Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Amina Yu and Version 2 by Amina Yu.
Surface functionalization of nanoparticles applies to the use of covalent and non-covalent bonds—such as hydrogen bonds, electrostatic force, and the van der Waals interactions—to integrate diverse organic and inorganic molecules at the nanoscale. Typically, multiple linker molecules are used to form covalent bonds between ligands and the surfaces of nanoparticles (NPs).
- nanomaterial
- bio-fabrication
- magnetic nanoparticles
- nanomedicine
- nanocarrier
1. Nanoparticles and Surface Functionalization
Recently, the level of nanoparticles (NPs) has been considered the most advanced, whether in scientific research or in commercial applications. A nanoparticle size is significant in its physiological and biochemical properties and diverse formats such as fullerenes, metal NPs, ceramic NPs, and polymeric NPs. Their nanoscale size and high surface area give NPs unique physical and chemical properties. NPs’ shape and structure affect their reactivity and toughness, their size affects other properties as well [1]. Surface recognition by NPs provides a possible tool to control the cellular and extracellular processes for various biological applications such as enzymatic inhibition, delivery, sensing, and transcription regulation (Figure 1). Depending on the core material, NP cores can be altered from more minor to larger sizes, giving an appropriate platform for the NP interactions with proteins and other biomolecules [2].
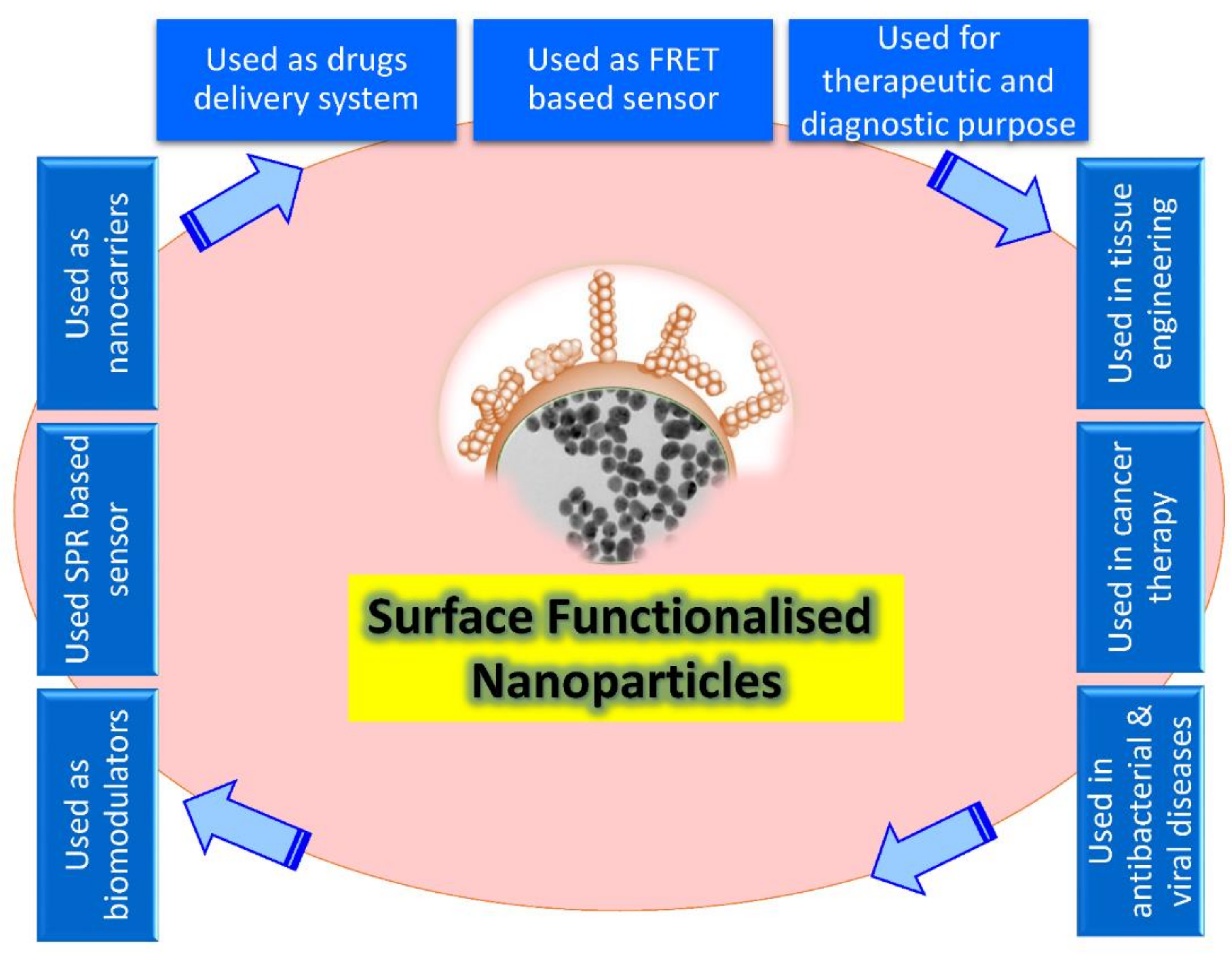
Figure 1. Schematic illustration showing the broad array of bio-applications of surface-functionalized nanoparticles.
Fabrication could be defined as the creation of complicated biological goods utilizing living tissue, molecules, extracellular arrays, and designed biomaterials based on these variables. However, this limits fabricated goods to living organs and tissues [3], but still suggests that natural resources have to include living or bio-inspired content, constituent procedures should be biology-based, and byproducts must be complex living cells [4]. On the contrary, according to a more comprehensive definition, bio fabrication includes a diverse set of physical, chemical, biological, and/or technological aspects with a variety of applications in biological sciences, including environmental and bioprocess monitoring, food quality control, agriculture, bioterrorism, and medical biosensor systems (Figure 1).
1.1. Interactions Involved in Surface Functionalization
Surface functionalization of nanoparticles applies to the use of covalent and non-covalent bonds—such as hydrogen bonds, electrostatic force, and the van der Waals interactions—to integrate diverse organic and inorganic molecules at the nanoscale [5]. Typically, multiple linker molecules are used to form covalent bonds between ligands and the surfaces of NPs. Pagels et al. [6] reported that PEG can be produced with specified functional groups at the terminals and employed as heterobifunctional linkers to execute a variety of functionalization activities. Non-covalent interactions have the advantage of simplicity and not influencing the structure of the molecules utilized or their interactions with target biological materials. However, the different factors—such as pH and ionic strength—can easily influence non-covalent changes [7]. Different types of nanostructures have distinct chemical characteristics and functional groups coated on their surfaces that can be employed in the early stages of surface functionalization. In general, the very first step of surface modification involves the use of homo- or hetero-bifunctional cross linkers with the goal of adding an organic functional group (R–NH2, RCOOH) and many more for binding biological molecules. There are three major aspects behind the surface functionalization of nanoparticles, including: firstly, the stabilization of nanoparticles against agglomeration and oxidation processes [8][9]; secondly, to make them compatible with a subsequent phase, i.e., when suitable ligands are linked to metal particles, they can be made water soluble [10]; thirdly, to help them organize themselves [11]. The surface alteration enhances the mechanical characteristics of the nanocomposite by avoiding homogeneity and compatibility issues between the two phases [12].
1.2. General Protocols and Material Required for Surface Functionalization
As it was known the size, shape, morphology, and dispersibility of nanoparticles can all have a significant impact on their biological applications. As a result, scientists are concentrating their efforts on surface modification of nanoparticles using various approaches to regulate their size, shape, and morphology while retaining variable and beneficial features. A number of synthesis approaches have been explored and reported to prepare the magnetic nanostructures such as chemical vapor deposition [13], flash nanoprecipitation [14], hydrothermal [15], thermal decomposition [16], electrochemical deposition [17], laser pyrolysis [18], microemulsion [19], solvothermal methods [20], sonochemical methods [21], the microwave-assisted method [22], aerosol pyrolysis [23], and UV aerosol synthesis [24] (Figure 2).
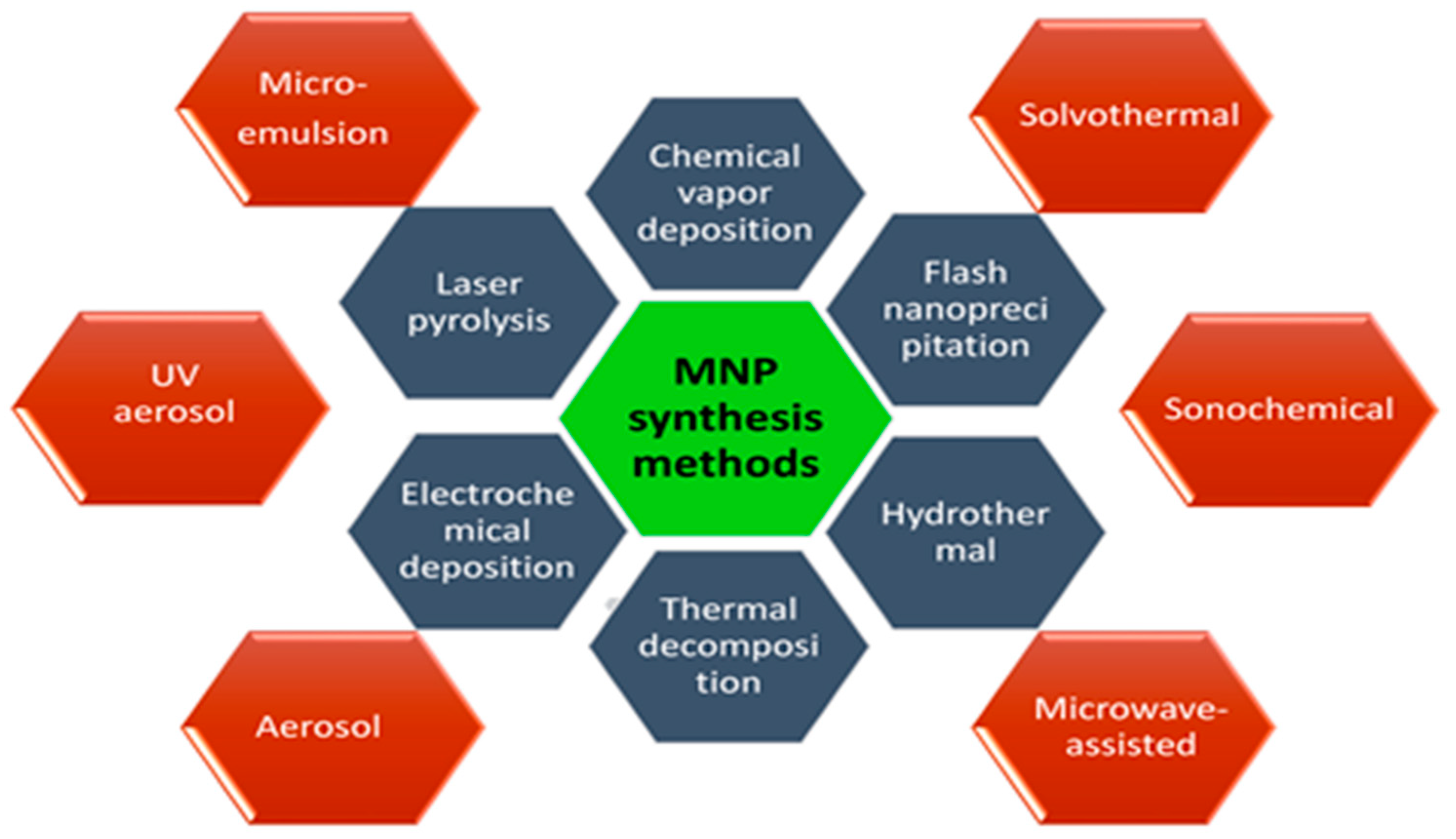
Figure 2. General methods of surface functionalization of magnetic nanoparticles (MNPs).
2. Mechanism of Surface Functionalization of NPs
Bioorganic and bioinorganic chemistry also provides a basis to join biotechnology with the advanced materials science. For example, bioorganic model systems give tools for probing the mechanisms of biological principles and developing chemical methods to manage natural components. Organic ligands and inorganic nanoparticle surface interaction facilitates coupling the biomolecular recognition systems in order to create novel materials (Figure 2). The formed multilayered laminate properties may be controlled in several ways, involving changing the adsorbing substances in the dipping solutions. The driving force for substance adsorption also depends on the nature of both the surface and adsorbing substance. Therefore, organic and inorganic compounds both significantly play a key role in the stabilization of newly synthesized nanoparticles through surface functionalization.
2.1. Surface Modification with Organic Molecules
Because of their simple structure, organic molecular agents can only be used in one way (ligand exchange or ligand adsorption) and monodentate ligands are commonly employed in ligand exchanges due to their ease of synthesis, simple structure, and other benefits. Recently, the organic materials have included citrates, phosphates, amines, thiols and various polymers (chitosan, dextran, PEG, polyvinyl alcohol (PVA), poly(lactic-co-glycolic acid) (PLGA), alginate, ployacrylic acid, pollunan and so on). Polymer-coated IONPs have received much attention in the past few years because of their variety of uses in numerous research fields, including nanomedicine. Two typical methods for making polymer coated IONPs are in situ and post-annealing coating [25]. Ali et al. [26] used an emulsion polymerization process to create Janus-like MNPs by modifying poly(methylmethacrylate-acrylic acid-divinylbenzene). The synthesized Janus-like MNPs ranged in size from 200 to 250 nm in diameter. The main advantage of MNPs with small molecular coatings is that large hydrodynamic sizes (>50 nm) may be addressed. MNPs can be successfully fabricated with specific groups—such as –COOH, –NH2, –OH, and –SH—which can be further manipulated with various bioactive molecule connections. Dheyab et al. recently used a quick and simple one-step co-participation technique to successfully construct highly magnetic and 19 nm-sized citric-acid-functionalized MNPs. The results showed a magnetic saturation of 54.8 emug−1 [27]. Chitosan is a nontoxic, alkaline, biocompatible, biodegradable, and hydrophilic polymer. To make a ferrofluid, Lee et al. [28] produced spherical MNPs and implanted them in chitosan. They discovered in vitro that MNP-chitosan nanoparticles enhanced MR image contrast significantly as compared to ferrofluids.
2.2. Surface Modification with Inorganic Molecules
Inorganic molecules consisting of silica, metals, and metal oxides [18][29][30][31][32] are in use. Magnetic nanoparticles (MNPs) surfaces modified with organic molecules have strong biocompatibility and biodegradability, in addition to the basic magnetic properties. For example, various organic compounds—including oleic acid, 1-octadecene, 1-tetradecene, and oleylamine—are typically added to the chemical reaction as stabilizers to obtain narrow size distribution iron oxide nanoparticles (IONPs). The well-known Stöber method is the most popular method for producing MNP@SiO2 nanomaterials [33]. In situ, SiO2 is produced through hydrolysis and condensation of a sol–gel precursor. The Stöber method is a versatile technique that can be utilized in both aqueous and organic conditions.
Surface coating of IONPs with metallic elements as an inorganic compound is also employed to improve their stability, biocompatibility, and disperstivity. The noble metal gold is the most widely utilized for surface coating. Generally, there are two ways to get a gold-shell coating on the surface of magnetic IONPs: direct and indirect [34]. When gold is covered with a strong sulfur conjugation, it delivers tremendous benefits. The chemical inertness of gold allows gold-coated MNPs to be perfectly stable. Wang et al. [35] used gold precursors to reduce iron oxide nanoparticles of various sizes as seeds to synthesize gold-coated iron oxide nanoparticles.
2.3. Stabilization of Nanoparticles
The stabilizing slows the nucleation activity and influences the adsorption of modifiers on nuclei and developing nanocrystals, potentially inhibiting IONP growth and favoring the development of micro IONPs. When compared to naked MNPs, nanocomposites functionalized with inorganic substances can have significantly improved antioxidant activities. The inorganic molecules—including silica, metals, metal oxides, nonmetals, and sulfides—are mainly exposed to atom surfaces. Surface modification of MNPs with silica (SiO2), gold (Au), and silver (Ag) is prevalent, producing core–shell patterns and ensuring nanoparticle integrity in solution. They also aid in the binding of numerous biological substances and medicines to the MNPs’ surface via appropriate functional moieties [36][37][38][39] (Figure 3).
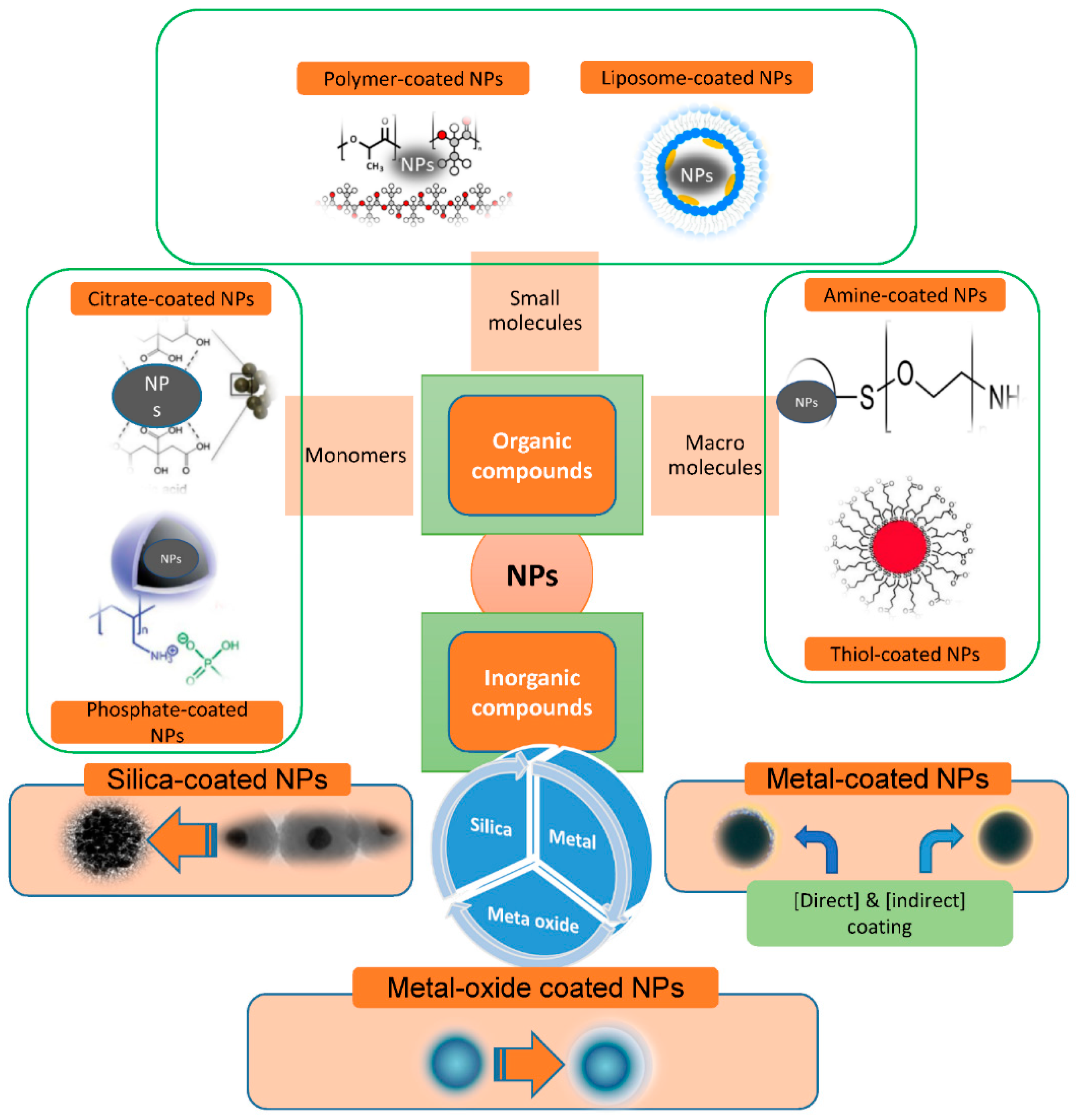
Figure 3. Scheme illustration for materials commonly used for nanoparticle (NP) functionalization.
The surface functionalization of nanoparticles can be achieved through two modes: (i) In situ modification, a single step method which involves the synthesis and surface functionalization simultaneously; (ii) Post-synthesis modification, a two-step procedure in which synthesis of NPs is followed by the modification of surface. The coating procedure is determined by the coating compounds’ properties as well as the desired application. Generally, ligand addition, ligand exchange, or encapsulation are used to modify the surface of NPs [40][41][42].
2.4. Ligand Addition
It is the process of adding a ligand to the exterior surface of produced NPs without removing any previously attached ligands (Figure 4A). The following strategies are required for ligand addition to NPs’ surface viz., (1) The ligand used is prepared with no capping agent; (2) Indirect addition of ligand, the formation of an inorganic layer on the surface of NPs followed by the direct adsorption of a ligand via ionic or other non-specific interactions; (3) Using the ‘hydrophobic attractive interaction’ to easily integrate hydrophobic molecules into NP’s hydrocarbon shell capped by desired ligands; and (4) A covalent connection is formed between the present ligand and the approaching ligand.
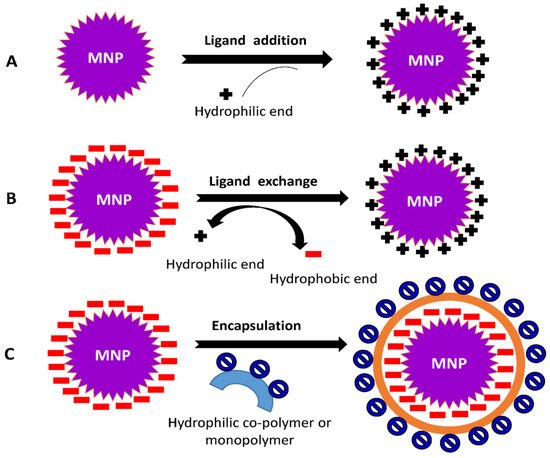
Figure 4. Common mechanisms involved in surface modification of NPs (A) ligand addition, (B) ligand exchange, and (C) encapsulation.
2.5. Ligand Exchange
This process involves changing a hydrophobic ligand into a hydrophilic one. These ligands are typically made up of hydrophilic and binding groups. The binding groups can attach to the NPs’ surface, exposing the hydrophilic groups to the external environment and enabling them to completely dissolve in the aqueous solution (Figure 4B).
2.6. Encapsulation
During the encapsulation procedure, NPs covered with hydrophobic ligands are coated with amphiphilic compounds. The hydrophobic component of materials is intercalated with the initial ligand on the surface of the MNPs, resulting in the hydrophilic portion facing the solution (Figure 4C). The head groups within the hydrophilic region of the substances allow MNPs to become water-compatible.
3. A Brief Note on the Unique Properties and Importance of Some Nanoparticles
3.1. Iron Oxide Nanoparticles (IONPs)
Iron oxide is the comprehensively investigated compound in biomedical techniques as it is characterized by high biocompatibility in relation to other magnetic resources, based on both the oxides and pure metal. Even though there are various forms of iron oxides that occur in the environment and they may be synthesized in the lab, only maghemite (γ-Fe2O3) and magnetite (Fe3O4) can meet the standards for biological applications. These parameters involve chemical stability, high magnetic moments, and low toxicity. In addition, there a simple and economical procedures to prepare these materials [29]. The efficacy of nanoparticles in diagnostic or therapeutic approaches is based upon a number of criteria, including the quantity of atomic order in iron oxide, crystallinity, and dispersity in regards to NP size and shape [43]. Furthermore, iron oxide NPs are nonporous. This helps to prevent delay caused by diffusion, so equilibrium is reached quickly and a quick response is possible. The drawback here is their self-condensation response, which results in multiple layer structure or accumulative deposition on surface layer. Phosphates, on the other hand, do not go through self-condensation interactions. These agents form a stable Fe–O–P structure by combining with surface-OH groups [9].
The inherent aggregation behavior of iron oxide NPs happens due to a high surfac- area-to-volume ratio and the attraction forces among magnetite, an important and a limiting component that diminishes intrinsic super paramagnetic properties [44], and activates opsonization procedure [45]. Hence, reducing aggregation is essential to engineering iron oxide NPs’ surface in order to prevent the particles’ aggregation in biological solution to achieve more effective stabilization [46]. The used approaches are grafting or coating to organic compounds, such as silica, metal or non-metal elementary content, metal oxide, or metal sulphide. Basically, defending shells could be used to normalize magnetic oxide NPs and improve NPs’ effectiveness as aptamers in sensing and (bio)chemical laboratory tests [47]. Silica is frequently used as a coating material over the surface of NPs as it is chemically inactive and encourages NP dispersion.
Use of nanoscale materials in combination with biomolecules has resulted in development of novel breed of hybrid modified electrodes to improve charge transfer as well as bioactivity retention [48].
3.2. Gold Nanoparticles (AuNPs)
The capability of gold NPs (AuNPs) to interact with cells and to enter them has helped the researchers to attach different biological macromolecules and compounds to gold. Because they tend to respond and clump with other NPs, gold NPs are available in various sizes and shapes [1]. AuNPs are typically made up of a thin gold shell encasing a dielectric core (an insulating material such as silica), which is referred to as nano-shells or AuNPs (typically spherical) with a size range of 0.8 to 250 nm. They are also distinguished by high absorption coefficients [49]. The optical properties of AuNPs are determined by their size and shape. The optical resonance of gold may be altered and moved as far as the mid-infrared region by changing the width of the core and outer shell of AuNPs [50]. Functionalization of nanoparticles is necessary for their stability, functionality, and biocompatibility. The main goal in functionalization is to preserve properties of both the AuNPs and the bound biological molecule. Surface-functionalization of AuNPs is critical in biological devices, diseased areas to interact selectively with cells, or biological molecules [51].
In general, the functionalization of AuNPs can be achieved by either using chemical functional groups or biological molecules. AuNPs with diameters just under 10 nm exhibit a variety of physiochemical and thermodynamic applications due to their increased surface-area-to-volume ratio. Ligands have thiol groups which bind covalently to Au atoms during the reduction of the HAuCl4 and assemble into an outer layer on the Au crystals [52]. For NPs with a 2 nm gold core, the covalently attached ligands are tightly packed and organized into structures similar to lattices in crystals. The choice of ligands and their organization will bear their biological properties and NP interaction with macromolecules in solutions and on target cells. Because of their long-term stabilization; ease in controlling size distribution; and good compatibility with biomolecules such as antibodies, antigens, proteins, DNA, and RNA, AuNPs have been commonly used in numerous immunoassay techniques in recent years [53]. Gold nanoparticles (AuNPs) are primarily suitable for advancement of quick tests. Photothermal AuNPs are being used in medical applications such as cancer treatment [54]. The surface of AuNPs could be handled to improve their stability and specificity, as well as to decrease accumulation. Functionalized AuNPs are being used for gene transfection and silencing, focused drug or gene delivery, intracellular recognition, bioimaging, cancer care, and as biosensors [55][56].
3.3. Platinum Nanoparticles (PtNPs)
Platinum nanoparticles (PtNPs) are extremely important due to its exceptional catalytic properties, that is influenced by ligands on their outer side. PtNPs can have a variety of surface functional groups that are hydrophilic, lipophilic, and chemically reactive [57]. Some platinum compounds are utilized as anticancer drugs because they are highly effective. This feature is related to DNA replication inhibition by adding PtNPs to DNA strands [58]. On the other hand, Hikosaka et al. [59] showed that PtNPs have a nicotinamide adenine dinucleotide (NADH) ubiquinone oxidoreductase-like activity. This finding suggests that PtNPs could be applied to ameliorate oxidative stress conditions defined by a lowered mitochondrial complex I [60]. Due to their affinity for DNA strands, PtNPs can be more effective as carriers for carrying drugs into bacteria. It is also a crucial element of fuel cells, where platinum acts as the very efficient electrocatalyst for oxygen reduction reaction (ORR) and fuel (which includes hydrogen, methanol, ethanol, and formic acid) oxidation reaction [61]. PtNPs catalytic activities mainly depend on their sizes, shapes, and structures [62].
Nowadays, there are various valid approaches to assembling nano-building materials, which include the use of preformed building blocks to construct NPs with high-ordered architecture. The methods of making well-organized NPs are categorized into biological and non-biological assemblies. These are utilized to produce complex hybrid nanomaterials containing unique biological or microbial compounds such as antigens and antibodies [63], glucose oxidase [64], DNA [65], and bacteria [66]. Ahmad et al. [67] reported a rapid detection of bacteria using antibody immunoglobulin G (h-IgG) to fabricate the platinum nanoparticle. The aggregation of NPs, particularly platinum, stimulated by immunoreactions provides a unique immunoassay process that uses light scattering identification to reach great sensitivity [68]. It was explained a potentially fast and effective immunoassay that used antibody-platinum NP adducts as a message particle [68]. This technology has many benefits: (a) easy to execute, (b) tiny quantity of reagent is being used, (c) provides a faster response, and (d) provides an effective and low-cost identification.
3.4. Silver Nanoparticles (AgNPs)
A distinctive emergent silver-based material has been shown to offer a lasting antimicrobial advantage that can offer environmental control of pathogenic bacteria on treated surfaces. Silver NPs (AgNPs) are handy labels. They have huge optical cross-sections with excellent photostabilities [69]. They are also responsive to multimodal imaging including optical, electron, and X-ray. Silver has been explained as a bulk material that is efficient against a wide range of pathogens [70]. Silver NPs have been examined and found to be highly attractive for use in several applications such as wound dressings, coatings for medical devices, and textile fabrics impregnation [39]. In spite of these benefits, it is challenging to prepare antibody functionalized AgNPs of a size that can generates sufficient signal for high temporal resolution optical imaging (20–40 nm) and remains stable in physiological buffers. Under these situations, screening the stabilizing charge of the NPs may lead to aggregation, and AgNPs can undergo oxidative corrosion [71]. Some improvement was achieved in synthesizing stable silver–DNA conjugates [72]. The direct method to biofunctionalized noble metal NPs is by using electrostatic attraction to have a non-covalent attachment of antibodies to the metal surface. The surface charge of AuNP complexes implies conformation of biomolecules on NPs and influences particle–biomolecule interactions, in addition to ensuring the stability of nanoconjugates [73]. Thiolation by organic thiol compounds is also a method of metal-surface functionalization. Under moderate circumstances, thiols generate stable metal–sulfur bonds using surface atoms in noble metals. Other thiol compounds immobilized on silver are 3-mercaptopropionic acid [74], 2-mercaptoethanol, 2-aminoethanethiol, and 2-mercaptoethanesulfonic acid sodium salt [75]. Functionalization of AgNP surfaces highly affects their characteristics. The optical properties of silver nanoparticles were altered after they were functionalized with alkanethiols. For each carbon atom in the alkane chain, the maximum of the local surface plasmon resonance spectra shifts linearly to the red by 3 nm. In contrast, only 60,000 alkanethiol molecules per nanoparticle cause spectral large shifts of up to 40 nm.
3.5. Silica-Coated Nanoparticle
Utilizing thin silica coating techniques, a variety of water-soluble, functionalized NPs can be synthesized [76]. Thin silica coating in the toluene stage was conducted utilizing triethoxysilane or trihydroxysilane, which takes the benefit of avoiding silica polymerization thus restricting the texture of silica shell, preventing particle–particle cross-linking, and generating narrower water-soluble NPs [1]. The thin silica coating was highly reproducible that can be used in a variety of hydrophobic NPs, including Au, Ag, Fe3O4, and quantum dots (QDs). The silanized granules that resulted were monodispersed, with high water solubility and colloidal consistency. PEG silane-coated NPs have recently been developed as contrast agents for magnetic resonance imaging (MRI) visualization of tumors [77]. A recenItly published article re was reported that the functionalized thin silica-coated NPs with aptamers and antibodies and used them in biological applications [78]. Koole et al. [79] outlined an evolutionary approach for coating silica particles with a closely packed monolayer of lipids without the use of coupling agents in two steps. Step one involved integrating strongly monodisperse silica particles with a single thread QD in their center and making diethylene triamine penta-acetic acid (DTPA) bistearyl amide (DSA) in hydrophobic lipid coating [79]. They were made target-specific by multiple Rvβ3-integrin specific RGD conjugation (arginine glycine–aspartic acid) peptides to enable their recognition with both fluorescence techniques and MRI. Using imaging technology, it was discovered that lipid coating improved bio-applicability and pharmacokinetics [79]. A fluorescent contrast material was used to functionalize silica-coated granules in a sequential manner. The drug image sensitizer palladium–porphyrin payload has been used for therapeutic treatment, and biomolecular ligands c(RGDyK) peptides on outermost exterior were used to target cancer cells’ Rv-3 integrins [37]. A membrane which consists of bis-sorbylphosphatidylcholine, has been used to enhance both the coating environmental and chemical stabilities [80]. This system decreased nonspecific interactions and allowed the functionalization of the particles. A novel delivery system termed a ‘nano-shuttle’ was described with a nano scale PEGylated-phospholipid coating and a 13-(chlorodimethylsilylmethyl) heptacosane-derived mesoporous silica NP [81].
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692.
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st century manufacturing paradigm. Biofabrication 2009, 1, 22001.
- Nguyen, P.Q.; Courchesne, N.M.D.; Duraj-Thatte, A.; Praveschotinunt, P.; Joshi, N.S. Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials. Adv. Mater. 2018, 30, 1704847.
- Holy, J.; Perkins, E.; Yu, X. Adhesion, proliferation and differentiation of pluripotent stem cells on multi-walled carbon nanotubes. IET Nanobiotechnology 2011, 5, 41–46.
- Pagels, R.F.; Pinkerton, N.M.; York, A.W.; Prud’homme, R.K. Synthesis of Heterobifunctional Thiol-poly(lactic acid)-b-poly(ethylene glycol)-hydroxyl for Nanoparticle Drug Delivery Applications. Macromol. Chem. Phys. 2020, 221, 1900396.
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J. Pharm. Sci. 2004, 93, 1980–1992.
- Sun, S.N.; Wei, C.; Zhu, Z.Z.; Hou, Y.L.; Venkatraman, S.S.; Xu, Z.C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 37503.
- Neouze, M.A.; Schubert, U. Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands. Mon. Für Chem. Chem. Mon. 2008, 139, 183–195.
- Doty, R.C.; Tshikhudo, T.R.; Brust, M.; Fernig, D.G. Extremely Stable Water-Soluble Ag Nanoparticles. Chem. Mater. 2005, 17, 4630–4635.
- Nguyen, D.T.; Kim, K.S. Functionalization of magnetic nanoparticles for biomedical applications. Korean J. Chem. Eng. 2014, 31, 1289–1305.
- Moon, J.H.; Shul, Y.G.; Han, H.S.; Hong, S.Y.; Choi, Y.S.; Kim, H.T. A study on UV-curable adhesives for optical pick-up: I. Photo-initiator effects. Int. J. Adhes. Adhes. 2005, 25, 301–312.
- Kumar, S.; Aziz, S.K.T.; Girshevitz, O.; Nessim, G.D. One-Step Synthesis of N-Doped Graphene Quantum Dots from Chitosan as a Sole Precursor Using Chemical Vapor Deposition. J. Phys. Chem. C 2018, 122, 2343–2349.
- Bteich, J.; McManus, S.A.; Ernsting, M.J.; Mohammed, M.Z.; Prud’Homme, R.K.; Sokoll, K.K. Using Flash Nanoprecipitation to Produce Highly Potent and Stable Cellax Nanoparticles from Amphiphilic Polymers Derived from Carboxymethyl Cellulose, Polyethylene Glycol, and Cabazitaxel. Mol. Pharm. 2017, 14, 3998–4007.
- Li, H.; Lu, Z.; Cheng, G.; Rong, K.; Chen, F.; Chen, R. HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological application. RSC Adv. 2015, 5, 5059–5067.
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303.
- Zhang, Z.; Feng, C.; Liu, C.; Zuo, M.; Qin, L.; Yan, X.; Xing, Y.; Li, H.; Si, R.; Zhou, S.; et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts. Nat. Commun. 2020, 11, 1215.
- Carmen Bautista, M.; Bomati-Miguel, O.; Del Puerto Morales, M.; Serna, C.J.; Veintemillas-Verdaguer, S. Surface characterisation of dextran-coated iron oxide nanoparticles prepared by laser pyrolysis and coprecipitation. J. Magn. Magn. Mater. 2005, 293, 20–27.
- Pineda-Reyes, A.M.; Olvera, M.D.L.L. Synthesis of ZnO nanoparticles from water-in-oil (w/o) microemulsions. Mater. Chem. Phys. 2018, 203, 141–147.
- Ooi, F.; Duchene, J.S.; Qiu, J.; Graham, J.O.; Engelhard, M.H.; Cao, G.; Gai, Z.; Wei, W.D. A Facile Solvothermal Synthesis of Octahedral Fe3O4 Nanoparticles. Small 2015, 11, 2649–2653.
- Zhang, W.; Yang, Y.; Ziemann, E.; Be’Er, A.; Bashouti, M.Y.; Elimelech, M.; Bernstein, R. One-step sonochemical synthesis of a reduced graphene oxide–ZnO nanocomposite with antibacterial and antibiofouling properties. Environ. Sci. Nano 2019, 6, 3080–3090.
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications. Nanomaterials 2019, 9, 212.
- Strobel, R.; Pratsinis, S.E. Flame aerosol synthesis of smart nanostructured materials. J. Mater. Chem. 2007, 17, 4743–4756.
- Sibeaud, M.; Croutxé-Barghorn, C.; Rigolet, S.; Michelin, L.; Josien, L.; Vidal, L.; Lebeau, B.; Wörner, M.; Chemtob, A. UV aerosol synthesis: A one-step route to silica, organic-silica and surfactant/silica nanostructured materials. RSC Adv. 2016, 6, 65047–65054.
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2009, 110, 2574.
- Ali, N.; Zhang, B.; Zhang, H.; Zaman, W.; Li, W.; Zhang, Q. Key synthesis of magnetic Janus nanoparticles using a modified facile method. Particuology 2014, 17, 59–65.
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Sci. Rep. 2020, 10, 10793.
- Lee, H.S.; Kim, E.H.; Shao, H.; Kwak, B.K. Synthesis of SPIO-chitosan microspheres for MRI-detectable embolotherapy. J. Magn. Magn. Mater. 2005, 293, 102–105.
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501.
- Lin, P.C.; Chou, P.H.; Chen, S.H.; Liao, H.K.; Wang, K.Y.; Chen, Y.J.; Lin, C.C.; Lin, P.C.; Chou, P.H.; Chen, S.H.; et al. Ethylene Glycol-Protected Magnetic Nanoparticles for a Multiplexed Immunoassay in Human Plasma. Small 2006, 2, 485–489.
- Fu, C.; Zhou, H.; Wang, Y.; Liu, D.; Li, J.; Deng, H.; Qi, X.; Chen, T.; Zhang, L.M.; Li, G. One-pot synthesis of dextran-coated iron oxide nanoclusters for real-time regional lymph node mapping. Int. J. Nanomed. 2017, 12, 3365.
- Qu, J.B.; Shao, H.H.; Jing, G.L.; Huang, F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: Preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2013, 102, 37–44.
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69.
- Moraes Silva, S.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540.
- Wang, L.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed Core−Shell Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601.
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small 2007, 3, 1341–1346.
- Cheng, S.H.; Lee, C.H.; Chen, M.C.; Souris, J.S.; Tseng, F.G.; Yang, C.S.; Mou, C.Y.; Chen, C.T.; Lo, L.W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics—The trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149–6157.
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504.
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449.
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230.
- Chu, X.; Yu, J.; Hou, Y.-L. Surface modification of magnetic nanoparticles in biomedicine. Chin. Phys. B 2015, 24, 14704.
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012.
- Ovejero, J.G.; Spizzo, F.; Morales, M.P.; Del Bianco, L. Nanoparticles for Magnetic Heating: When Two (or More) Is Better Than One. Materials 2021, 14, 6416.
- Moghimi, S.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318.
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021.
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265.
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415.
- Hung, C.W.; Holoman, T.R.; Kofinas, P.; Bentley, W.E. Towards oriented assembly of proteins onto magnetic nanoparticles. Biochem. Eng. J. 2008, 38, 164–170.
- Zeng, H.J.; Zhao, R.L.; Wang, D.S.; Li, C.X.; Liu, Y.Y. Optical Analysis of the Interaction of Mercaptan Derivatives of Nanogold Particles with Carcinoembryonic Antigen. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 478–481.
- Acunto, M.D.; Moroni, D.; Salvetti, O. Nanoscale Biomolecular Detection Limit for Gold Nanoparticles Based on Near-Infrared Response. Adv. Opt. Technol. 2012, 2012, 278194.
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53.
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278.
- Jazayeri, M.H.; Aghaie, T.; Avan, A.; Vatankhah, A.; Ghaffari, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sens. Res. 2018, 20, 1–8.
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 1449.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124.
- Yang, G.; Liu, Y.; Wang, H.; Wilson, R.; Hui, Y.; Yu, L.; Wibowo, D.; Zhang, C.; Whittaker, A.K.; Middelberg, A.P.J.; et al. Bioinspired Core–Shell Nanoparticles for Hydrophobic Drug Delivery. Angew. Chem. Int. Ed. 2019, 58, 14357–14364.
- Mohammadi, H.; Abedi, A.; Akbarzadeh, A.; Mokhtari, M.J.; Shahmabadi, H.E.; Mehrabi, M.R.; Javadian, S.; Chiani, M. Evaluation of synthesized platinum nanoparticles on the MCF-7 and HepG-2 cancer cell lines. Int. Nano Lett. 2013, 3, 28.
- Sawosz, E.; Chwalibog, A.; Szeliga, J.; Sawosz, F.; Grodzik, M.; Rupiewicz, M.; Niemiec, T.; Kacprzyk, K. Visualization of gold and platinum nanoparticles interacting with Salmonella Enteritidis and Listeria monocytogenes. Int. J. Nanomed. 2010, 5, 631.
- Hikosaka, K.; Kim, J.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Platinum nanoparticles have an activity similar to mitochondrial NADH:ubiquinone oxidoreductase. Colloids Surf. B Biointerfaces 2008, 66, 195–200.
- Watanabe, A.; Kajita, M.; Kim, J.; Kanayama, A.; Takahashi, K.; Mashino, T.; Miyamoto, Y. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology 2009, 20, 455105.
- Menshikov, V.S.; Novomlinsky, I.N.; Belenov, S.V.; Alekseenko, A.A.; Safronenko, O.I.; Guterman, V.E. Methanol, Ethanol, and Formic Acid Oxidation on New Platinum-Containing Catalysts. Catalysts 2021, 11, 158.
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Zhong, L.W. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735.
- Shenton, W.; Davis, S.; Mann, S. Directed self-assembly of nanoparticles into macroscopic materials using antibody-antigen recognition. Adv. Mater. 1999, 11, 449–452.
- Claussen, J.C.; Hengenius, J.B.; Wickner, M.M.; Fisher, T.S.; Umulis, D.M.; Porterfield, D.M. Effects of carbon nanotube-tethered nanosphere density on amperometric biosensing: Simulation and experiment. J. Phys. Chem. C 2011, 115, 20896–20904.
- Yao, H.; Yi, C.; Tzang, C.H.; Zhu, J.; Yang, M. DNA-directed self-assembly of gold nanoparticles into binary and ternary nanostructures. Nanotechnology 2006, 18, 15102.
- Berry, V.; Saraf, R.F.; Berry, V.; Saraf, R.F. Self-Assembly of Nanoparticles on Live Bacterium: An Avenue to Fabricate Electronic Devices. Angew. Chem. Int. Ed. 2005, 44, 6668–6673.
- Ahmad, F.; Siddiqui, M.A.; Babalola, O.O.; Wu, H.F. Biofunctionalization of nanoparticle assisted mass spectrometry as biosensors for rapid detection of plant associated bacteria. Biosens. Bioelectron. 2012, 35, 235–242.
- Lin, H.C.; Wang, I.L.; Lin, H.P.; Chang, T.C.; Lin, Y.C. Enhancement of an immunoassay using platinum nanoparticles and an optical detection. Sensors Actuators B Chem. 2011, 154, 185–190.
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2002, 107, 668–677.
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874.
- Skewis, L.R.; Reinhard, B.M. Control of Colloid Surface Chemistry through Matrix Confinement: Facile Preparation of Stable Antibody Functionalized Silver Nanoparticles. ACS Appl. Mater. Interfaces 2009, 2, 35–40.
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865.
- Li, J.; Wei, X.; Yuan, Y. Synthesis of magnetic nanoparticles composed by Prussian blue and glucose oxidase for preparing highly sensitive and selective glucose biosensor. Sensors Actuators B Chem. 2009, 139, 400–406.
- Kudelski, A.; Michota, A.; Bukowska, J. Monolayers of sulfur-containing molecules at metal surfaces as studied using SERS: 3, 3′-thiodipropionic acid and 3-mercaptopropionic acid adsorbed on silver and copper. J. Raman Spectrosc. 2005, 36, 709–714.
- Gui, J.Y.; Stern, D.A.; Frank, D.G.; Lu, F.; Zapien, D.C.; Hubbard, A.T. Adsorption and surface structural chemistry of thiophenol, benzyl mercaptan, and alkyl mercaptans. Comparative studies at silver(111) and platinum(111) electrodes by means of Auger spectroscopy, electron energy loss spectroscopy, low energy electron diffraction and electrochemistry. Langmuir 2002, 7, 955–963.
- Jana, N.R.; Earhart, C.; Ying, J.Y. Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 2007, 19, 5074–5082.
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Birkedal, H.; Vorup-Jensen, T.; Jakobsen, M.H.; Ostergaard, L.; Horsman, M.R.; Besenbacher, F.; Howard, K.A.; et al. Size-dependent accumulation of pegylated silane-coated magnetic iron oxide nanoparticles in murine tumors. ACS Nano 2009, 3, 1947–1951.
- Zhong, Z.; Li, M.; Xiang, D.; Dai, N.; Qing, Y.; Wang, D.; Tang, D. Signal amplification of electrochemical immunosensor for the detection of human serum IgG using double-codified nanosilica particles as labels. Biosens. Bioelectron. 2009, 24, 2246–2249.
- Koole, R.; Van Schooneveld, M.M.; Hilhorst, J.; Castermans, K.; Cormode, D.P.; Strijkers, G.J.; Donegá, C.D.M.; Vanmaekelbergh, D.; Griffioen, A.W.; Nicolay, K.; et al. Paramagnetic Lipid-Coated Silica Nanoparticles with a Fluorescent Quantum Dot Core: A New Contrast Agent Platform for Multimodality Imaging. Bioconjugate Chem. 2008, 19, 2471–2479.
- Senarath-Yapa, M.D.; Phimphivong, S.; Coym, J.W.; Wirth, M.J.; Aspinwall, C.A.; Saavedra, S.S. Preparation and characterization of poly(lipid)-coated, fluorophore-doped silica nanoparticles for biolabeling and cellular imaging. Langmuir 2007, 23, 12624–12633.
- Wang, L.S.; Wu, L.C.; Lu, S.Y.; Chang, L.L.; Teng, I.T.; Yang, C.M.; Ho, J.A.A. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: Improved water suspensibility and decreased nonspecific protein binding. ACS Nano 2010, 4, 4371–4379.
More
