Creatine is a naturally occurring compound, functioning in conjunction with creatine kinase to play a quintessential role in both cellular energy provision and intracellular energy shuttling. An extensive body of literature solidifies the ergogenic benefits gained following dietary creatine supplementation; however, recent findings have further indicated a potential therapeutic role for creatine in several pathologies such as myopathies, neurodegenerative disorders, metabolic disturbances, chronic kidney disease and inflammatory diseases. Furthermore, creatine has been found to exhibit non-energy-related properties, such as serving as a potential antioxidant and anti-inflammatory. Despite the therapeutic success of creatine supplementation in varying clinical populations, there is scarce information regarding the potential application of creatine for combatting the current leading cause of mortality, cardiovascular disease (CVD). Taking into consideration the broad ergogenic and non-energy-related actions of creatine, it could be hypothesize that creatine supplementation may be a potential therapeutic strategy for improving vascular health in at-risk populations such as older adults or those with CVD. This entry gives a short background on creatine, its cellular function and metabolism, in addition to the pleiotropic applications of creatine within just a few clinical populations. Furthermore, this entry concludes by eluding to the potential in which creatine may possess to benefit vascular health and to combat the pathology the underlies CVD.
- creatine
- vascular health
- oxidative stress
- inflammation
1. Introduction
Creatine is an organic compound that is both synthesized endogenously and found exogenously in various food sources such as meats and fish. Since creatine’s isolation and extraction from animal skeletal muscle by French chemist Michel Eugène Chevreul in 1832, the function of creatine has been extensively researched. Furthermore, studies such as those by Chanutin [1][1], Walker [2][2], and Harris et al. in 1992[3] [3] have all shown that supplemental creatine can augment natural human intramuscular creatine stores. These studies, among others, pioneered the current understanding of creatine and the use of creatine supplementation to promote energy provision and to benefit skeletal muscle performance and health. Considering the undeniably important role that creatine and phosphocreatine (PCr) play in rapid energy provision, it is of no surprise that the primary focus of creatine research has centered around the ergogenic effects of creatine supplementation to improve exercise performance. The accumulation of creatine-focused research has contributed to a vast body of knowledge and has led to several authors declaring creatine as being one of the most effective and underrated nutritional supplements [4–6][4][5][6]. Furthermore, with clear scientific support and expanding mainstream popularity, creatine remains one of the most dominant sports supplements on the market, accumulating more than $400 million in annual sales [7,8][7][8].
In addition to the well-known ergogenic value of creatine [5[5][9],9], there has been an emerging interest in the clinical application of creatine. Creatine has been cited as a potential adjuvant therapy for the treatment of a variety of diseases such as myopathies, dystrophies, inflammatory diseases, neurodegenerative disorders, metabolic disturbances, and joint syndromes [4][4]. With advancing understanding, it is clear that the function of creatine goes far beyond that of a primary role in metabolism and energetics. In fact, recent evidence indicates that creatine supplementation results in a multitude of non-energy-related beneficial effects on a wide range of cellular targets. Among these promising effects includes the antioxidant potential of creatine, scavenging and neutralizing the reactive oxygen species (ROS) that underly many pathologies [10,11][10][11].
2. Brief Overview of Creatine Metabolism and the Cellular Actions of Creatine
In addition to being consumed in an omnivorous diet, creatine is also synthesized endogenously. Endogenous synthesis of creatine is an interorgan process and requires the investment of three major amino acids: glycine, arginine, and methionine; together with two primary enzymes: L-arginine: glycine amidinotransferase (AGAT) and guanidinoacetate N-methyltransferase (GAMT). The first step of creatine biosynthesis occurs in the kidneys, when AGAT catalyzes the transfer of an amidino residue from arginine to glycine, resulting in the formation of L-ornithine and guanidinoacetate (GAA). GAA then exits the kidneys and is transported to the liver where GAMT functions to transfer a methyl group from S-adenosylmethionine (SAM) to GAA, resulting in the final production of creatine. Cellular uptake of creatine is mediated by a specific creatine transporter (CRT), also known as SLC6A8. This transporter is sodium- and chloride-dependent, requiring at least two sodium ions and one chloride ion for the transport of one creatine molecule [22][12]. Given its vital role in metabolism and energy provision, the largest stores of creatine are found in skeletal muscle (~95%); however, other notable stores include the brain, kidneys, and liver [5][5]. Intracellularly, creatine can exist in a free form or in a phosphorylated form, PCr. Both creatine and PCr are metabolized and lost naturally throughout the day via a non-enzymatic, spontaneous reaction into creatinine, which is then excreted at a rate of ~2 g/day by the kidneys in the urine [23–25] [13][14][15](Figure 1).
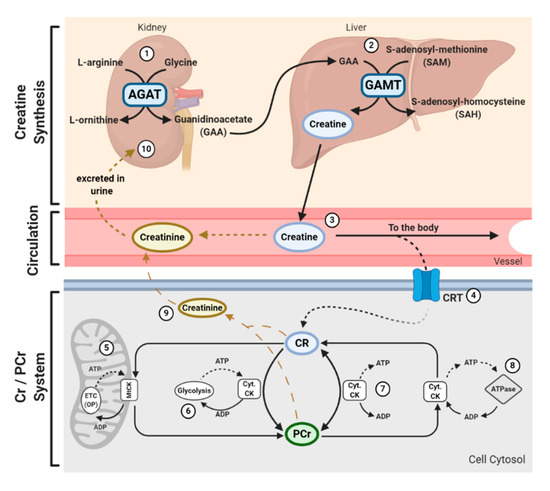
Figure 1. Physiological Journey of Creatine: Synthesis of creatine (Cr) happens at a rate of ~1 g/day[7] [7] via an interorgan process. (1) Within the kidneys, L-arginine: glycine amidinotransferase (AGAT) transfers an amidino group from L-arginine to glycine, resulting in the formation of L-ornithine and guanidinoacetate (GAA). (2) GAA is then transferred and processed in the liver. Guanidinoacetate N-methyltransferase (GAMT) transfers a methyl group from the methyl donor S-adenosylmethionine (SAM) to GAA, resulting in the formation of Cr and S-adenosylhomocysteine (SAH). SAH can thereon be hydrolyzed into homocysteine by S-adenosylhomocysteine hydrolase (not shown). (3) Cr is released from the liver into circulation, where Cr is transported to varying tissues such as the skeletal muscle, brain, kidney, and heart. (4) Cellular uptake of Cr is mediated by a creatine transporter (CRT), or SLC6A8. Cr carries both positive and negative charges, and is transported via secondary-active transport, driven by a sodium/chloride-ATPase generated gradient. Once in the cell, Cr has a multitude of fates. (5) Cellular Cr can be transformed into phosphocreatine (PCr) by mitochondrial creatine kinase (mtCK) which is coupled to oxidative phosphorylation (OP) via the electron transport chain (ETC). (6) Cr can be converted into PCr by cytosolic creatine kinase (Cyt. CK) coupled to glycolysis. (7) The cellular Cr/PCr pool is utilized to maintain adenosine triphosphate (ATP)/ adenosine diphosphate (ADP) ratios through ATP resynthesis or “buffering.” (8) Cyt. CKs located throughout the cytosol can utilize the high-energy PCr stores to shuttle and utilize energy at sites of ATP demand, or ATP-dependent processes, via ATPase enzymes. Such processes include ATP-gated ion channels, ATP-regulated receptors, ATP-regulated ion pumps; contractile processes, cell motility, cell signaling, or organelle transport. (9) Both Cr and PCr are naturally metabolized into creatinine via a non-enzymatic, spontaneous reaction. Creatinine diffuses freely into the circulation to be transported to the kidneys. (10) Creatinine is fully excreted in the urine.
Creatine and PCr, together with creatine kinase (CK) isoenzymes, function as quintessential high-energy compounds crucial for metabolism. In the case of low adenosine triphosphate (ATP) levels or high ATP demand, CK will catalyze the transfer of the N-phosphoryl group from PCr to adenosine diphosphate (ADP) to resynthesize ATP. This process quickly replenishes the ATP pool, maintaining ATP:ADP ratios and cellular homeostasis. Conversely, when ATP production from either glycolytic or oxidative pathways are greater than ATP utilization, CK can function in reverse to capture and store this cellular energy by replenishing PCr stores. There is a long-held belief that the primary function of the creatine-phosphocreatine system (Cr-PCr system) is to serve as a temporal high-energy phosphate buffer [23,26] [13][16](Figure 1). The presence of specific CKs throughout the cell are integral to the function of the Cr-PCr system. CKs exist in a variety of isoforms, which, in addition to the subcellular distribution and compartmentalization of such CKs, led to the proposal that the Cr-PCr system plays a far more complex role in energy metabolism than once believed. Cytosolic CKs (Cyt.CKs) exist as dimers, composed of either muscle (M) type or brain (B) type; therefore, three cytosolic isoenzymes exist: muscle-muscle creatine kinase (MM-CK); brain-brain creatine kinase (BB-CK); and, muscle-brain creatine kinase (MB-CK) [27][17]. Specific mitochondrial CKs (MtCKs) also exist, such as sarcomeric MtCK (sMtCK) found in striated muscle and ubiquitous MtCK (uMtCK) found in other tissues such as the brain [28][18]. MtCKs are found between the inner and outer mitochondrial membranes, and when in the presence of creatine, ensure the bulk of ATP from oxidative phosphorylation is converted into PCr [23,28][13][18]. Cyt.CKs, on the other hand, are found within the cytoplasm and at sites of high energy consumption or demand (e.g., cellular ATPases, myofibrils, sarcoplasmic reticulum, plasma membrane) [23][13]. With a sophisticated variety of CKs and their subcellular localization, the Cr-PCr system is capable of functioning as an energy shuttle of high energy phosphates, shuttling energy between sites of mitochondrial ATP production and sites of ATP utilization [26] [16](Figure 1).
The function of the Cr-PCr system as a temporal high-energy phosphate buffer and a spatial high-energy shuttle are not mutually exclusive and coexist in varying degrees. The masterful interplay between both shuttle and buffering abilities enables the Cr-PCr system to intricately monitor and stabilize ATP:ADP ratios within the cell, minimize adenine nucleotide loss, maintain cellular pH via hydrogen ion buffering, and to reduce free inorganic phosphates [29–31][19][20][21]. Furthermore, it has been speculated that it is the interaction between MtCKs and Cyt.CKs that ensures the maintenance of ATP:ADP ratios within the mitochondrial matrix, thereby stimulating healthy respiratory chain function [32][22]. This therefore leads to a reduction in electron leakage and reduced production of harmful mitochondrial-specific ROS.
From the above, it is clear that the Cr-PCr system plays a vital role in cellular function. Those readers interested in expanding their knowledge on the function, compartmentalization, and pharmacokinetics of the Cr-PCr system are directed to read reviews by Wallimann et al.[23] [33] and Perksy and Brazeau [34][24].
3. Pleiotropic Application of Creatine
Creatine supplementation has been widely utilized by healthy individuals and athletes as an ergogenic aid to improve intermittent high-intensity exercise capacity due to the Cr-PCr system sustaining rapid ATP resynthesis. The total creatine pool (creatine + PCr) of a 70 kg individual is approximately 120 mmol/kg of dry muscle mass, or around 60–80% saturation [5][5]. Pivotal research conducted by Dr. Roger Harris and colleagues demonstrated that oral creatine supplementation is capable of increasing muscle creatine and PCr stores by around 20% [3][3]. Although consumed naturally in the diet (~1–2 g/day) and synthesized daily, with intramuscular creatine metabolism and excretion of around 2 g/day [23][13], additional dietary supplementation with creatine analogs, such as creatine monohydrate, remains the most efficient way of increasing creatine stores. A common supplementation protocol usually involves a loading phase of 4 × 5 g of creatine for five to seven days, followed by a maintenance phase of 3 to 5 g/day [3,5][3][5]. A low dose protocol of consuming 3 g/day for approximately 28 days, however, can still result in increased intramuscular creatine stores [3][3]. Considering the extensive body of literature on the efficacy of creatine supplementation, there is evidence that creatine supplementation can enhance many exercise-related variables such as exercise capacity [35–38][25][26][27][28], recovery [5[5][29][30],39,40], resistance to fatigue [41–43][31][32][33], and lean body mass [5[5][34],44], in both young and older individuals. For in-depth reviews highlighting the ergogenic value of creatine supplementation, readers are directed to those by Kreider et al.[5] [5] and Butts et al. [8][8].
With significant success as an ergogenic aid, the potential application of creatine supplementation in clinical populations has gained attention. Creatine supplementation has been shown to impart a variety of benefits upon skeletal muscle, such as the enhancement of force output during skeletal muscle contraction [45][35], the augmentation of lean body mass [46][36], fatigue resistance [41,42][31][32], and the improvement of intracellular calcium handling [47][37]. Furthermore, it has been proposed that creatine supplementation may impart further favorable effects on skeletal muscle physiology and metabolism, such as enhancing growth and hypertrophy through direct modulation of components of the mammalian target of rapamycin (mTOR), secretion of myokines such as myostatin and insulin-like growth factor-1, and increasing the expression of myogenic regulatory factors which can stimulate satellite cell mitotic activity [48–50][38][39][40]. Considering the beneficial impact of creatine upon muscle, several investigators have studied the effects of creatine on myopathies. Low creatine and PCr stores have been observed in those suffering from muscular disorders, contributing to poor cellular bioenergetics and muscle integrity [51][41]. These findings led to the proposed hypothesis that creatine supplementation may serve as a therapeutic intervention for myopathies. Tarnopolsky and Martin supported this hypothesis, reporting substantial increases in muscle strength, exercise capacity, and body mass in patients with mitochondrial cytopathies, neuropathic disorders, dystrophies/congenital myopathies, and inflammatory myopathies [52][42]. Further trials by Tarnopolsky et al. [53][43], Walter et al. [54][44], and Louis et al. [55][45] provide additional evidence that supports the use of creatine for the therapeutic management of various myopathies; however, the complexity and difference between myopathies has limited the ability to make an overarching conclusion. In addition, the potential therapeutic application of creatine for neurological diseases has similarly been hypothesized. The brain, despite having a relatively small mass, represents one of the largest sources of energy consumption, accounting for approximately 20% of resting metabolism [56,57][46][47]. While all energy systems play a vital role in ATP provision, the presence of brain-specific CKs suggests a vital role of the Cr-PCr system within the brain [58,59][48][49]. Furthermore, considering genetic creatine deficiency syndromes are often characterized by cognitive impairment, developmental delay, autistic behavior, and seizures, it is clear that creatine contributes to healthy brain function [60][50]. Researchers have since tested this hypothesis and have shown that creatine supplementation can aid in the improvement of cognitive processes such as memory and attention in both young[51] [61] and older individuals [62,63][52][53]. It has also been shown that creatine exhibits potential anti-depressant properties [64][54]. Animal and in vitro models have further been used to assess the efficacy of creatine supplementation for certain neurodegenerative disorders such as Parkinson’s, Huntington’s, and Alzheimer’s disease, some of which report promising results in regard to offering neuroprotection against oxidative stress and neurotoxicity [65–67][55][56][57]. Results of clinical trials, however, remain mixed, with some trials reporting potential benefit following creatine supplementation that warrants further investigation [68–71][58][59][60][61], and others reporting minimal or no benefits [72,73][62][63].
The physiological benefits of creatine do not stop at the muscular and neurological systems. Creatine supplementation has also been found to help ameliorate hyperglycemia[64] [74] and improve glycemic control in those suffering from type 2 diabetes [75][65], improve function in those suffering from fibromyalgia [76][66], protect the integumentary system from age-related deterioration and damage [77][67], increase bone mineral density and tensile strength in elderly individuals [78][68], decrease triglyceride accumulation and increase liver health in models of non-alcoholic fatty liver disease [79][69], and protect both mitochondrial and nuclear deoxyribonucleic acid (DNA) from markers of oxidative and inflammatory damage [11,18,80][11][70][71].
Despite the above described potential of creatine for the management of various metabolic, muscular, and neurological diseases, there is surprisingly very little information on the use of creatine supplementation to reduce the current leading cause of mortality in the United States (US): cardiovascular disease (CVD). Major examples of CVDs can include coronary heart disease, heart failure, stroke, atherosclerosis, hypertension, and peripheral artery disease. It has been estimated that approximately 610,000 deaths are caused by CVDs in the US every year [81][72], with more than 43.7 million adults aged >60 years suffering from one or more CVDs in 2016 alone [82][73]. Deteriorations in vascular integrity such as arterial thickening, stiffening, endothelial dysfunction, and inflammation are associated with most CVDs, and are all related to, or augmented by, the accumulation of ROS [83–86][74][75][76][77]. Considering creatine’s proposed antioxidant properties and promising application within varying clinical populations, the sparse amount of research on the effect of creatine supplementation on vascular function and health is surprising and highlights a major gap in the literature. A recently published review entitled "The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health?" by Clarke et al. delves further into the literature surrounding the application of creatine as an antioxidant, anti-inflammatory agent, and as a substance capable of reducing homocysteine levels; all of which are proposed mechanisms in which creatine may be able to benefit vascular health. Readers are directed to this review for a deeper understanding of the relatively under researched areas in which creatine has yet to show its full pleiotropic potential.
References
- Chanutin, A.; The fate of creatine when administered to man. J. Biol. Chem. 1926, 67, 29–41.
- James B. Walker; Creatine: Biosynthesis, Regulation, and Function. Advances in Enzymology - and Related Areas of Molecular Biology 2006, 50, 177-242, 10.1002/9780470122952.ch4.
- Roger C. Harris; Karin Soderlund; Eric Hultman; Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clinical Science 1992, 83, 367-374, 10.1042/cs0830367.
- Gualano, B.; Artioli, G.G.; Poortmans, J.R.; Lancha Junior, A.H. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010, 38, 31–44.
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18.
- Wallimann, T.; Harris, R. Creatine: A miserable life without it. Amino Acids 2016, 48, 1739–1750.
- Momaya, A.; Fawal, M.; Estes, R. Performance-enhancing substances in sports: A review of the literature. Sports Med. 2015, 45, 517–531.
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sports Health 2018, 10, 31–34.
- Robert Cooper; Fernando Naclerio; Judith Allgrove; Alfonso Jimenez; Creatine supplementation with specific view to exercise/sports performance: an update. Journal of the International Society of Sports Nutrition 2012, 9, 33-33, 10.1186/1550-2783-9-33.
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52.
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396
- Rodney J. Snow; Robyn M. Murphy; Creatine and the creatine transporter: A review. Molecular and Cellular Biochemistry 2001, 224, 169-181, 10.1023/a:1011908606819.
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 2007, 27, 241–261.
- Kan, H.E.; van der Graaf, M.; Klomp, D.W.; Vlak, M.H.; Padberg, G.W.; Heerschap, A. Intake of 13C-4 creatine enables simultaneous assessment of creatine and phosphocreatine pools in human skeletal muscle by 13C MR spectroscopy. Magn. Reson. Med. 2006, 56, 953–957.
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41.
- Theo Wallimann; M Wyss; D Brdiczka; Klaas Nicolay; H M Eppenberger; Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemical Journal 1992, 281, 21-40, 10.1042/bj2810021.
- Ulrich K.M. Decking; Christiane Alves; Theo Wallimann; Markus Wyss; Jürgen Schrader; Functional aspects of creatine kinase isoenzymes in endothelial cells.. American Journal of Physiology-Cell Physiology 2001, 281, C320-C328, 10.1152/ajpcell.2001.281.1.c320.
- Uwe Schlattner; Malgorzata Tokarska-Schlattner; Theo Wallimann; Mitochondrial creatine kinase in human health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2006, 1762, 164-180, 10.1016/j.bbadis.2005.09.004.
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213.
- Wallimann, T.; Hemmer, W. Creatine kinase in non-muscle tissues and cells. Mol. Cell. Biochem. 1994, 133, 193–220.
- Greenhaff, P.L. The creatine-phosphocreatine system: There’s more than one song in its repertoire. J. Physiol. 2001, 537, 657
- Johannes H. G. M. Van Beek; Faculty Opinions recommendation of Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity.. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2008, 281, 37361–37371, 10.3410/f.1098979.555158.
- Theo Wallimann; Max Dolder; Uwe Schlattner; Michael Eder; Thorsten Hornemann; Eddie O'gorman; Alex Rück; Dieter Brdiczka; Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. BioFactors 1998, 8, 229-234, 10.1002/biof.5520080310.
- Adam M. Persky; G A Brazeau; Clinical pharmacology of the dietary supplement creatine monohydrate.. Pharmacological Reviews 2001, 53, 161–176.
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82.
- Volek, J.S.; Ratamess, N.A.; Rubin, M.R.; Gómez, A.L.; French, D.N.; McGuigan, M.M.; Scheett, T.P.; Sharman, M.J.; Häkkinen, K.; Kraemer, W.J. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur. J. Appl. Physiol. 2004, 91, 628–637.
- Aguiar, A.F.; Januário, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996.
- Tarnopolsky, M.A. Potential benefits of creatine monohydrate supplementation in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 497–502.
- Cooke, M.B.; Rybalka, E.; Williams, A.D.; Cribb, P.J.; Hayes, A. Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individuals. J. Int. Soc. Sports Nutr. 2009, 6, 13.
- Smith, S.A.; Montain, S.J.; Matott, R.P.; Zientara, G.P.; Jolesz, F.A.; Fielding, R.A. Creatine supplementation and age influence muscle metabolism during exercise. J. Appl. Physiol. 1998, 85, 1349–1356
- Urbanski, R.L.; Vincent, W.J.; Yaspelkis, B.B., 3rd. Creatine supplementation differentially affects maximal isometric strength and time to fatigue in large and small muscle groups. Int. J. Sport Nutr. 1999, 9, 136–145.
- Rawson, E.S.; Stec, M.J.; Frederickson, S.J.; Miles, M.P. Low-dose creatine supplementation enhances fatigue resistance in the absence of weight gain. Nutrition 2011, 27, 451–455.
- Stout, J.R.; Sue Graves, B.; Cramer, J.T.; Goldstein, E.R.; Costa, P.B.; Smith, A.E.; Walter, A.A. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J. Nutr. Health Aging 2007, 11, 459–464
- Philip D Chilibeck; Mojtaba Kaviani; Darren G Candow; Gordon A Zello; Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access Journal of Sports Medicine 2017, 8, 213-226, 10.2147/oajsm.s123529.
- Mark A. Tarnopolsky; Dan P. MacLennan; Creatine Monohydrate Supplementation Enhances High-Intensity Exercise Performance in Males and Females.. International Journal of Sport Nutrition and Exercise Metabolism 2000, 10, 452-463, 10.1123/ijsnem.10.4.452.
- Sasa Mihic; Jay R. Macdonald; Scott McKenzie; Mark A. Tarnopolsky; Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Medicine & Science in Sports & Exercise 2000, 32, 291, 10.1097/00005768-200002000-00007.
- S.M Pulido; A.C Passaquin; W.J Leijendekker; C Challet; T Wallimann; U.T Rüegg; Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Letters 1998, 439, 357-362, 10.1016/s0014-5793(98)01399-4.
- Farshidfar, F.; Pinder, M.A.; Myrie, S.B. Creatine Supplementation and Skeletal Muscle Metabolism for Building Muscle Mass-Review of the Potential Mechanisms of Action. Curr. Protein Pept. Sci. 2017, 18, 1273–1287.
- Parise, G.; Mihic, S.; MacLennan, D.; Yarasheski, K.E.; Tarnopolsky, M.A. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J. Appl. Physiol. 2001, 91, 1041–1047.
- Olsen, S.; Aagaard, P.; Kadi, F.; Tufekovic, G.; Verney, J.; Olesen, J.L.; Suetta, C.; Kjaer, M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J. Physiol. 2006, 573, 525–534.
- M.A. Tarnopolsky; G. Parise; Direct measurement of high‐energy phosphate compounds in patients with neuromuscular disease. Muscle & Nerve 1999, 22, 1228-1233, 10.1002/(sici)1097-4598(199909)22:9<1228::aid-mus9>3.3.co;2-y.
- Mark Tarnopolsky; Joan Martin; Creatine monohydrate increases strength in patients with neuromuscular disease.. Neurology 1999, 52, 854-854, 10.1212/wnl.52.4.854.
- M. A. Tarnopolsky; D. J. Mahoney; J. Vajsar; C. Rodriguez; T. J. Doherty; B. D. Roy; D. Biggar; Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy.. Neurology 2004, 62, 1771-1777, 10.1212/01.wnl.0000125178.18862.9d.
- Maggie C. Walter; Hanns Lochmuller; P. Reilich; T. Klopstock; R. Huber; M. Hartard; Michael Hennig; D. Pongratz; Wolfgang Müller-Felber; Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study.. Neurology 2000, 54, 1848-1850, 10.1212/wnl.54.9.1848.
- Magali Louis; Jean Lebacq; Jacques R. Poortmans; Marie-Claude Belpaire-Dethiou; Jean-Pierre Devogelaer; Paul Van Hecke; Francis Goubel; Marc Francaux; Beneficial effects of creatine supplementation in dystrophic patients. Muscle & Nerve 2003, 27, 604-610, 10.1002/mus.10355.
- Hyder, F.; Rothman, D.L.; Bennett, M.R. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc. Natl. Acad. Sci. USA 2013, 110, 3549–3554.
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216.
- Hemmer, W.; Wallimann, T. Functional aspects of creatine kinase in brain. Dev. Neurosci. 1993, 15, 249–260.
- Béard, E.; Braissant, O. Synthesis and transport of creatine in the CNS: Importance for cerebral functions. J. Neurochem. 2010, 115, 297–313.
- National Organization of Rare Disorders Creatine Transporter Deficiency. Available online: https://rarediseases.org/rare-diseases/creatine-transporter-deficiency/ (accessed on 27 August 2020).
- Caroline Rae; Alison L. Digney; Sally R. McEwan; Timothy C. Bates; Oral creatine monohydrate supplementation improves brain performance: a double–blind, placebo–controlled, cross–over trial. Proceedings of the Royal Society B: Biological Sciences 2003, 270, 2147-2150, 10.1098/rspb.2003.2492.
- McMorris, T.; Harris, R.C.; Swain, J.; Corbett, J.; Collard, K.; Dyson, R.J.; Dye, L.; Hodgson, C.; Draper, N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology 2006, 185, 93–103.
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362.
- Francis L. Pazini; Mauricio P. Cunha; Ana Lúcia S. Rodrigues; The possible beneficial effects of creatine for the management of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2019, 89, 193-206, 10.1016/j.pnpbp.2018.08.029.
- Matthews, R.T.; Ferrante, R.J.; Klivenyi, P.; Yang, L.; Klein, A.M.; Mueller, G.; Kaddurah-Daouk, R.; Beal, M.F. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999, 157, 142–149.
- Andres, R.H.; Ducray, A.D.; Perez-Bouza, A.; Schlattner, U.; Huber, A.W.; Krebs, S.H.; Seiler, R.W.; Wallimann, T.; Widmer, H.R. Creatine supplementation improves dopaminergic cell survival and protects against MPP+ toxicity in an organotypic tissue culture system. Cell Transpl. 2005, 14, 537–550.
- Brewer, G.J.; Wallimann, T.W. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 2000, 74, 1968–1978
- Li, Z.; Wang, P.; Yu, Z.; Cong, Y.; Sun, H.; Zhang, J.; Zhang, J.; Sun, C.; Zhang, Y.; Ju, X. The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson′s disease. Eur. Neurol. 2015, 73, 205–211.
- Bender, A.; Koch, W.; Elstner, M.; Schombacher, Y.; Bender, J.; Moeschl, M.; Gekeler, F.; Müller-Myhsok, B.; Gasser, T.; Tatsch, K.; et al. Creatine supplementation in Parkinson disease: A placebo-controlled randomized pilot trial. Neurology 2006, 67, 1262–1264.
- Hersch, S.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2′ dG. Neurology 2006, 66, 250–252.
- Investigators, N.N.-P. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 2006, 66, 664–671.
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593.
- Verbessem, P.; Lemiere, J.; Eijnde, B.O.; Swinnen, S.; Vanhees, L.; Van Leemputte, M.; Hespel, P.; Dom, R. Creatine supplementation in Huntington′s disease: A placebo-controlled pilot trial. Neurology 2003, 61, 925–930.
- Robert J. Ferrante; Ole A. Andreassen; Bruce G. Jenkins; Alpaslan Dedeoglu; Stefan Kuemmerle; James K. Kubilus; Rima Kaddurah-Daouk; Steven M. Hersch; M F Beal; Neuroprotective Effects of Creatine in a Transgenic Mouse Model of Huntington's Disease. The Journal of Neuroscience 2000, 20, 4389-4397, 10.1523/JNEUROSCI.20-12-04389.2000.
- Bruno Gualano; Vitor De Salles Painelli; Hamilton Roschel; Rebeca Lugaresi; Egidio Dorea; Guilherme Giannini Artioli; Fernanda Rodrigues Lima; Maria Elizabeth Rossi Da Silva; Maria Rosária Cunha; Antonio Carlos Seguro; et al.Maria Heloisa ShimizuMaria Concepción García OtaduyMarcelo Tatit SapienzaCláudia Da Costa LeiteEloisa BonfáAntonio Herbert Lancha Junior Creatine supplementation does not impair kidney function in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2010, 111, 749-756, 10.1007/s00421-010-1676-3.
- Christiano R. R. Alves; Bianca M. Santiago; Fernanda R. Lima; Maria C. G. Otaduy; Ana Luisa Calich; Aline C. C. Tritto; Ana Lúcia De Sá Pinto; Hamilton Roschel; Cláudia C. Leite; Fabiana B. Benatti; et al.Eloisa BonfáBruno Gualano Creatine Supplementation in Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care & Research 2013, 65, 1449-1459, 10.1002/acr.22020.
- Holger Lenz; Melanie Schmidt; Vivienne Welge; Uwe Schlattner; Theo Wallimann; Hans-Peter Elsässer; Klaus-Peter Wittern; Horst Wenck; Franz Stäb; Thomas Blatt; et al. The Creatine Kinase System in Human Skin: Protective Effects of Creatine Against Oxidative and UV Damage In Vitro and In Vivo. Journal of Investigative Dermatology 2005, 124, 443-452, 10.1111/j.0022-202x.2004.23522.x.
- Philip D. Chilibeck; Darren G. Candow; Tim Landeryou; Mojtaba Kaviani; Lisa Paus-Jenssen; Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Medicine & Science in Sports & Exercise 2015, 47, 1587-1595, 10.1249/mss.0000000000000571.
- Robin P. Da Silva; Karen B. Kelly; Kelly-Ann Leonard; René L. Jacobs; Creatine reduces hepatic TG accumulation in hepatocytes by stimulating fatty acid oxidation. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2014, 1841, 1639-1646, 10.1016/j.bbalip.2014.09.001.
- R Rahimi; Creatine Supplementation Decreases Oxidative DNA Damage and Lipid Peroxidation Induced by a Single Bout of Resistance Exercise. Journal of Strength and Conditioning Research 2011, 25, 3448-3455, 10.1519/jsc.0b013e3182162f2b.
- Chiara Guidi; Lucia Potenza; Piero Sestili; Chiara Martinelli; Michele Guescini; Laura Stocchi; Sabrina Zeppa; Emanuela Polidori; Giosuè Annibalini; Vilberto Stocchi; et al. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochimica et Biophysica Acta (BBA) - General Subjects 2008, 1780, 16-26, 10.1016/j.bbagen.2007.09.018.
- Sherry L Murphy; Jiaquan Xu; Kenneth D Kochanek; Elizabeth Arias; Mortality in the United States, 2017.. NCHS data brief 2018, 328, 1-8.
- Donna Tippett; Mozaffarian D; Go As; Arnett Dk; Blaha Mj; Cushman M; Das Sr; De Ferranti S; Després JP; Fullerton Hj; et al.Howard VjHuffmanIsasi CrJiménez McJudd SeKissela BmLichtman JhLisabeth LdMackey RhMagid DjMohler ErMoy CsMuntner PMussolino MeNasir KNeumar RwNichol GPalaniappan LRodriguez CjRosamond WSorlie PdStein JTowfighi ATuran TnVirani SsTurner MbStroke Statistics SubcommitteeBenjamin EjLiu SMcGuire DkPandey DkReeves MjWoo DYeh RwAmerican Heart Association Statistics CommitteeWriting Group Members Faculty Opinions recommendation of Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association.. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2017, 133, e38–e360, 10.3410/f.726023516.793533696.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375.
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pr. 2014, 2014, 291–308.
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening: Causes and consequences. Front. Genet. 2015, 6, 112
