Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Miura Kenji and Version 3 by Catherine Yang.
Heat stress (HS) is a prevalent negative factor affecting plant growth and development, as it is predominant worldwide and threatens agriculture on a large scale. PHYTOCHROMES (PHYs) are photoreceptors that control plant growth and development, and the stress signaling response partially interferes with their activity.
- tomato
- PHYTOCHROME A
- PHYTOCHROME B1B2
1. Introduction
Abiotic stress caused by environmental changes negatively affects plant growth and development [1]. Global warming has greatly impacted agriculture [2], and in many areas of the world, heat stress (HS) is one of the most crucial threats to plant growth and development, as it leads to a severe reduction in economic yield by causing morpho-anatomical, physiological, and biochemical changes in plants [3]. From 2050 to 2100, HS will likely negatively affect tomato growth and productivity in the open field and decrease the optimal areas for cultivation [4]. An approach to mitigate the adverse effects of HS is to develop crop plants with improved plant stress tolerance using various genetic methods [3].
Tomato (Solanum lycopersicum L.) is an economically important crop worldwide that is sensitive to a series of abiotic stresses, particularly extreme temperatures [5]. The optimum temperature for tomato growth, fruit set, and yield ranges between 21 and 29.5 °C during the day and between 18.5 and 21 °C during the night [6].
PHYTOCHROMES (PHYs), which absorb red and far-red light, are the most characterized photoreceptors in plants [7]. Plant growth and development (from seed germination to flowering) can be controlled by PHYs. PHYs regulate both biotic stress and stress induced by abiotic factors, such as high and low temperatures, salinity, drought, toxic metals, ultraviolet B radiation, and herbivory [8], by changing a wide range of biochemical and molecular responses [7]. The number and types of PHYs vary among plant species. Tomato and Arabidopsis contain five PHYs in their genomes. Tomato plants have PHYA, PHYB1, PHYB2, PHYE, and PHYF [9], whereas Arabidopsis contains PHYA–PHYE [10]. In rice, plants have only three PHYs, PHYA–PHYC [11]. Bioinformatics analysis of microarrays has clarified the performance of Arabidopsis PHYA and PHYB photoreceptors in plant HS responses [12] and revealed that PHYB also functions as a photoreceptor and temperature sensor in Arabidopsis plants [13].
2. Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress
Tomato phyA and phyB1B2 exhibited tolerance to HS during seed germination and the vegetative growth phase and for a short time of HS during the flowering stage. A long period of HS during the flowering or fruiting stage returned a response similar to that of the WT, which showed a sensitive response under all growth phases. For the seed germination stage, both phy mutants showed nonsignificant differences in seed germination rate under normal and HS conditions, whereas the WT germination rate was negatively affected by HS, which indicates that both phy mutants can grow in hot seasons. During the vegetative growth stage, phyA and
Tomato phyA and phyB1B2 exhibited tolerance to HS during seed germination and the vegetative growth phase and for a short time of HS during the flowering stage. A long period of HS during the flowering or fruiting stage returned a response similar to that of the WT, which showed a sensitive response under all growth phases.
For the seed germination stage, both phy mutants showed nonsignificant differences in seed germination rate under normal and HS conditions, whereas the WT germination rate was negatively affected by HS, which indicates that both phy mutants can grow in hot seasons.
During the vegetative growth stage, phyA and phyB1B2 exhibited high tolerance to HS by enhancing the plant mechanism to cope with high-temperature stress. Inducing damage to plasma membranes in plants is an important side effect of HS [70]. Tomato phyA and phyB1B2 exhibited lower electrolyte leakage compared to WT under HS, even though the tomato ‘Moneymaker’ is classified as having moderate heat tolerance [71], indicating that tomato phyA and phyB1B2 phyB1B2are highly tolerant mutants of HS that enhance membrane thermostability. Increased leaf temperature is a result of HS, which in turn inhibits the activity of enzymatic antioxidants and considerably increases the malondialdehyde (MDA) level in the leaves [72,73]. Tomato phyA inhibited the production of MDA under HS, which could be an indicator of the inhibition of polyunsaturated fatty acid peroxidation in the cells and the reduction in oxidative damage to membrane lipids, which then enhance heat tolerance. The accumulation of osmoprotectants is an adaptive mechanism in plants against environmental stresses such as heat tolerance [74] and increases plant survival by protecting the cellular structure [75]. Under high temperatures, plants accumulate different osmolytes, such as proline [76]. Tomato phyA
exhibited high tolerance to HS by enhancing the plant mechanism to cope with high-temperature stress. Inducing damage to plasma membranes in plants is an important side effect of HS [14]. Tomato phyA and phyB1B2 exhibited lower electrolyte leakage compared to WT under HS, even though the tomato ‘Moneymaker’ is classified as having moderate heat tolerance [15], indicating that tomato phyA and phyB1B2 are highly tolerant mutants of HS that enhance membrane thermostability. Increased leaf temperature is a result of HS, which in turn inhibits the activity of enzymatic antioxidants and considerably increases the malondialdehyde (MDA) level in the leaves [16][17]. Tomato phyA inhibited the production of MDA under HS, which could be an indicator of the inhibition of polyunsaturated fatty acid peroxidation in the cells and the reduction in oxidative damage to membrane lipids, which then enhance heat tolerance. The accumulation of osmoprotectants is an adaptive mechanism in plants against environmental stresses such as heat tolerance [18] and increases plant survival by protecting the cellular structure [19]. Under high temperatures, plants accumulate different osmolytes, such as proline [20]. Tomato phyA enhanced proline accumulation under HS conditions via enhancing the expression of P5CS and P5CR genes that control proline biosynthesis, suggesting that proline accumulation is involved in the heat tolerance of phyA .
In addition, under the vegetative growth stage, both phy mutants exhibited a reduced number of stomata compared to the WT under HS, and phyA
enhanced proline accumulation under HS conditions via enhancing the expression of P5CS and P5CR genes that control proline biosynthesis, suggesting that proline accumulation is involved in the heat tolerance of phyA .
In addition, under the vegetative growth stage, both phy mutants exhibited a reduced number of stomata compared to the WT under HS, and phyA
exhibited a decrease in stomatal aperture. In Arabidopsis, spch
exhibited a decrease in stomatal aperture. In Arabidopsis, spch mutant did not produce stomata or lineages [64]. Tomato phyA
and phyB1B2
and phyB1B2
mutants showed a downregulation in the expression level of SPCH gene compared to WT plants (Figure S6A), which may be one of the reasons for the stomata number reduction in phy
mutants showed a downregulation in the expression level of SPCH gene compared to WT plants, which may be one of the reasons for the stomata number reduction in phy mutants. It is plausible that water preservation also enhanced the heat tolerance of phyA
mutants. It is plausible that water preservation also enhanced the heat tolerance of phyA
and
and phyB1B2. Decreased stomatal closure and accelerated water loss speed enhanced HS sensitivity in transgenic OsMDHAR4-overexpressing rice [77]. Moreover, ABA is a key molecule that activates plant reactions to stress conditions, such as HS. It induces the accumulation of different proteins involved in stress acclimation and regulates stomatal closure under HS conditions [78].
Furthermore, the basic network among HSFA1a, HSFA2, and HSFB1 activity controls the HS response in tomatoes [79]. In addition, HSP accumulation protects the cell system from HS and enhances several functions and mechanisms to cope with HS [80]. In tomato
phyB1B2. Decreased stomatal closure and accelerated water loss speed enhanced HS sensitivity in transgenic OsMDHAR4-overexpressing rice [22]. Moreover, ABA is a key molecule that activates plant reactions to stress conditions, such as HS. It induces the accumulation of different proteins involved in stress acclimation and regulates stomatal closure under HS conditions [23].
Furthermore, the basic network among HSFA1a, HSFA2, and HSFB1 activity controls the HS response in tomatoes [24]. In addition, HSP accumulation protects the cell system from HS and enhances several functions and mechanisms to cope with HS [25]. In tomato phyB1B2, the upregulation of
, the upregulation of
HSFA2
,
HSFB1
,
HSFA4a, and HSP90 expression levels enhanced thermotolerance. The results showed that the expression levels of HSFA2 and HSFB1 were upregulated in phyA. HSFA2 is an ideal transcriptional activator during the HS response [26]. Meanwhile, HSFA4a is involved in the regulation of abiotic stress tolerance, such as salt tolerance in Arabidopsis [27] and cadmium tolerance in wheat and rice [28]. GRP is involved in the defense system of plants under biotic and abiotic stresses and has a high glycine content [29]. Tomato phyA showed a high expression level of GRP under HS, which might play a role in heat tolerance.
HS negatively affects pollen development and fertility, which reduces fruit setting [30]. Over a short period (less than 14 days) under fluctuating high temperature in the GH, phyA exhibited a significantly enhanced percentage of developed flowers compared to WT and phyB1B2. However, phyB1B2 produced fertile pollen for a longer time under HS, followed by phyA, in comparison with WT pollen, which quickly reacted to high temperatures. Stigma exertion and antheridia cone splitting are floral characteristics used to evaluate HS tolerance [31]. PhyA achieved a significantly lower percentage of stigma-exerted and abnormal flowers compared to WT within 2 weeks under fluctuating high temperature; at the same time, no marked difference occurred when compared to phyB1B2. Although phyA showed heat tolerance by floral characteristics during the early exposure period to HS, its abnormal floral structure was enhanced by long HS application during the flowering phase. In tomato, the homozygous mutation in TAP3 showed a classic B-class gene loss-of-function phenotype, which formed a complete transformation of the petals into sepaloid structures and the stamens into carpel-like organs [32]. This might explain the conversion in the flower structure of phyA due to the downregulation of TAP3 by showing a lower expression level compared to WT and phyB1B2 after exposure to HS for 35 days. The long sepals were confirmed by the results of fruit calyx, which significantly increased in phyA compared to WT and phyB1B2. In addition, in phyA, the enhanced heat-responsive genes and GRP that were upregulated during the vegetative growth stage did not show a marked difference compared to WT under long periods of HS during the flowering stage. The
, and HSP90 expression levels enhanced thermotolerance. The results showed that the expression levels of HSFA2 and HSFB1 were upregulated in phyA. HSFA2 is an ideal transcriptional activator during the HS response [81]. Meanwhile, HSFA4a is involved in the regulation of abiotic stress tolerance, such as salt tolerance in Arabidopsis [82] and cadmium tolerance in wheat and rice [83]. GRP is involved in the defense system of plants under biotic and abiotic stresses and has a high glycine content [84]. Tomato phyA showed a high expression level of GRP under HS, which might play a role in heat tolerance.
HS negatively affects pollen development and fertility, which reduces fruit setting [85]. Over a short period (less than 14 days) under fluctuating high temperature in the GH, phyA exhibited a significantly enhanced percentage of developed flowers compared to WT and phyB1B2. However, phyB1B2 produced fertile pollen for a longer time under HS, followed by phyA, in comparison with WT pollen, which quickly reacted to high temperatures. Stigma exertion and antheridia cone splitting are floral characteristics used to evaluate HS tolerance [45]. PhyA achieved a significantly lower percentage of stigma-exerted and abnormal flowers compared to WT within 2 weeks under fluctuating high temperature; at the same time, no marked difference occurred when compared to phyB1B2. Although phyA showed heat tolerance by floral characteristics during the early exposure period to HS, its abnormal floral structure was enhanced by long HS application during the flowering phase. In tomato, the homozygous mutation in TAP3 showed a classic B-class gene loss-of-function phenotype, which formed a complete transformation of the petals into sepaloid structures and the stamens into carpel-like organs [69]. This might explain the conversion in the flower structure of phyA due to the downregulation of TAP3 by showing a lower expression level compared to WT and phyB1B2 after exposure to HS for 35 days. The long sepals were confirmed by the results of fruit calyx, which significantly increased in phyA compared to WT and phyB1B2. In addition, in phyA, the enhanced heat-responsive genes and GRP that were upregulated during the vegetative growth stage did not show a marked difference compared to WT under long periods of HS during the flowering stage. The phyB1B2 showed a nonsignificant variance in the expression level of all studied heat-responsive genes during the long exposure to HS during the flowering stage). These results confirm that phyA and phyB1B2 did not enhance HS tolerance during the flowering stage when exposed to a long period of high-temperature stress, as did the WT.
In tomatoes, small parthenocarpic fruit is one of the side effects of high temperature, which leads to a decrease in yield [86]. All observed fruit in WT plants and phy mutants were parthenocarpic, and the average FW of the fruits was low, indicating that phyA and phyB1B2 phyB1B2
showed a nonsignificant variance in the expression level of all studied heat-responsive genes during the long exposure to HS during the flowering stage). These results confirm that phyA
did not enhance HS tolerance during the fruiting stage as observed with WT.
Taken together, tomato phy
and phyB1B2 did not enhance HS tolerance during the flowering stage when exposed to a long period of high-temperature stress, as did the WT.
In tomatoes, small parthenocarpic fruit is one of the side effects of high temperature, which leads to a decrease in yield [33]. All observed fruit in WT plants and phy mutants were parthenocarpic, and the average FW of the fruits was low, indicating that phyA and phyB1B2 did not enhance HS tolerance during the fruiting stage as observed with WT.
Taken together, tomato phy
mutants have a different response to high-temperature stress, as determined by the growth stage. The vegetative growth stage had the greatest growth, showing a high HS tolerance. Therefore, tomato phyA
mutants have a different response to high-temperature stress, as determined by the growth stage. The vegetative growth stage had the greatest growth, showing a high HS tolerance. Therefore, tomato phyA is a thermotolerant mutant that enhances membrane stability, proline accumulation, and water preservation by reducing stomata number and stomatal aperture and upregulating the expression levels of
is a thermotolerant mutant that enhances membrane stability, proline accumulation, and water preservation by reducing stomata number and stomatal aperture and upregulating the expression levels of
HSFA2
,
HSFB1
, and GRP. In addition, it inhibited MDA accumulation in the leaves (
Figure 1A). Tomato phyB1B2 is a heat-tolerant mutant that enhances membrane stability and expression levels of
0A). Tomato phyB1B2 is a heat-tolerant mutant that enhances membrane stability and expression levels of
HSFA2
,
HSFB1
,
HSFA4a
, and HSP90. In addition, it inhibited water loss by decreasing the number of stomata (
Figure 1B).
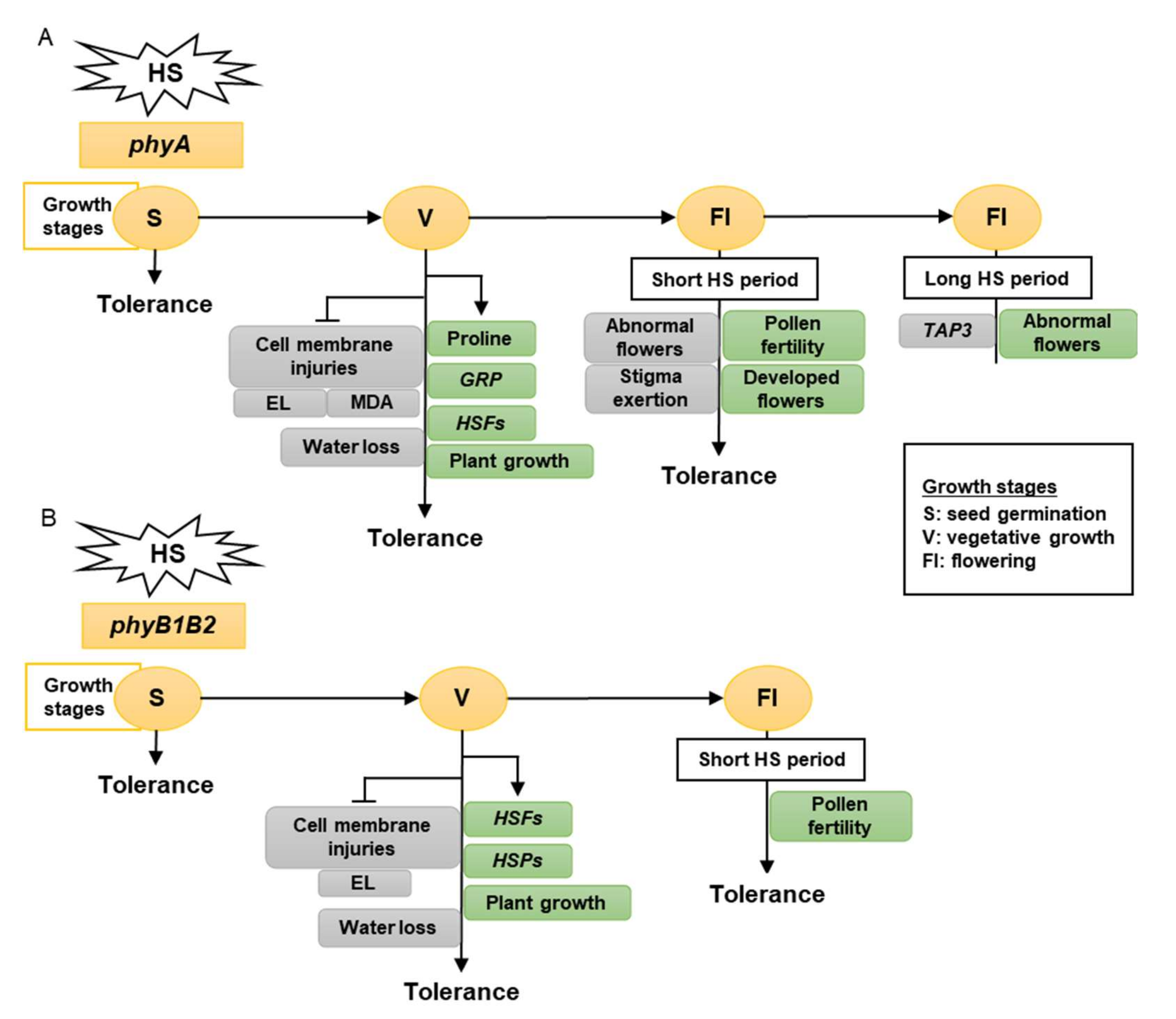

0B).
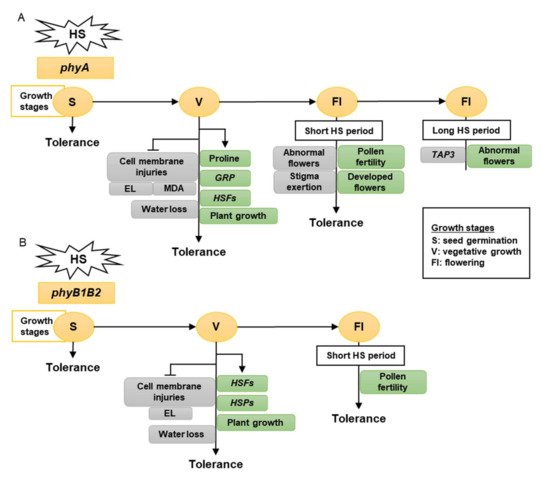

Figure 10.
The model shows the response of phyA (
A
) and phyB1B2 (
B) mutations at different growth stages under heat stress (HS). The gray rectangles show the factors inhibited while the green rectangles illustrate the factors enhanced by stress application. The growth stages that were shortened in S, V, and Fl were the seed germination, vegetative growth, and flowering stages, respectively. The phy mutants exhibited tolerance to HS during S, V, and Fl stages. In S stage, the seed germination rates of both phy mutants were not significantly affected by HS compared to control conditions (A, B). In V stage, phyA enhanced the proline production, HSFs,
) mutations at different growth stages under heat stress (HS). The gray rectangles show the factors inhibited while the green rectangles illustrate the factors enhanced by stress application. The growth stages that were shortened in S, V, and Fl were the seed germination, vegetative growth, and flowering stages, respectively. The phy mutants exhibited tolerance to HS during S, V, and Fl stages. In S stage, the seed germination rates of both phy mutants were not significantly affected by HS compared to control conditions (A, B). In V stage, phyA enhanced the proline production, HSFs, GRP, and plant growth (A), while phyB1B2 enhanced the upregulation of HSFs GRP
, and plant growth (A), while phyB1B2 enhanced the upregulation of HSFs and
and HSPs HSPsas well as plant growth (B). In addition, both phy
as well as plant growth (B). In addition, both phy
mutants inhibited cell membrane injuries (A, B). In Fl stage, phy
mutants inhibited cell membrane injuries (A, B). In Fl stage, phy mutants enhanced pollen fertility for a longer time compared to WT (A, B). Moreover, phyA exhibited an increase in the percentage of developed flowers and an inhibition in the percentage of abnormal and stigma-exerted flowers compared to WT. During a long HS application in the Fl stage,
mutants enhanced pollen fertility for a longer time compared to WT (A, B). Moreover, phyA phyA
exhibited enhanced abnormal flower formation via downregulation of TAP3 which enhanced sepal and petal conversion (A).
Finally, using plant genome editing on PHYA or B genes in other plant crops could be a good method of enhancing crop tolerance to HS. The genome editing method is a powerful technique for producing varieties; thus, several genome-edited plants are currently being produced [34][35]. Mutations in HSFA6a and HSFA6b, created by CRISPR/Cas9, are more tolerant to abiotic stresses such as ABA, mannitol, and sodium chloride [36]. Researchers have developed several mutants, such as the tomato ead1 mutant and soybean hsp90A2 mutant, using CRISPR/Cas9. The tomato ead1 mutant exhibited short roots and increased sensitivity to ABA [37]. The soybean hsp90A2
exhibited an increase in the percentage of developed flowers and an inhibition in the percentage of abnormal and stigma-exerted flowers compared to WT. During a long HS application in the Fl stage, phyA
mutant is sensitive to HS, has decreased chlorophyll content, and has high levels of lipid peroxidation [38]. Generally, genome editing techniques introduce mutations in the targeted genes. Mutations in negative regulators enhance tolerance to abiotic stress. Thus,
exhibited enhanced abnormal flower formation via downregulation of TAP3 PHYwhich enhanced sepal and petal conversion (A).
and PHY
or
B1B2 are good target genes for enhancing abiotic stress tolerance.
genes in other plant crops could be a good method of enhancing crop tolerance to HS. The genome editing method is a powerful technique for producing varieties; thus, several genome-edited plants are currently being produced [87,88]. Mutations in HSFA6a and HSFA6b, created by CRISPR/Cas9, are more tolerant to abiotic stresses such as ABA, mannitol, and sodium chloride [89]. Researchers have developed several mutants, such as the tomato ead1 mutant and soybean hsp90A2 mutant, using CRISPR/Cas9. The tomato ead1 mutant exhibited short roots and increased sensitivity to ABA [90]. The soybean hsp90A2 mutant is sensitive to HS, has decreased chlorophyll content, and has high levels of lipid peroxidation [91]. Generally, genome editing techniques introduce mutations in the targeted genes. Mutations in negative regulators enhance tolerance to abiotic stress. Thus, PHYA and PHYB1B2 are good target genes for enhancing abiotic stress tolerance.
