Fluorescein is a fluorescent organic dye used as tracer, contrasting agent or a diagnostic tool in various fields of medicine and natural sciences in general. Although fluorescein is a compound with relatively low toxicity, after photoactivation, it releases potentially toxic molecules, such as singlet oxygen and, as have been recently demonstrated, also carbon monoxide. Since both of these molecules are biologically active, it is essential to explore potential biological effects of fluorescein photochemistry.
- fluorescein
- irradiation
- singlet oxygen
- carbon monoxide
- cytotoxicity
- metabolism
- proliferation
1. Introduction
Fluorescein is a fluorescent small-molecule organic dye that exhibits strong fluorescence at neutral and alkalic pH in aqueous media. In these conditions, its main absorption peak is at 490 nm (ε490 = 76 900 M−1 cm−1) and is commonly employed in cellular biology as a the qurantum yield of the subsequent fluorescence is 0.93[1]cer. Its is a dark orange powder soluble in water and when dissolved in proper conditions, emitting bright green fluorescence (with maximum emission at 515 nm[2]). It bsodium salt is widely used in clinical medicinelongs, to a group of xanthene dyes that includes substances like eosin Y or rhodamine B. It was first synthesized by Adolf von Baeyer around 1871 by condensation of resorcinol and particularly as a diagnostic tool in ophthalic anhydride catalmologyzed with zinc chloride[31][4].
2. Application
Fluorescein isIt a broadly applied substance in various fields. For instance, it is commonly employed as a tracer. Some examples of this fluoresceinhas also been studied for new therapeutic application are utilization as a ground-water tracer[5], and use for ins in vestigating molecular movement in the bone lacunar-canalicular system[6]rious or for examining extravasation to study blood-spinal cord barrier permeability on animal modelields, such as[7][8]. Fluorescein can be also applied as a topical fluorescent contrast agent for microendoscoprology of gastrointestinal[2][93] or urinary tract[104] and as a pH sensitive corrosion indicatoeurosur[11][12].
Morgeover, it is extensively employed in ophthalmology as a diagnostic tool[135][14][15][166]. Having an important role in the diagnostics of ocular diseases, fluorescein has been included on the List of Essential Medicines, published by the World Health Organization[177]. An example of a well-established and frequently used fluorescein diagnostic technique is fluorescein angiography[13][18][19]. During diagnostic procedures, it can be administered locally[208], orally[219], or intravenously[2210] with subsequent irradiation of the area of interest using blue light[131][233][244] (≈490 nm[2511][2612]). T
Althere are also studies that points to ugh fluorescein’s utilization in the labeling of brain tumor tissue (high-grade gliomas) to obtain better results of its resection[27][28][29] is considered to be generally safe, it is a photoactive compound whose biological effects associated with this activity have been neglected to date. For example, . Ffluorescein is capable of accumulating in cerebral areas with damaged blood-brain barrierknown to photosensitize oxygen to form singlet oxygen (1O2)[13][3014]. and therefore ensure better resection due to contrast-enhanced borderline of malignant and healthy tissue[28].Furthermore, it has been recently demonstrated that a fluorescein analog, xanthene-9-carboxylic In addition, there exist studies supporting use in urology, specifically, in bladder[31][23]cid, releases carbon monoxide (CO) upon photoactivation by and penile[24] cancer surgery.
3. New discoveries
Fluorescein Irradiation Produces Biologically Active Compounds
Fluorescein in lightself[15] vis a relatively nontoxic substance (LD50a decarbonylation of the = 6.7 g/kg for rats[32], which is almost 20x greater than LD50 rboxyl group. Although fluor caffeine[33]escein oris 2x more than this value for NaCstructurall[34]).y Howdiffever, it is a photoactive compound whose biological effects associated with this activity have been neglected to date. For example, fluorescein is known to photosensitize oxygen to form singlet oxygen (1O2)[35][36]. Addirent, it was hypothesized that it may also undergo the photodecarbonylation reaction. This hypothesis has been recentionally, Martinek et al.[37] andy confirmed, as Srankova et al.[3816] had shown that carbon monoxide (CO)CO is produced through photochemical degradation of fluorescein in a relatively high chemical yield of 40%.
Both 1O2 and CO are biologically active molecules that affect physiological processes in the human body[3917][4018][4119]. Over the past three decades, they have also been thoroughly studied for their use in the treatment of various diseases. 1O2 is a very reactive molecule, the cytotoxic properties of which are utilized in medicine, e.g., in photodynamic therapy[4220]. CO, in low concentration, acts in the body as a s an important gasotransmitter mediatingwith anti-inflammatory[4321], antiapoptotic[4422], and antiproliferative properties[23]. Its anticancer action was also studied in our laboratory, showing a positive effect on the survival rate of mice xenotransplanted with pancreatic cancer[4524]. Although 1O2 and CO are being investigated for their potential therapeutic use in the treatment of various diseases, theyapplications, both exert cytotoxicity at higher concentrations, particularly when their transport to target sites is not strictly controlled. While 1O2 causes oxidative damage and cell death[4625][4726][4827][4928], the toxicity of CO is related to its high binding affinity to blood hemoglobin[5029][5130][5231][5332] or the heme moiety of extravascular hemoproteins[5433][5534] such as cytochrome c oxidase[5635], affecting their oxygen carrier properties or enzymatic activities, respectively. In addition, CO can trigger oxidative stress[5736] and lipoperoxidation[5837].
Flu
2. Impact of Fluorescein Irradiation on Biological Systems
Cytorescein Irradiation Impacts Physiological Processes of HepG2 cellsoxicity of fluorescein
Cytotoxicity of fluorescein irradiation
AFluorescein is has been relatively nontoxic compound (LD50 = 6.7 g/kg for rats[38]), and it is widelready mentioned in this entry,y used in medicine for diagnostic purposes, as it exhibits strong fluorescein posnce in aqueous media[1][2][4][6]. This dyeses relatively is also used as a fluorescent label in target tissues[1][5]. lLow toxicity. Intracellular was also observed for its stable photoproducts, created by exhaustive fluorescein (administrated in the formdiacetate (FDA) irradiation (FDA is used to study intracellular effects of fluorescein diacetate (FDA) which, as, unlike fluorescein, it is able to penetrate the cellular membrane where it is hydrolyzed to fluorescein[59]) also showed minimal toxicity (Figure 1, graphs A and B[38]). Low toxicity was also observed for its stable photoproducts (Figure 1, graphs C and D). However, simultaneous irradiation of cells treated with fluorescein led to a significant and time-dependent decrease in cell viability (Figure 1, graphs E and F), suggesting that one or more photoproducts formed during irradiation, which were not present in the solutions upon exhaustive irradiation (= stable photoproducts), were responsible for the observed cytotoxicity. This means that these species must be either volatile or short-lived (although reactive), pointing to CO and 1O2[3816].
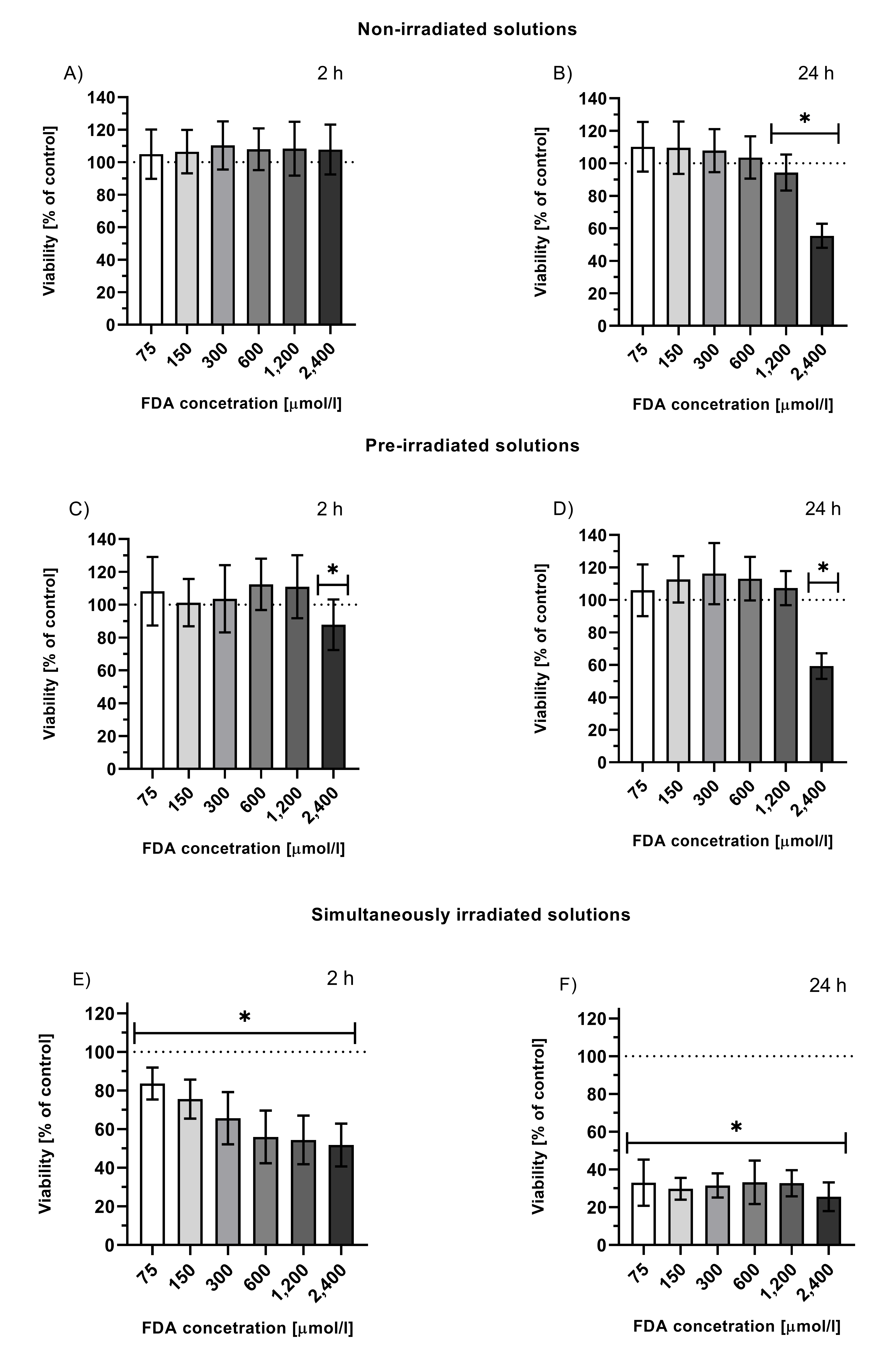
Figure 1. Viability of HepG2 cells treated with solutions of non-irradiated FDA solution (A, B), solution of fluorescein photoproducts (C, D; tir = 24 h, I = 160 mW/cm2 prior to the treatment, FDA concentration = initial concentration of FDA prior irradiation), or simultaneously irradiated solutions of FDA (E, F; irradiation throughout the entire incubation time 2 or 24 h, I = 160 mW/cm2, FDA concentration = FDA initial concentration prior irradiation); *p ≤ 0.05 vs. untreated control.
Effect on Krebs Cycle
ExThis data was, however, obtained upon long irradiation timesure of . Therefore, to obtain more clinically relevant data, as diagnostic fluorescein angiography lasts only a few minutes, HepG2 cells to irtreated with FDA or fluorescein were irradiation oed for 30 min, and analyzed immediately, or after further incubation for 2 or 24 h in the dark (Figure 3 of the article). Irradiatintracellon of cells treated with FDA showed a significant decrease in cell viability (≤ 80%) in the majority of the analyzed concentrations (19-300 μmol/l) for 2 and 24 h incubation intervalar s; whereas this effect was much weaker when cells were treated with fluorescein (Figure 3 of the article)[16].
Effect on Krebs Cycle
To elucidate the effects of fluorescein irradiation on cellular metabolism, the concentrations ofurthermore, Krebs cycle intermediates and its anaplerotic pathways were analyzed in HepG2 cells. HepG2 cells treated with FDA were irradiated directly to find the immediate impact of the photochemical transformation of fluorescein to photoproducts, including 1O2 and CO. This resulted in a significant decrease in the majority of metabolites, with the most significant changes in the concentrations of lactate, 2-hydroxyglutarateHG, 2-oxoglutarateOG, and citrate (<30% those of controlFigure 5A of the article)[3816]. This indicates that the above-mentioned biologically active by-products of fluorescein photoexcitation might affect the overall cellular energetic metabolism.
To test the hypothesis that the produced CO is responsible for decreased cell metabolism, HepG2 cells were incubated in an atmosphere containing 100 ppm of CO. A significant decrease in the concentrations of the Krebs cycle intermediates (2-hydroxyglutarateHG, glutamate, 2-oxoglutarateOG, and citrate) was also oobserved following CO treatment (enrichedfor 2 h (Figure 5B COof atmosphere, 100 ppmthe article)[3816], confirming the key role of this molecule in theis observed attenuation of cell metabolism caused by fluorescein irradiationprocess. These results correspond to those observed upon CO exposure that demonstrated the inhibition of respiration and glycolysis and a decrease in some Krebs cycle metabolites[6039]. On the other hand, some published data have proved that CO can promote oxidative phosphorylation[6140][6241], mitochondrial biogenesis[6342], and even an increase in cytochrome c oxidase activity[6443], suggesting that the effect of CO is concentration- and tissue-dependent and reflects the overall cell/tissue status or oxygen level. In our case, significant inhibition of the Krebs cycle was observed, which coincided with a relatively high FDA concentration and a long exposure to generated and simultaneously irradiated fluorescein.
Effect on Cell Cycle
Effect on Cell Cycle
Irradiation of FDA-treated cells also resulted in a significant increase in the G0 phase and a simultaneous decrease in G2/M phase (18% decrease when compared to control) of cell cycle, indicating reduced proliferation and thus the antiproliferative and anticancer potential of fluorescein. No significant effectTo assess the function of CO (enriched , an analogous experiment was performed under a CO atmosphere, 100 ppm). No significant effect of CO was observed on cell cycle progression, indicating no involvement of the CO released during the photoreaction in this process (Figure 6 of the article)[3816]. However, CO has been suggested to affect the cell cycle[6544][6645], showing that this effect might be both dose- and cell-dependent.
Comparison of Different Fluorescein Administration Modes
Comparison of Different Fluorescein Administration Modes
TCompareatment of cells with freeing the effects of the two modes of fluorescein compared to ttreatment with FDA gave different results. Comparing the effects of these two modes of f(free fluorescein and cell-targeted fluorescein treatmentin in the form of FDA) helped to assess the biological effects of 1O2 and CO when produced both intra- and extracellularly. Comparisons of the cell viability indicated that when administered as a free acid, fluorescein’s negative impact on viability is significantly smaller[3816]. In this case, the 1O2 molecules released during the photoreaction do not necessarily reach the intracellular compartment because of the short half-life of 1O2 (τ1/2 = 3–4 μs[6746]). On the other hand, the long-lived CO (τ1/2 = 3–4 h) can freely pass through the plasma membrane and affect cellular processes when generated extracellularly, as shown by Lazarus et al.[6847], who studied the intra- vs. extracellular delivery of CO using two types of CO-releasing molecules (CORMs) differing in their cellular localization. They showed that extracellular CO production exhibited a lower toxic effect on cells, whereas anti-inflammatory cell signaling processes were similar to those of intracellular delivery.
Impact of O
2
Concentration on Cytotoxicity of Fluorescein Irradiation
Fluorescein irradiation cytotoExicity experiments performed in a hypoxic chamber (a 9% O2 level was set according to the measured O2 level in rat livers[48]) showed that hypoxia was associated with a significantly lower drop in the viability of cells treated when ith either fluorescein or FDA when compared to thatwith cells under normoxic conditions. Three different ways of how the O2 level may influence this parameter were proposed. A lower O2 level can result in: a lower yield of 1O2 (fewer O2 molecules available for sensitization); reduced efficiency of the fluorescein photoreaction (if 1O2 is responsible for its degradation) and thus less efficient CO release; or a different cellular metabolic status, any of which ultimately affects the cell’s survival[3816].
43. Conclusion and Outlook
FluoIrescein is a widely used fluorescent dye that has found its applicradiation in many fields of natural sciences. Medicine is no exception, where of fluorescein plays the role of a tracer, contrast compound or diagnostic agent, etc. For many of these applications is fluorescein irradiated to yield fluorescence. It was found that this irradiation results in the results in the production of the biologically active molecules 1O2 and CO. Experiments on HepG2 cells also showed cyThese molecules might be responsible for the phototoxicity, attenuation of metabolism and de of fluorescein, which increased proliferation as a result of fluorescein s with increasing dosage, times of irradiation. This may indicate that volatile and reactive molecules produced during, and tissue oxygenation levels. Moreover, the fluorescein photochemical reaction, from which products 1O2 andffect CO were identified, are responsible for some adverse effects observed with fluorescein administcell metabolism and proliferation to patients. On the other hand, as fluorescein. As it releases CO in substantial amounts, it might even be used therapeutically as a photoCORM to release CO into target tissues. Another possibility of application is to employ both bioactive molecules, 1O2 and CO, for t irradiated withe therapeutic action, for example, in the treatment of cancerlight.
