- W M Star; H P Marijnissen; A E Van Den Berg-Blok; J A Versteeg; K A Franken; H S Reinhold; Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers.. Cancer Research 1986, 46, -.
- Norman I. Krinsky; Singlet oxygen in biological systems. Trends in Biochemical Sciences 1977, 2, 35-38, 10.1016/0968-0004(77)90253-5.
- Michael J. Davies; Singlet oxygen-mediated damage to proteins and its consequences. Biochemical and Biophysical Research Communications 2003, 305, 761-770, 10.1016/s0006-291x(03)00817-9.
- Ivan Blumenthal; Carbon Monoxide Poisoning. Journal of the Royal Society of Medicine 2001, 94, 270-272, 10.1177/014107680109400604.
- C. G. Douglas; J. S. Haldane; The laws of combination of haemoglobin with carbon monoxide and oxygen. The Journal of Physiology 1912, 44, 275-304, 10.1113/jphysiol.1912.sp001517.
- Bernard, C. . Leçons sur Les Effets des Substances Toxiques et Médicamenteuses; Librairie, J.B., Ed.; Baillière et Fils: Leon, France, 1857; pp. -.
- John Haldane; J. Lorrain Smith; The Absorption of Oxygen by the Lungs. The Journal of Physiology 1897, 22, 231-258, 10.1113/jphysiol.1897.sp000689.
- Norman Nomof; James Hopper; Ellen Brown; Kenneth Scott; Reidar Wennesland; Simultaneous Determinations of the Total Volume of Red Blood Cells by Use of Carbon Monoxide and Chromium51 IN HEALTHY AND DISEASED HUMAN SUBJECTS1. Journal of Clinical Investigation 1954, 33, 1382-1387, 10.1172/jci103015.
- Ronald F. Coburn; THE CARBON MONOXIDE BODY STORES. Annals of the New York Academy of Sciences 1970, 174, 11-22, 10.1111/j.1749-6632.1970.tb49768.x.
- Òscar Miró; Jordi Casademont; Antoni Barrientos; Álvaro Urbano-Márquez; Francesc Cardellach; Mitochondrial Cytochrome c Oxidase Inhibition during Acute Carbon Monoxide Poisoning. Pharmacology & Toxicology 1998, 82, 199-202, 10.1111/j.1600-0773.1998.tb01425.x.
- J Zhang; C A Piantadosi; Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain.. Journal of Clinical Investigation 1992, 90, 1193-1199, 10.1172/jci115980.
- S. R. Thom; Carbon monoxide-mediated brain lipid peroxidation in the rat. Journal of Applied Physiology 1990, 68, 997-1003, 10.1152/jappl.1990.68.3.997.
- Samuel L. Yankell; Joseph J. Loux; Acute Toxicity Testing of Erythrosine and Sodium Fluorescein in Mice and Rats. Journal of Periodontology 1977, 48, 228-231, 10.1902/jop.1977.48.4.228.
- Rotman, B.; Papermaster, B.W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc. Natl. Acad. Sci. USA 1966, 55, 134.
- Patrycja Kaczara; Barbara Sitek; Kamil Przyborowski; Anna Kurpinska; Kamil Kus; Marta Stojak; Stefan Chlopicki; Antiplatelet Effect of Carbon Monoxide Is Mediated by NAD + and ATP Depletion. Arteriosclerosis, Thrombosis, and Vascular Biology 2020, 40, 2376-2390, 10.1161/atvbaha.120.314284.
- Marialuisa Lavitrano; Ryszard T. Smolenski; Antonino Musumeci; Massimo Maccherini; Ewa Slominska; Ernesto Florio; Adele Bracco; Antonio Mancini; Giorgio Stassi; Mariella Patti; et al.Roberto GiovannoniAlberto FroioFelicetta SimeoneMonica ForniMaria Laura BacciGiuseppe D'AliseEmanuele CozziLeo E. OtterbeinMagdi H. YacoubFritz H. BachFulvio Calise Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. The FASEB Journal 2004, 18, 1093-1095, 10.1096/fj.03-0996fje.
- Tung-Yu Tsui; Yeung-Tung Siu; Hans J. Schlitt; Sheung-Tat Fan; Heme oxygenase-1-derived carbon monoxide stimulates adenosine triphosphate generation in human hepatocyte. Biochemical and Biophysical Research Communications 2005, 336, 898-902, 10.1016/j.bbrc.2005.08.187.
- Hagit B. Suliman; Martha S. Carraway; Lynn G. Tatro; Claude A. Piantadosi; A new activating role for CO in cardiac mitochondrial biogenesis. Journal of Cell Science 2007, 120, 299-308, 10.1242/jcs.03318.
- Cláudia Sf Queiroga; Ana S Almeida; Paula M Alves; Catherine Brenner; Helena LA Vieira; Carbon monoxide prevents hepatic mitochondrial membrane permeabilization. BMC Cell Biology 2011, 12, 10-10, 10.1186/1471-2121-12-10.
- Ruiping Song; Raja S. Mahidhara; Fang Liu; Wen Ning; Leo E. Otterbein; Augustine M. K. Choi; Carbon Monoxide Inhibits Human Airway Smooth Muscle Cell Proliferation via Mitogen-Activated Protein Kinase Pathway. American Journal of Respiratory Cell and Molecular Biology 2002, 27, 603-610, 10.1165/rcmb.4851.
- Hyun-Ock Pae; Gi-Su Oh; Byung-Min Choi; Soo-Cheon Chae; Young-Myeong Kim; Khee-Rhin Chung; Hun-Taeg Chung; Carbon Monoxide Produced by Heme Oxygenase-1 Suppresses T Cell Proliferation via Inhibition of IL-2 Production. The Journal of Immunology 2004, 172, 4744-4751, 10.4049/jimmunol.172.8.4744.
- Francis Wilkinson; W. Phillip Helman; Alberta B. Ross; Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. An Expanded and Revised Compilation. Journal of Physical and Chemical Reference Data 1995, 24, 663-677, 10.1063/1.555965.
- Livia S. Lazarus; Casey Simons; Ashley Arcidiacono; Abby Benninghoff; Lisa M. Berreau; Extracellular vs Intracellular Delivery of CO: Does It Matter for a Stable, Diffusible Gasotransmitter?. Journal of Medicinal Chemistry 2019, 62, 9990-9995, 10.1021/acs.jmedchem.9b01254.
