Obesity has reached global epidemic proportions and it affects the development of insulin resistance, type 2 diabetes, fatty liver disease and other metabolic diseases. Membrane lipids are important structural and signaling components of the cell membrane.
1. Introduction
The primary defect in the pathogenesis of NAFLD is dysregulated lipid metabolism and the excessive accumulation of fatty acids and cholesterol within hepatocytes known as hepatic lipotoxicity [1][6]. Lipids play very important role in cellular functions and are major components of biological membranes. Cell membranes are the barriers that delineate the various organelles of the cells into separate but interrelated sections. It is a lipid bilayer and contains proteins that act as integral, peripheral and transmembrane proteins. The major lipids found in the cell membrane of eukaryotes are phospholipids, sphingolipids, cholesterol and sterol lipids [2][10]. In mammals, phospholipids are formed from two hydrophobic fatty acyl chains and one hydrophilic group, sphingolipids are synthesized from ceramides as the main precursors, while cholesterol with a steroid backbone is the main sterol component of the membrane [3][11]. Carbohydrates interact with both lipids and proteins to form glycolipids and glycoproteins, respectively [4][12]. Apart from serving as the major components of cell membrane and precursors for many hormones, membrane lipids play very important functions in lipid homeostasis, cellular signaling, energy storage and provide an appropriate environment for protein function [2][5][10,13]. The organizational and functional properties of cell membranes is unique considering that the composition of lipids differs between the cell membrane and membrane surrounding cell organelles or other compartments [6][14].
2. Role of Sphingomyelin-Mediated Ceramide in Fatty Liver Disease
Sphingolipids are important constituents of cell membrane acting both intracellularly and extracellularly to regulate cell proliferation, differentiation, cell death and immunological responses [7]. Sphingomyelin is also an important structural component of biological membrane and is one of the endpoints in the synthesis of sphingolipids, which are a class of membrane lipids for vital structural and signaling bioactive molecules [8][9]. Ceramides are members of the sphingolipid family and are one of the major lipid constituents in the lipid bilayer of the cell membrane [10]. Since ceramides and sphingomyelin are important components in the double membrane-bound sphingolipids, dysregulated metabolisms of ceramide and sphingomyelin play a critical role in pathologies of the liver, including NAFLD and NASH [11]. Ceramides are generated through three pathways that include de novo synthesis, sphingomyelin hydrolysis, and salvage pathway. The de novo synthesis of ceramide begins with the condensation of serine and palmitoyl-CoA through serine palmitoyltransferase, followed by the activity of 3-ketodihydrosphingosin reductase, dihydroceramide synthase, and dihydroceramide desaturase [12]. The de novo synthesis of ceramide occurs in the endoplasmic reticulum. Then, ceramide is subsequently transported to the Golgi apparatus to be metabolized to other sphingolipids, such as sphingomyelin [10]. Ceramide is also generated by the sphingomyelin hydrolysis through sphingomyelinases [12][13]. In the salvage pathway, ceramide generated by sphingomyelin hydrolysis is further hydrolyzed by ceramidases to sphingosine, which is reacylated through ceramide synthases to regenerate ceramide [14]. Sphingolipids are important constituents of cell membrane acting both intracellularly and extracellularly to regulate cell proliferation, differentiation, cell death and immunological responses [15]. Sphingomyelin is also an important structural component of biological membrane and is one of the endpoints in the synthesis of sphingolipids, which are a class of membrane lipids for vital structural and signaling bioactive molecules [16,17]. Ceramides are members of the sphingolipid family and are one of the major lipid constituents in the lipid bilayer of the cell membrane [18]. Since ceramides and sphingomyelin are important components in the double membrane-bound sphingolipids, dysregulated metabolisms of ceramide and sphingomyelin play a critical role in pathologies of the liver, including NAFLD and NASH [19]. Ceramides are generated through three pathways that include de novo synthesis, sphingomyelin hydrolysis, and salvage pathway. The de novo synthesis of ceramide begins with the condensation of serine and palmitoyl-CoA through serine palmitoyltransferase, followed by the activity of 3-ketodihydrosphingosin reductase, dihydroceramide synthase, and dihydroceramide desaturase [20]. The de novo synthesis of ceramide occurs in the endoplasmic reticulum. Then, ceramide is subsequently transported to the Golgi apparatus to be metabolized to other sphingolipids, such as sphingomyelin [18]. Ceramide is also generated by the sphingomyelin hydrolysis through sphingomyelinases [20,21]. In the salvage pathway, ceramide generated by sphingomyelin hydrolysis is further hydrolyzed by ceramidases to sphingosine, which is reacylated through ceramide synthases to regenerate ceramide [22].
3. Role of Glycolipids in NAFLD
Glycolipids are a type of complex carbohydrate that contains both a glycan and a lipid component [15]. They are important components of cellular membranes and may act as receptors, are involved in cell aggregation and dissociation, and are responsible for cellular interaction and signal transmission [15]. The glyco-components are mono- or oligosaccharide chains that are commonly branched linked to ceramide or other glycerol derivatives and may be replaced with acetyl or sulfate groups. They are classified as glycosphingolipids (GSLs) or glycoglycerolipids, based on the structure of the lipid moiety. GSLs comprise about 100,000 species divided into four subfamilies (phosphosphingolipids, glycosphingolipids, sphingoid bases, and ceramides), all of which have the sphingoid structure [16][17][18]. They are a broad range of biomolecules consisting of both hydrophilic as well as hydrophobic domains that are embedded in the cell membranes of all eukaryotic organisms [5], and are believed to be involved in regulating major cellular processes [19]. As it is well known that NAFLD is linked to a variety of lipid abnormalities in the liver [20], sphingolipid has received a lot of interest in this respect. In recent years, there has been growing interest in the role of glycolipids, particularly sphingolipids, during NAFLD progression [21]. They are thought to have a role in the development of NAFLD through a variety of mechanisms, including obesity, inflammation, insulin resistance, and oxidative stress. The liver has a far greater concentration of sphingolipids, particularly ceramide and sphingomyelin, than any other tissue [22][23]. As a result, the liver is vulnerable to sphingolipotoxicity. Studies have shown that hepatic ceramide and sphingomyelin levels are considerably higher in the livers of rats fed a high-fat diet (HFD) or mice overexpressing acyl-CoA: Diacylglycerol acyltransferase 2 in hepatocytes [24][25][26][27]. At the ceramide genetic level, heterozygous CerS6 knockout mice (CerS6+/) had higher Beta-oxidation and lower CD36/FAT expression, leading to lower lipid accumulation [28]. Furthermore, NAFLD, at the transcription level, is linked to increased expression of genes implicated in three distinct pathways that lead to ceramide formation (de novo synthesis, sphingomyelin hydrolysis, and the salvage route) [24][29]. Recent studies investigated the effects of some natural products on glycolipid disorders amelioration of fatty liver disease. To examine the effect of beta-glycophospholipid in improving liver injury, Zigmond E. et al. utilized an animal model of NASH-Psammomys obesus fed on high diet [30]. They reported that beta-glycophospholipid administration in these mice reduced cholesterol and TGs levels and improvement in liver injury [30]. Another study investigated the role of epigallocatechin-3-gallate (EGCG) on glycolipid disorder [31]. Administration of EGCG in a diabetic mouse model demonstrate that this natural product improved the incidence of glycolipid disorder by restoring normal lipid metabolism [31]. Other studies showed that tauroursodeoxycholic acid (TUDCA) ameliorates glycolipid disorder in obese mice by reversing impaired autophagy [32].
4. Phosphatidylcholine and Phosphatidylethanolamine-Mediated NAFLD
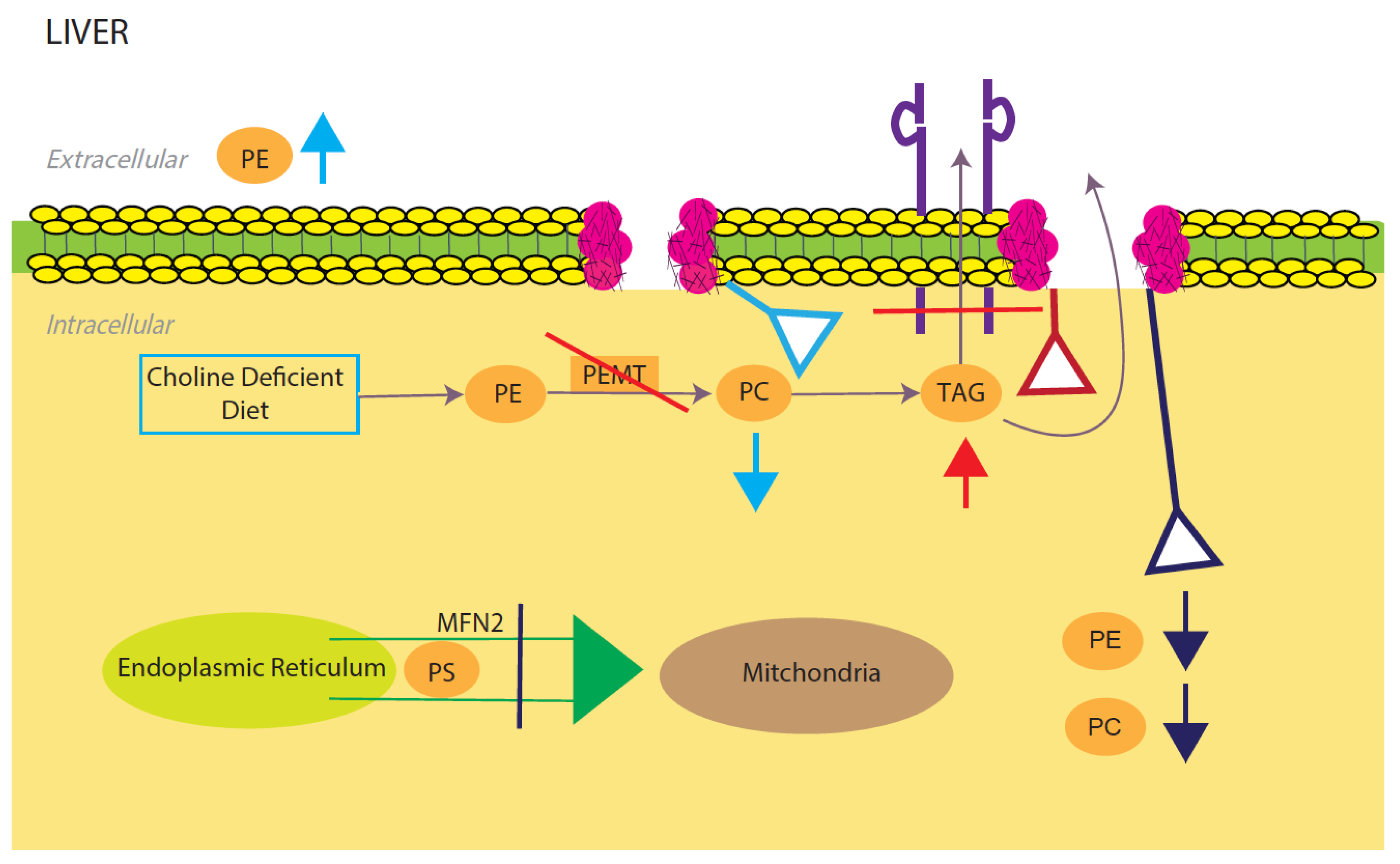
Phosphatidylcholine (PE) and phosphatidylethanolamine (PC) comprise the vast majority of the phospholipids present in the mammalian cells and as such constitute the major structural components of the cell membrane [33][34]. In the liver, PC is derived from choline through the CDP-choline pathway and conversion from PE to PC through three methylation reactions catalyzed by the PE N-methyltransferase (PEMT) [35][36]. The integrity of the plasma membrane is maintained by the ratio of PC and PE. Therefore, abnormalities in this structural property of the membrane predispose the hepatic cellular membrane to various insults including toxic lipids, immune messenger and activators leading to NAFLD, inflammation and cell death [34] (
3. Role of Glycolipids in NAFLD
Glycolipids are a type of complex carbohydrate that contains both a glycan and a lipid component [51]. They are important components of cellular membranes and may act as receptors, are involved in cell aggregation and dissociation, and are responsible for cellular interaction and signal transmission [51]. The glyco-components are mono- or oligosaccharide chains that are commonly branched linked to ceramide or other glycerol derivatives and may be replaced with acetyl or sulfate groups. They are classified as glycosphingolipids (GSLs) or glycoglycerolipids, based on the structure of the lipid moiety. GSLs comprise about 100,000 species divided into four subfamilies (phosphosphingolipids, glycosphingolipids, sphingoid bases, and ceramides), all of which have the sphingoid structure [23,52,53]. They are a broad range of biomolecules consisting of both hydrophilic as well as hydrophobic domains that are embedded in the cell membranes of all eukaryotic organisms [13], and are believed to be involved in regulating major cellular processes [54]. As it is well known that NAFLD is linked to a variety of lipid abnormalities in the liver [55], sphingolipid has received a lot of interest in this respect. In recent years, there has been growing interest in the role of glycolipids, particularly sphingolipids, during NAFLD progression [56]. They are thought to have a role in the development of NAFLD through a variety of mechanisms, including obesity, inflammation, insulin resistance, and oxidative stress. The liver has a far greater concentration of sphingolipids, particularly ceramide and sphingomyelin, than any other tissue [31,57]. As a result, the liver is vulnerable to sphingolipotoxicity. Studies have shown that hepatic ceramide and sphingomyelin levels are considerably higher in the livers of rats fed a high-fat diet (HFD) or mice overexpressing acyl-CoA: Diacylglycerol acyltransferase 2 in hepatocytes [58,59,60,61]. At the ceramide genetic level, heterozygous CerS6 knockout mice (CerS6+/) had higher Beta-oxidation and lower CD36/FAT expression, leading to lower lipid accumulation [62]. Furthermore, NAFLD, at the transcription level, is linked to increased expression of genes implicated in three distinct pathways that lead to ceramide formation (de novo synthesis, sphingomyelin hydrolysis, and the salvage route) [58,63]. Recent studies investigated the effects of some natural products on glycolipid disorders amelioration of fatty liver disease. To examine the effect of beta-glycophospholipid in improving liver injury, Zigmond E. et al. utilized an animal model of NASH-Psammomys obesus fed on high diet [64]. They reported that beta-glycophospholipid administration in these mice reduced cholesterol and TGs levels and improvement in liver injury [64]. Another study investigated the role of epigallocatechin-3-gallate (EGCG) on glycolipid disorder [65]. Administration of EGCG in a diabetic mouse model demonstrate that this natural product improved the incidence of glycolipid disorder by restoring normal lipid metabolism [65]. Other studies showed that tauroursodeoxycholic acid (TUDCA) ameliorates glycolipid disorder in obese mice by reversing impaired autophagy [66].
4. Phosphatidylcholine and Phosphatidylethanolamine-Mediated NAFLD
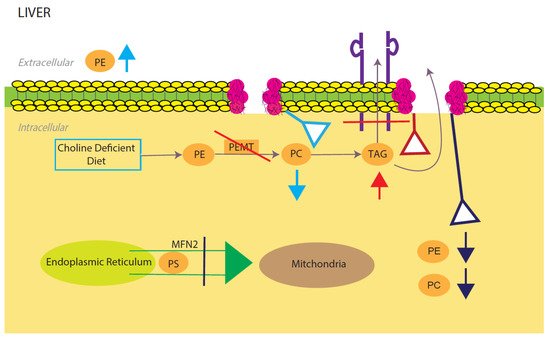
Phosphatidylcholine (PE) and phosphatidylethanolamine (PC) comprise the vast majority of the phospholipids present in the mammalian cells and as such constitute the major structural components of the cell membrane [67,68]. In the liver, PC is derived from choline through the CDP-choline pathway and conversion from PE to PC through three methylation reactions catalyzed by the PE N-methyltransferase (PEMT) [69,70]. The integrity of the plasma membrane is maintained by the ratio of PC and PE. Therefore, abnormalities in this structural property of the membrane predispose the hepatic cellular membrane to various insults including toxic lipids, immune messenger and activators leading to NAFLD, inflammation and cell death [68] ( Model for phosphatidylcholine (PC)/phosphatidylethanolamine (PE)-induced fatty liver disease. Light blue, red, and dark blue are three different PE/PC membrane disruption pathways. Light blue shows the effects of a low choline diet, which significantly lowers intracellular PC levels due to lack of its primary reactant. The liver will try to compensate for this lowering by increasing extracellular PE. Red shows the effects of disrupting the PEMT pathway, which significantly decreases TAG secretion and increases TAG intracellular accumulation. Dark blue shows the effects of silencing the expression of mitochondrial protein Mfn2. In the ER, PS is converted to PE, which is then sent into the mitochondria for modification to PC. Knocking out this membrane protein causes a NAFLD/NASH phenotype due to the halting of PE and PC creation. All three disruptions upset the intracellular PE/PC ratio, which in turn causes membrane destabilization.
5. Oxidized Phospholipids Contribution to NAFLD/NASH
Oxidized PC and PE constitute the major oxidized phospholipids (OxPLs) and they are generally synthesized in platelets, neutrophils and monocytes [37][38][39]. In addition, in macrophages, oxidized cholesteryl esters derived from 15-lipoxygenase can generate OxPLs by transferring the oxidized fatty acyl group to phospholipids [39]. Early studies indicate an essential role of myeloperoxidase-derived oxidative stress in forming oxidized phospholipids in distinct localization in the liver thereby promoting the progression of fatty liver disease [40]. Recently, Sun X. et al. reported that amylin liver NASH (AMLN)-fed Ldlr−/− mice exhibit increased plasma oxidized phospholipids (OxPLs), hepatic steatosis, inflammation and fibrosis compared with mice fed a control diet [41]. They utilized a mouse model that express a functional single-chain variable fragment of a natural antibody that neutralizes OxPLs, called E06, to determine whether neutralization of oxidized phospholipid alleviates the symptoms of NASH and incidence of liver cancer [41]. E06 binds the PC headgroup of OxPLs but not to unoxidized phospholipids. The binding of E06 with oxidized phospholipids blocks the uptake of oxidized LDL by macrophages which inhibits the proinflammatory properties of OxPLs. Comparison of OxPLs content in the livers of AMLN-fed E06-scFv Ldlr−/− and Ldlr−/− mice shows that AMLN-fed E06-tscFv Ldlr−/− has reduced OxPLs [41]. In contrast, they exhibit similar levels of serum cholesterol and TGs. Furthermore, E06 significantly improves hepatic inflammation, steatosis, hepatocellular injury, and fibrosis. The progression from NASH to HCC was also reduced by neutralizing OxPLs in AMLN-fed E06-scFv Ldlr−/− compared with Ldlr−/− mice, as indicated by significant reduction in tumor number, tumor volume and tumor incidence. More studies have demonstrated inhibition of NAFLD using lecinoxoid, an oxidized phospholipid small molecule [42], while others showed that a combination of HFD and oxidized low-density lipoprotein promotes NAFLD [43]. Oxidized phospholipids promote these alterations as reactive oxygen species (ROS) bind to phospholipids of the mitochondrial membrane [44]. An increase in oxidative stress leads to a reduction in cardiolipins due to oxidation. Cardiolipins are mitochondrial membrane phospholipids that hold their structure and function to stabilize respiratory chain complexes and carrier proteins [45]. Oxidized cardiolipins destabilize the inner membrane and allow ROS and cytochrome c to leak out of the mitochondria, causing cell death [45]. These studies demonstrate an important role for oxidized phospholipids in the development of NASH and some mitigation factors include using antioxidant processes and antibodies that target and neutralize the oxidized phospholipid head and could potentially translate clinically with a similar naturally occurring antibody in humans.
6. Role of Cholesterol in NAFLD/NASH
In mammals, cholesterol is an important structural component of cell membranes and play a key role in membrane permeability and signaling processes [46][47]. Cholesterol serves as the precursor for all steroids hormones and bile acids [48]. The synthesis of cholesterol occurs in the cytoplasm and endoplasmic reticulum (ER) from acetyl-CoA via the mevalonic acid (MVA) pathway [48]. 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) is the rate-limiting enzyme in the cholesterol biosynthetic pathway. In order to preserve a low sterol level, cholesterol and its precursors move out of the ER and attached to the cell membrane and participate in cellular signal transduction [49]. In addition, cholesterol is also converted into cholesterol esters by acyl-CoA acyl-transferase (ACAT) to reduce free cholesterol accumulation in the intracellular membranes and plasma [49]. Whole-body and cellular cholesterol homeostasis is a tightly regulated process in order to prevent accumulation of excess cholesterol or deficiency [50]. The liver plays a key role in the regulation of distorted cholesterol levels in the plasma through a complex metabolic process involving sterol transporters, lipoprotein and nuclear receptors, and transcriptional gene expression [51].
A disturbance in the cholesterol pathways that perform synthesis, transport and conversion of cholesterol is believed to play a significant role in developing liver damage [52]. The non-inflammatory intracellular fat disposition can result from imbalance between lipid disposition and lipid removal from the liver, which can lead to cirrhosis, hepatocellular carcinoma, and hepatic fibrosis [53]. Furthermore, ER controls cellular levels of cholesterol, and a protein misfolding in the ER can lead to irregular cholesterol synthesis and clearance, leading to NAFLD.
Light blue, red, and dark blue are three different PE/PC membrane disruption pathways. Light blue shows the effects of a low choline diet, which significantly lowers intracellular PC levels due to lack of its primary reactant. The liver will try to compensate for this lowering by increasing extracellular PE. Red shows the effects of disrupting the PEMT pathway, which significantly decreases TAG secretion and increases TAG intracellular accumulation. Dark blue shows the effects of silencing the expression of mitochondrial protein Mfn2. In the ER, PS is converted to PE, which is then sent into the mitochondria for modification to PC. Knocking out this membrane protein causes a NAFLD/NASH phenotype due to the halting of PE and PC creation. All three disruptions upset the intracellular PE/PC ratio, which in turn causes membrane destabilization.
5. Oxidized Phospholipids Contribution to NAFLD/NASH
Oxidized PC and PE constitute the major oxidized phospholipids (OxPLs) and they are generally synthesized in platelets, neutrophils and monocytes [83,84,85]. In addition, in macrophages, oxidized cholesteryl esters derived from 15-lipoxygenase can generate OxPLs by transferring the oxidized fatty acyl group to phospholipids [85]. Early studies indicate an essential role of myeloperoxidase-derived oxidative stress in forming oxidized phospholipids in distinct localization in the liver thereby promoting the progression of fatty liver disease [86]. Recently, Sun X. et al. reported that amylin liver NASH (AMLN)-fed Ldlr−/− mice exhibit increased plasma oxidized phospholipids (OxPLs), hepatic steatosis, inflammation and fibrosis compared with mice fed a control diet [87]. They utilized a mouse model that express a functional single-chain variable fragment of a natural antibody that neutralizes OxPLs, called E06, to determine whether neutralization of oxidized phospholipid alleviates the symptoms of NASH and incidence of liver cancer [87]. E06 binds the PC headgroup of OxPLs but not to unoxidized phospholipids. The binding of E06 with oxidized phospholipids blocks the uptake of oxidized LDL by macrophages which inhibits the proinflammatory properties of OxPLs. Comparison of OxPLs content in the livers of AMLN-fed E06-scFv Ldlr−/− and Ldlr−/− mice shows that AMLN-fed E06-tscFv Ldlr−/− has reduced OxPLs [87]. In contrast, they exhibit similar levels of serum cholesterol and TGs. Furthermore, E06 significantly improves hepatic inflammation, steatosis, hepatocellular injury, and fibrosis. The progression from NASH to HCC was also reduced by neutralizing OxPLs in AMLN-fed E06-scFv Ldlr−/− compared with Ldlr−/− mice, as indicated by significant reduction in tumor number, tumor volume and tumor incidence. More studies have demonstrated inhibition of NAFLD using lecinoxoid, an oxidized phospholipid small molecule [88], while others showed that a combination of HFD and oxidized low-density lipoprotein promotes NAFLD [89]. Oxidized phospholipids promote these alterations as reactive oxygen species (ROS) bind to phospholipids of the mitochondrial membrane [90]. An increase in oxidative stress leads to a reduction in cardiolipins due to oxidation. Cardiolipins are mitochondrial membrane phospholipids that hold their structure and function to stabilize respiratory chain complexes and carrier proteins [91]. Oxidized cardiolipins destabilize the inner membrane and allow ROS and cytochrome c to leak out of the mitochondria, causing cell death [91]. These studies demonstrate an important role for oxidized phospholipids in the development of NASH and some mitigation factors include using antioxidant processes and antibodies that target and neutralize the oxidized phospholipid head and could potentially translate clinically with a similar naturally occurring antibody in humans.
6. Role of Cholesterol in NAFLD/NASH
In mammals, cholesterol is an important structural component of cell membranes and play a key role in membrane permeability and signaling processes [92,93]. Cholesterol serves as the precursor for all steroids hormones and bile acids [94]. The synthesis of cholesterol occurs in the cytoplasm and endoplasmic reticulum (ER) from acetyl-CoA via the mevalonic acid (MVA) pathway [94]. 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) is the rate-limiting enzyme in the cholesterol biosynthetic pathway. In order to preserve a low sterol level, cholesterol and its precursors move out of the ER and attached to the cell membrane and participate in cellular signal transduction [95]. In addition, cholesterol is also converted into cholesterol esters by acyl-CoA acyl-transferase (ACAT) to reduce free cholesterol accumulation in the intracellular membranes and plasma [95]. Whole-body and cellular cholesterol homeostasis is a tightly regulated process in order to prevent accumulation of excess cholesterol or deficiency [96]. The liver plays a key role in the regulation of distorted cholesterol levels in the plasma through a complex metabolic process involving sterol transporters, lipoprotein and nuclear receptors, and transcriptional gene expression [97].
A disturbance in the cholesterol pathways that perform synthesis, transport and conversion of cholesterol is believed to play a significant role in developing liver damage [98]. The non-inflammatory intracellular fat disposition can result from imbalance between lipid disposition and lipid removal from the liver, which can lead to cirrhosis, hepatocellular carcinoma, and hepatic fibrosis [99]. Furthermore, ER controls cellular levels of cholesterol, and a protein misfolding in the ER can lead to irregular cholesterol synthesis and clearance, leading to NAFLD.