Microparticles (MPs) are extracellular vesicles with a size ranging from 0.1 to 1.0 μm. They carry cargo (mRNA, DNA, lipid and specific proteins) from originating cells and transfer to recipient cells, allowing cell-to-cell communication.
- Microparticles,
- Extracellular vesciles
- vascular inflammation
- macrophage
- endothelial cell
- vascular smooth muscle cell
1. Introduction
MP release is triggered by inducer that can cause cell apoptosis or activation[1] and are generated by any type of body cells such as platelets, endothelial cells, leukocytes, smooth muscle cells, and erythrocytes[2]. In the past, MPs were considered as “cell debris/dust” but now, it is clear that MPs are not just cell debris; however, the detailed process of their generation is yet to be elucidated[3]. Nowadays, it is known that the release of MPs from cells involves the reconstitution of a phospholipid bilayer with the outside of the membrane exposed with phosphatidylserine and change in the cellular organization with the disruption of cytoskeleton architecture[4]. Recently, the role of MPs in the pathogenesis of central nervous system disorder[5], diabetes mellitus[6], cancer[7], inflammation[8], systemic lupus erythematosus[9], endothelial dysfunctions[10] has been explored by clinical/experimental research. In the human systemic circulation, elevated levels of endothelial MPs (EC-MP) are linked with the progression of various cardiovascular diseases, primarily initiated by endothelial dysfunction[11]. An in vitro study revealed that EC-MP treatment affects the various angiogenesis parameters by reducing endothelial cell (EC) proliferation, decreasing capillary formation and increasing apoptosis[12]. Hidenobu Koga et al. observed a higher level of the cluster of differentiation (CD)-144 or vascular endothelial (VE)-cadherin positive EC-MP in the systemic circulation of patients with diabetic mellitus and coronary artery disease, including atherosclerosis. This suggests VE-cadherin positive EC-MP in serum can be a hallmark for analyzing atherosclerosis. Thus, various cardiovascular complications can be efficiently prevented by the therapeutics approach in atherosclerosis patients focusing on assessments of blood CD144-EMP levels[13].
Microparticles are produced by macrophage cells in response to various stimuli such as lipopolysaccharide (LPS), poly (I:C), actinomycin D[14][15]. LPS is a toll-like receptor (TLR)-4 ligand, and poly (I:C) is a TLR3 ligand that can stimulate macrophage microparticles (MØ-MP) via TLR. MØ-MP release induced by LPS or poly(I:C) is correlated with nitric oxide (NO) production, and treatment with the inducible nitric oxide synthase (iNOS) inhibitor 1400W decreased particle release and NO production. Furthermore, the treatment of RAW 264.7 cells with NO donors induced MPs production[14]. MPs from activated macrophages could carry tumor necrosis factor-alpha (TNF-α) and contribute to the propagation of inflammatory signals leading to myocardial infarction. Milbank E et al. hypothesized that MPs from human carotid atherosclerotic plaques might contain active TNF-α, which could contribute to MPs-induced inflammatory signals in human atherosclerotic lesions[15].
Pro-atherogenic inducer such as TNF-α, thrombin, and lysophosphatidylcholine can generate MPs by vascular smooth muscle cells (VSMC)[16]. VSMC-microparticles (VSMC-MP) possesses protein such as caveolin-1[16] and alpha-smooth muscle actin (α-SMA)[17] obtained from originating VSMC, making it delectable in an experimental setting by labeling VSMC-MP with either caveolin-1 or α-SMA. EC, VSMC and macrophage are three important cell types involved in the pathogenesis of atherosclerosis either in initiation steps or later progression of vascular inflammation[16]. G. Chiva-Blanch et al. found that aspirin therapy inhibits vascular wall cell activation and microparticle shedding by VSMC, suggesting a therapeutic target to lower microparticles released from cells can prevent the progression of the disease such as diabetes mellitus and atherosclerosis[17]. Additionally, MPs generated from apoptotic VSMC (commonly observed in atherosclerotic plaques) can, in turn, induce endothelial dysfunction as shown by its decreased NO production and vasodilatory capacity[18]. Previously, we have shown that VSMC-MP promotes the proliferation of VSMC through the upregulation of the mitogen-activated protein kinase pathway (MAPK) and proliferative cell nuclear antigen to facilitate vascular inflammation[19]. Similarly, in a recent review paper, we have highlighted the role of circulating EC-MP in the progression of atherosclerosis through oxidative stress, and upregulation of iNOS, cyclooxygenase (COX-2) and nuclear factor kappaB (NF-κB) pathway[20]. As cell-to-cell communication mediated by cargos of microparticles has been recently explored, there exists a potential research platform to investigate potent drug candidates that can target various pathways to alleviate the disease progression.
2. Isolation, Characterization and Quantification of Microparticles
As microparticles are released by the process of cell activation or apoptosis, researchers use various stimulants in high concentrations for high-yield production of microparticles. Gauley et al. treated LPS at a dose ranging from 0.05 to 50 µg/mL in the RAW264.7 cell line and found the highest yield of microparticles and a corresponding increase in nitrite (a marker of inflammation) at 50 µg/mL LPS[14]. In our study, we also use high doses of LPS for EC and RAW264.7 for the high yield of microparticles. Briefly, confluent EC and RAW264.7 were stimulated with 10 µg/mL and 50 µg/mL LPS respectively for 24 h. Confluent VSMC were stimulated with 20 ng/mL of TNF-α for 16 h[16]. The cell supernatant from these stimulated cells (Five 100mm cell culture plates for each cell) was collected and subjected to multiple centrifugation steps to obtain an MP pellet of respective cells and the process was repeated to obtain enough MPs for all experiments. Each cell MP was pooled into a single Eppendorf tube mixed in PBS and stored at -70°C until further use.Characterization of microparticles was carried out by immunofluorescence. EC-MP was stained with ICAM primary antibody followed by Alexa fluor-488 secondary antibody[21], VSMC-MP were stained with α-SMA, followed by Alexa fluor-488 secondary antibody[17], and MØ-MP were stained with annexin V-FITC as a general marker of microparticles[14]. Fluorescence images were taken at different magnifications using confocal or microscope.Quantification of microparticles was performed by measuring protein concentration. For each cell microparticle, 10 µL of MP was mixed with 200 µL of 20% Bio-Rad protein assay reagent, and absorbance of resultant blue color was read at 570nm. The protein concentration of microparticles in terms of mg/mL was calculated with reference to the standard curve equation obtained from a serial dilution of bovine serum albumin.
In our study, microparticles were successfully isolated from VSMC and macrophage and EC, in vitro. Figure 1A represents the confocal microscopic image of in vitro isolated EC-MP stained with ICAM-Alexa 488. Similarly, Figure 1B (copyright@American Scientific Publishers) represents the confocal image of VSMC-MP stained with α-SMA-Alexa 488[19]. Likewise, Figure 1C represents the confocal image of Annexin-V-FITC labeled MØ-MP generated in vitro from macrophages (RAW264.7 cells).
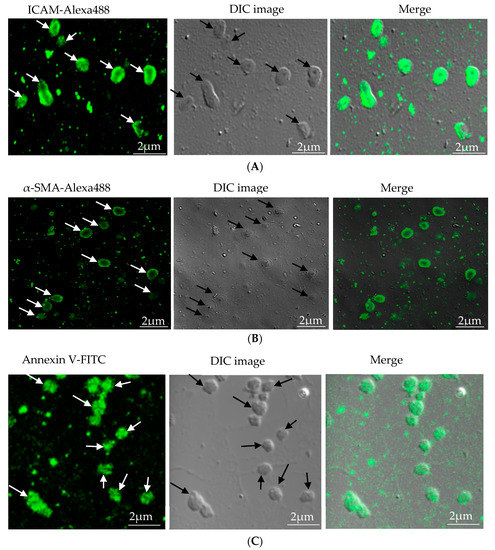
Figure 1. Characterization of microparticles isolated from various cell lines. (A) Endothelial microparticles (EC-MP) (shown by arrowhead) obtained from in vitro lipopolysaccharide (LPS)-treated endothelial cell (EC) were stained with ICAM followed by Alexa-488 and photographs were taken with a confocal microscope at a magnification of 630 X Zoom 4. (B) Vascular smooth muscle cell (VSMC)-MP (shown by arrowhead) obtained from tumor necrosis factor-alpha (TNF-α)-stimulated VSMC were stained with α-smooth muscle actin (α-SMA), and photographs were taken with a confocal microscope at a magnification of 630 X Zoom. (C) Macrophage microparticles (MØ-MP) obtained from LPS treated macrophage (RAW264.7) were stained with Annexin V-FITC, and photographs were taken with a confocal microscope at a magnification of 630 X Zoom 4.
References
- Marja J. Vanwijk; E. VanBavel; A. Sturk; R. Nieuwland; Microparticles in cardiovascular diseases. Cardiovascular Research 2003, 59, 277-287, 10.1016/s0008-6363(03)00367-5.
- Carolina Nunes França; Maria Cristina De Oliveira Izar; Jônatas Bussador Do Amaral; Daniela Melo Tegani; Francisco Antonio Helfenstein Fonseca; Microparticles as Potential Biomarkers of Cardiovascular Disease. Arquivos Brasileiros de Cardiologia 2014, 104, 169-174, 10.5935/abc.20140210.
- Silke Patz; Christa Trattnig; Gerda Grünbacher; Birgit Ebner; Christian Gülly; Alexandra Novak; Beate Rinner; Gerd Leitinger; Markus Absenger; Oana A. Tomescu; et al.Gerhard G. ThallingerUlrike FaschingSonja WissaJuan Archelos-GarciaUte Schäfer More than Cell Dust: Microparticles Isolated from Cerebrospinal Fluid of Brain Injured Patients Are Messengers Carrying mRNAs, miRNAs, and Proteins. Journal of Neurotrauma 2013, 30, 1232-1242, 10.1089/neu.2012.2596.
- David Connor; T. Exner; D. D. F. Ma; J. E. Joseph; The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thrombosis and Haemostasis 2010, 103, 1044-1052, 10.1160/th09-09-0644.
- Stephanie M. Schindler; Jonathan Little; Andis Klegeris; Microparticles: A New Perspective in Central Nervous System Disorders. BioMed Research International 2014, 2014, 1-17, 10.1155/2014/756327.
- A. F. Tramontano; R. Lyubarova; J. Tsiakos; T. Palaia; J. R. DeLeon; L. Ragolia; Circulating Endothelial Microparticles in Diabetes Mellitus. Mediators of Inflammation 2010, 2010, 1-8, 10.1155/2010/250476.
- Maria Augusta Roos; Luisa Gennero; Tetyana Denysenko; Stefano Reguzzi; Giovanni Cavallo; Gian Piero Pescarmona; Antonio Ponzetto; Microparticles in physiological and in pathological conditions. Cell Biochemistry and Function 2010, 28, 539-548, 10.1002/cbf.1695.
- Jörg H. W. Distler; David S. Pisetsky; Lars C. Huber; Joachim R. Kalden; Steffen Gay; Oliver Distler; Microparticles as regulators of inflammation: Novel players of cellular crosstalk in the rheumatic diseases. Arthritis & Rheumatism 2005, 52, 3337-3348, 10.1002/art.21350.
- Javier Pérez-Hernández; Raquel Cortes; Extracellular Vesicles as Biomarkers of Systemic Lupus Erythematosus. Disease Markers 2015, 2015, 1-7, 10.1155/2015/613536.
- Thassila Nogueira Pitanga; Luciana De Aragão França; Viviane Costa Junqueira Rocha; Thayna Meirelles; Valeria M. Borges; Marilda S. Gonçalves; Lain Pontes-De-Carvalho; Alberto Augusto Noronha-Dutra; Washington Luís Conrado Dos Santos; Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biology 2014, 15, 21-21, 10.1186/1471-2121-15-21.
- Ziad Mallat; B. Hugel; J Ohan; G Lesèche; J M Freyssinet; A. Tedgui; Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity.. Circulation 1999, 99, 348–353.
- Alexandre Mezentsev; Roeland M. H. Merks¶; Edmond O'riordan; Jun Chen; Natalia Mendelev; Michael S. Goligorsky; Sergey V. Brodsky; Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. American Journal of Physiology-Heart and Circulatory Physiology 2005, 289, H1106-H1114, 10.1152/ajpheart.00265.2005.
- Hidenobu Koga; Seigo Sugiyama; Kiyotaka Kugiyama; Keisuke Watanabe; Hironobu Fukushima; Tomoko Tanaka; Tomohiro Sakamoto; Michihiro Yoshimura; Hideaki Jinnouchi; Hisao Ogawa; et al. Elevated Levels of VE-Cadherin-Positive Endothelial Microparticles in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease. Journal of the American College of Cardiology 2005, 45, 1622-1630, 10.1016/j.jacc.2005.02.047.
- Julie Gauley; David S. Pisetsky; The release of microparticles by RAW 264.7 macrophage cells stimulated with TLR ligands. Journal of Leukocyte Biology 2010, 87, 1115-1123, 10.1189/jlb.0709465.
- Edward Milbank; Raffaella Soleti; Emilie Martinez; Badreddine Lahouel; Grégory Hilairet; M Carmen Martinez; Ramaroson Andriantsitohaina; Jacques Noireaud; Microparticles from apoptotic RAW 264.7 macrophage cells carry tumour necrosis factor-? functionally active on cardiomyocytes from adult mice.. Journal of Extracellular Vesicles 2015, 4, 28621.
- Esther Serrano-Pertierra; Lorena Benavente Esther Serrano-Pertierra; Lysophosphatidylcholine Induces Vascular Smooth Muscle Cell Membrane Vesiculation: Potential Role in Atherosclerosis through Caveolin-1 Regulation. Journal of Proteomics & Bioinformatics 2014, 7, 332, 10.4172/jpb.1000336.
- Gemma Chiva-Blanch; Rosa Suades; Teresa Padró; Gemma Vilahur; Esther Pena; Juan Ybarra; Jose M. Pou; Lina Badimon; Microparticle Shedding by Erythrocytes, Monocytes and Vascular Smooth Muscular Cells Is Reduced by Aspirin in Diabetic Patients. Revista Española de Cardiología (English Edition) 2016, 69, 672-680, 10.1016/j.rec.2015.12.033.
- Essayagh, S.; Brisset, A.C.; Terrisse, A.D.; Dupouy, D.; Tellier, L.; Navarro, C.; Arnal, J.F.; Sie, P.; Microparticles from apoptotic vascular smooth muscle cells induce endothelial dysfunction, a phenomenon prevented by beta3-integrin antagonists. Thromb. Haemost. 2005, 94, 853–858.
- Keshav Raj Paudel; Min-Ho Oak; Dong-Wook Kim; Smooth Muscle Cell Derived Microparticles Acts as Autocrine Activation of Smooth Muscle Cell Proliferation by Mitogen Associated Protein Kinase Upregulation.. Journal of Nanoscience and Nanotechnology 2020, 20, 5746-5750, 10.1166/jnn.2020.17661.
- Keshav Raj Paudel; Nisha Panth; Dong-Wook Kim; Circulating Endothelial Microparticles: A Key Hallmark of Atherosclerosis Progression. Scientifica 2016, 2016, 1-9, 10.1155/2016/8514056.
- Biswanath Dinda; Subhajit Dinda; Saikat Dassharma; Rajarshi Banik; Ankita Chakraborty; Manikarna Dinda; Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. European Journal of Medicinal Chemistry 2017, 131, 68-80, 10.1016/j.ejmech.2017.03.004.
