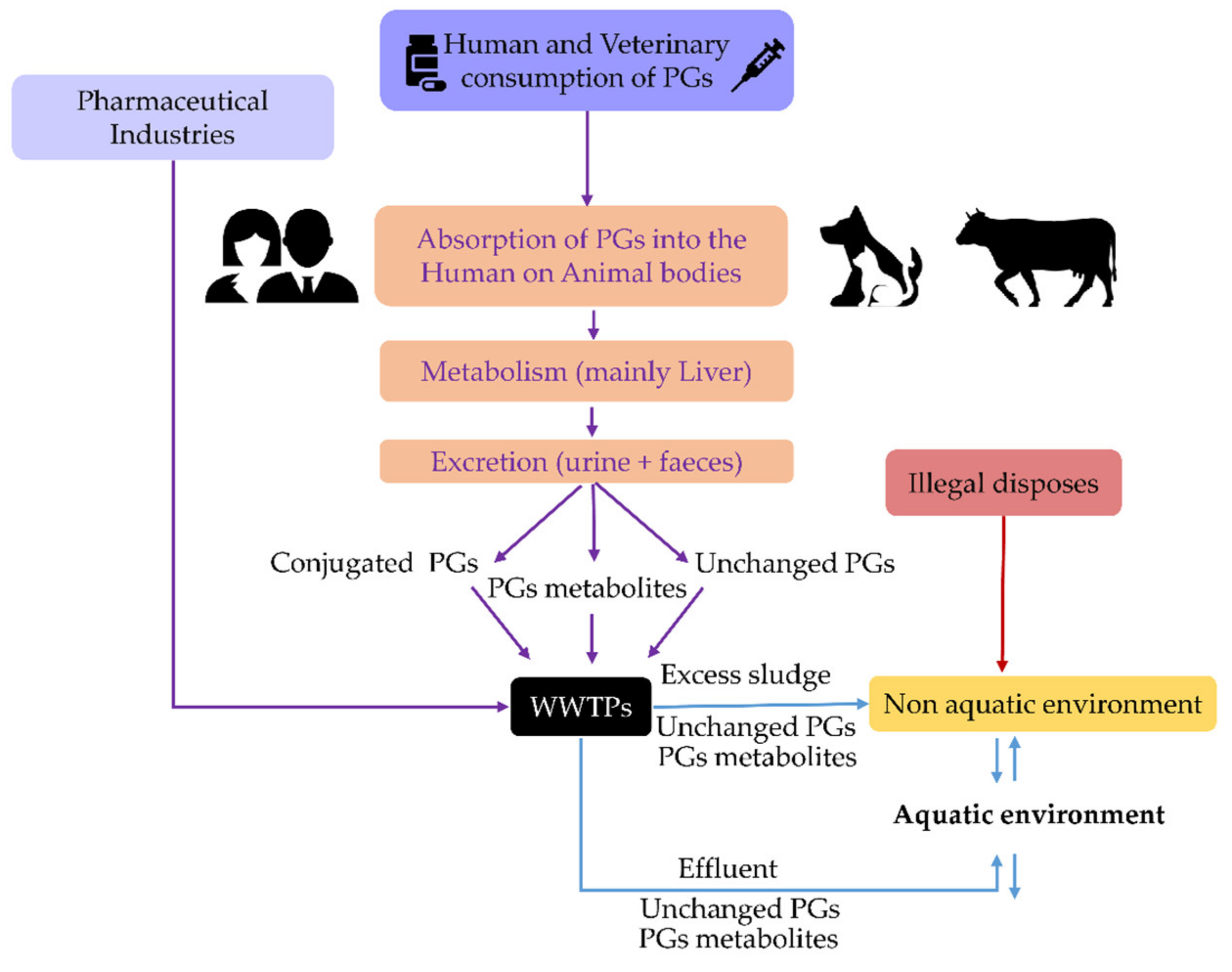
Synthetic progestins (PGs) are a large family of hormones used in continuously growing amounts in human and animal contraception and medicinal therapies. Because wastewater treatment plants (WWTPs) are unable to eradicate PGs after excretion, they are discharged into aquatic systems, where they can also be regenerated from conjugated PG metabolites. The PGs were considered of particular interest due to their wide use, activity, and hormonal derivation (from testosterone, progesterone, and spirolactone). It is concluded that PGs had been analysed in WWTPs influents and effluents and, to a lesser extent, in other matrices, including surface waters, where their concentrations range from ng/L to a few µg/L. Because of their high affinity for cell hormone receptors, PGs are endocrine disruptor compounds that may alter the reproductive fitness and development of biota. This review focused on their biological effects in fish, which are the most used aquatic model organisms to qualify the impacts of PGs, highlighting the risks that environmental concentrations pose to their health, fecundity, and fertility.
- drospirenone
- EDCs
- estranes
- gestagens
- gonanes
- norpregnanes
- pregnanes
- risk assessment
1. Introduction
2. Classification and Properties of the Most Prominent PGs in Aquatic Environments
PGs are typically classified considering their structural derivation and “generation” (| Hormone | Family | Common Name (Acronym) & CAS | Structure | & Molecular Formula | Generation | & Activity | ||||||
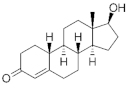 |  | 19-Nortestosterone | Gonanes (C17)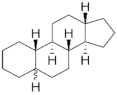 or LNG family | Gonanes (C17) or LNG family |
Gestodene ( | GES | ) 60282-87-4 |
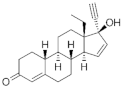 C21H28O2 |  C21H28O2 |
3rd Generation 1986 (+++) |
||
| Levonorgestrel ( | LNG | ) 797-63-7 |
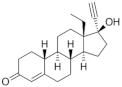 C21H28O2 |  C21H28O2 |
2nd Generation 1966 (+++) |
|||||||
| Norgestrel ( | NET | ) 6533-00-2 |
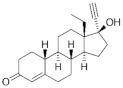 C21H28O2 |  C21H28O2 |
2nd Generation 1966 (+++) |
|||||||
| Etonogestrel ( | ENG | ) 54048-10-1 |
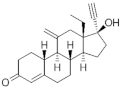 C22H28O2 |  C22H28O2 |
3rd Generation 1998 (+) |
|||||||
Estranes (C18)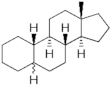 or NTD family | Estranes (C18) or NTD family |
Norethisterone ( | NTD | ) 68-22-4 |
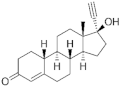 C20H26O2 |  C20H26O2 |
1st Generation 1951 (++) |
|||||
| Norethisterone acetate ( | NTDA | ) 51-98-9 |
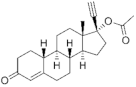 C22H28O3 |  C22H28O3 |
1st Generation 1951 (++) |
|||||||
| Dienogest ( | DIE | ) 65928-58-7 |
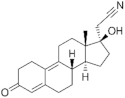 C22H28O2 |  C22H28O2 |
4th Generation 1978 (-) |
|||||||
| Progesterone derivatives | 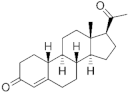 |  | 19-Norprogesterone | Norpregnanes (C20) | Norpregnanes (C20) |
Nomegestrol acetate ( | NOMAC | ) 58652-20-3 |
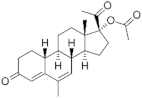 C23H30O4 |  C23H30O4 |
4th Generation 1986 (-) |
|
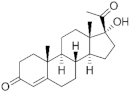 17α-Hydroxyprogesterone |  17α-Hydroxyprogesterone |
Pregnanes (C21)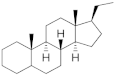 | Pregnanes (C21) |
Medroxyprogesterone ( | MEP | ) 520-85-4 |
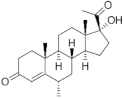 C22H32O3 |  C22H32O3 |
1st Generation 1957 (+) |
|||
| Medroxyprogesterone acetate ( | MPA | ) 71-58-9 |
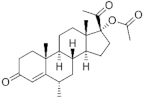 C24H34O4 |  C24H34O4 |
1st Generation 1957 (+) |
|||||||
| Megestrol acetate ( | MGA | ) 595-33-5 |
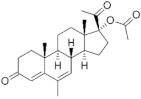 C22H30O4 |  C22H30O4 |
1st Generation 1963 (-) |
|||||||
| Spironolactone | derivative | 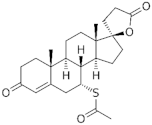 Spironolactone |  Spironolactone |
Drospirenone ( | DSP | ) 67392-87-4 |
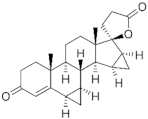 C24H30O3 |  C24H30O3 |
4th Generation 1976 (-) |
|||
3. Waste and Surface Waters Concentrations of Synthetic Progestins
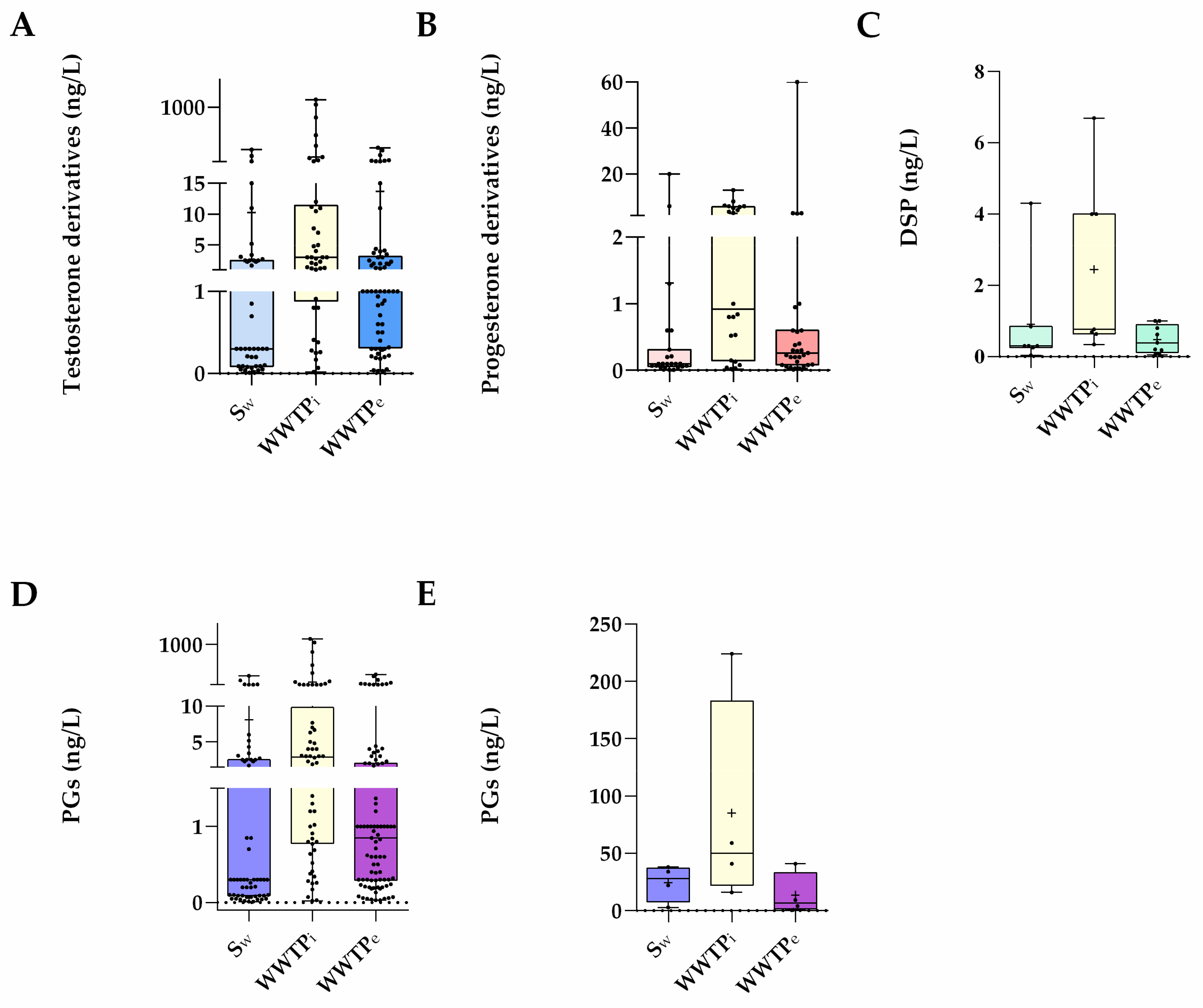
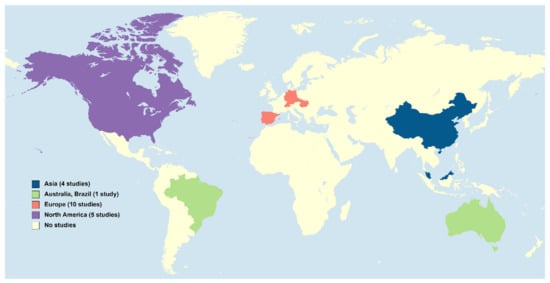
PGs are considered emerging micropollutants in aquatic ecosystems, where they are usually present in concentrations in the order of ng/L. However, accurately knowing their concentrations in waters is crucial since such tiny amounts are potentially harmful to (at least) fish [6][7]. Likely because analysing PGs requires trace analytical methods for their extraction and quantification, the number of studies concerning the environmental levels of these compounds is still scarce and, in a majority, focused on the concentrations of these hormones in influents and effluents from wastewater treatment plants (WWTPs). In addition, the surveyed areas are still limited in space (3. Waste and Surface Waters Concentrations of Synthetic Progestins
PGs are considered emerging micropollutants in aquatic ecosystems, where they are usually present in concentrations in the order of ng/L. However, accurately knowing their concentrations in waters is crucial since such tiny amounts are potentially harmful to (at least) fish [6,7]. Likely because analysing PGs requires trace analytical methods for their extraction and quantification, the number of studies concerning the environmental levels of these compounds is still scarce and, in a majority, focused on the concentrations of these hormones in influents and effluents from wastewater treatment plants (WWTPs). In addition, the surveyed areas are still limited in space (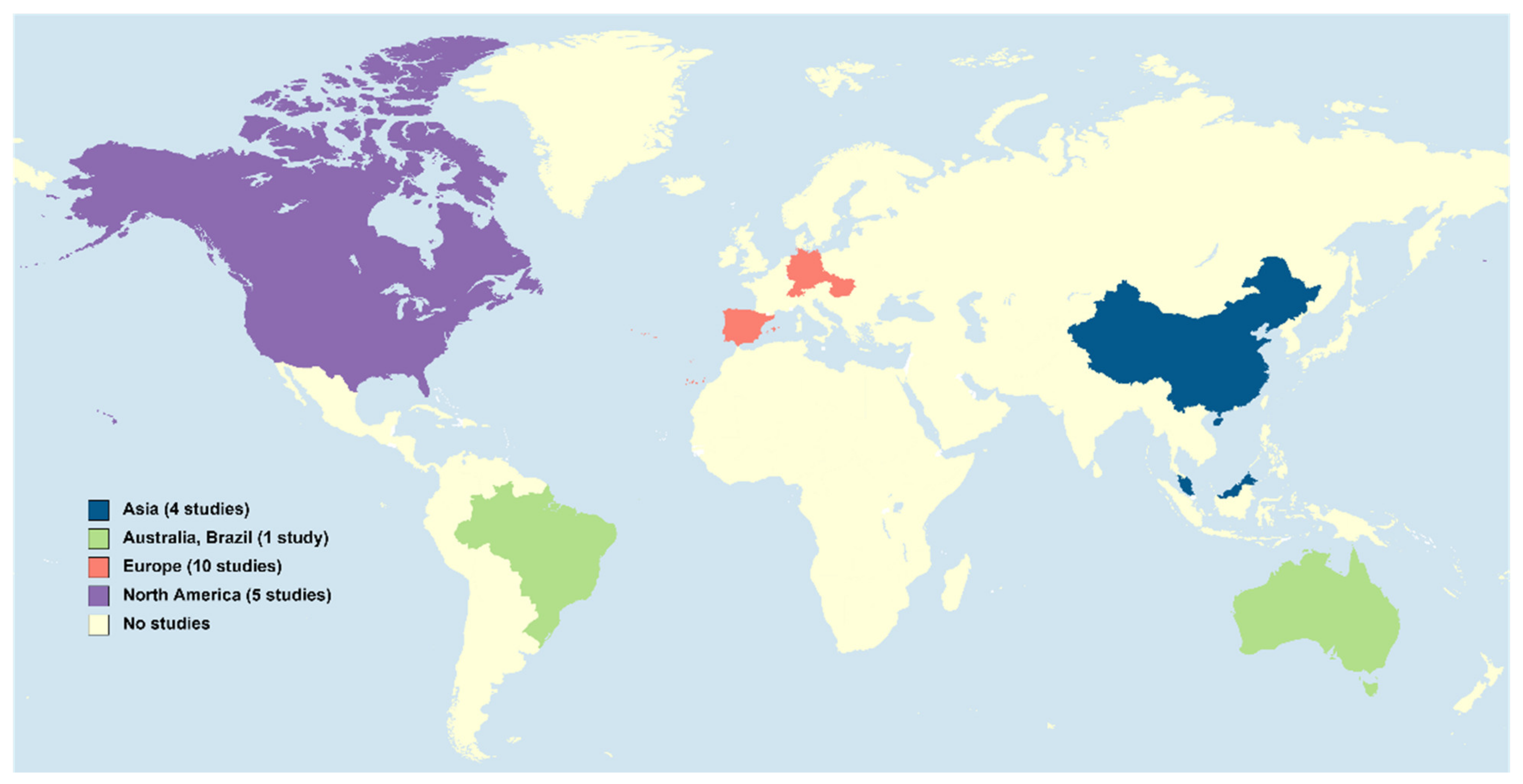
| Testosterone derivatives (Gonanes) | PGs | QM | Sw | (ng/L) | WWTPi (ng/L) | WWTPe | (ng/L) | Local (Country) | References | ||
| GES | (1) | 0.2 | 3 | 1 | Basel and canton Zürich WWTPs (Switzerland). | [31] | |||||
| (2) | <0.05 | <0.38–7.7 | <0.29–0.71 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (2) | <0.64 | <0.41–7.0 | <0.19–<3.5 | Several WWTPs (Czech and Slovak Republics) |
[35] | ||||||
| (1) | <0.3 | n.e. | <1.0 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (3) | <0.2 | <3.0 | <1.0 | Jona River and WWTPs (Switzerland). | [36] | ||||||
| (4) | <21.5 | <21.5 | <21.5 | Five WWTPs (Portugal). | [37] | ||||||
| LNG | (1) | <2.5–117 | 493–811 | 32–39 | Langat River Basin (Malaysia). | [38] | |||||
| (5) | <2.5 | n.e. | <2.5 | Southeast Queensland (Australia). | [39] | ||||||
| (6) | 0.85–3.40 | n.e. | n.e. | Lake Balaton (Hungry). | [40] | ||||||
| (7) | <15 | n.e. | <15 | Two WWTPs in Quebec (Canada). | [41] | ||||||
| (2) | <0.08 | <0.26–<2.1 | <0.22–<0.83 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (2) | <0.09 | <0.07–<1.2 | <0.03–<0.32 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (1) | <0.05–<0.7 | n.e. | <0.3–<1.0 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (1) | ND | ND–38.4 | ND–20.1 | Several WWTPs, Quebec (Canada). | [42] | ||||||
| (8) | <2.5 | <5–299 ± 17 | <3.0 | Québec and Ontario (Canada). | [43] | ||||||
| (4) | n.e. | 2.81 | 1.37 | 21 WWTPs (China). | [34] | ||||||
| (4) | n.e. | n.e. | <1.0 | Several WWTPs effluents (Germany). | [44] | ||||||
| NET | (4) | n.e. | n.e. | <2.0 | Gran Canaria (Spain) | [45] | |||||
| (4) | n.e. | 11.2 | 1.92 | 21 WWTPs (China). | [34] | ||||||
| ENG | (2) | <0.07 | <0.28–<1.4 | <0.21–<0.89 | Blanice River and WWTPs (Czech Republic). | [32] | |||||
| (2) | <0.09 | <0.25–<1.2 | <0.18–<0.94 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (1) | <0.3 | n.e. | <0.5 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (4) | n.e. | n.e. | <1.2 | Several WWTPs effluents (Germany). | [44] | ||||||
| Testosterone derivatives (Estranes) | NTD | (1) | <2.5–230 | 1048–1137 | 218–265 | Langat River Basin (Malaysia). | [38] | ||||
| (4) | n.e. | n.e. | <2.0 | Gran Canaria (Spain). | [45] | ||||||
| (9) | ND–5.20 | 1.02–94.7 Av. = 25.7 |
ND–1.68 Av. = 1.25 |
Four WWTPs, Shanghai (China). | [46] | ||||||
| (5) | <0.21–3.1 | n.e. | n.e. | Freshwater aquaculture (China). | [47] | ||||||
| (1) | <0.3 | <3 | <0.6 | Basel and canton Zürich WWTPs (Switzerland). | [31] | ||||||
| (7) | <11 | n.e. | <11 | Two WWTPs in Quebec (Canada). | [41] | ||||||
| (2) | <0.04 | <0.02–<0.17 | <0.03–0.85 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (2) | <0.01 | <0.02–<0.91 | <0.02–<4.1 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (1) | n.e. | n.e. | <0.40 | Pharmaceutical manufacturing facility discharges (USA). | [48] | ||||||
| (3) | <0.3 | <3 | <0.6 | Jona River and several WWTPs (Switzerland). | [36] | ||||||
| (1) | <0.1–<0.3 | n.e. | <1.0 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (8) | 1.7 ± 0.05–2.7 ± 0.17 | <4.8 | 2 ± 0.2–132 ± 2.2 | Québec and Ontario (Canada). | [43] | ||||||
| (10) | <2.3 | <2.3 | <2.3 | Basque Country (Spain). | [49] | ||||||
| (1) | ND | ND–78.8 | ND–31.8 | Several WWTPs, Quebec (Canada). | [42] | ||||||
| (4) | n.e. | 4.02 | 0.20 | 21 WWTPs (China). | [34] | ||||||
| (4) | n.e. | n.e. | <1.0 | Several WWTPs effluents (Germany). | [44] | ||||||
| NTDA | (4) | n.e. | 10.5 | 0.24 | 21 WWTPs (China). | [34] | |||||
| (1) | <0.3 | n.e. | <0.5 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (4) | n.e. | n.e. | <1.0 | Several WWTPs (Germany). | [44] | ||||||
| DIE | (1) | <0.3 | <0.8 | <0.3 | Basel and canton Zürich WWTPs (Switzerland). | [31] | |||||
| (3) | <0.3 | <0.8 | <0.3 | Jona River and several WWTPs (Switzerland). | [36] | ||||||
| (2) | <0.09 | 1.9–11.0 | <0.05–1.0 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (2) | <0.04 | 1.3–12 | <0.04–<4.0 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (1) | <0.02–2.3 | n.e. | 1.3–4.4 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (4) | n.e. | n.e. | 0.3–3.7 | Several WWTPs effluents (Germany). | [44] | ||||||
| Progesterone derivatives | NOMAC | (2) | <0.07 | <0.08–3.6 | <0.03–0.26 | Blanice River and WWTPs (Czech Republic). | [32] | ||||
| MEP | (5) | <0.07–1.3 | n.e. | n.e. | Freshwater aquaculture (China). | [47] | |||||
| (1) | <0.6 | <6 | <3 | Basel and canton Zürich WWTPs (Switzerland). | [31] | ||||||
| (3) | <0.6 | <6 | <3 | Jona River and several WWTPs (Switzerland). | [36] | ||||||
| (2) | <0.06 | <0.02–<0.13 | <0.03–0.23 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (1) | ND | ND–5.7 | ND–2.9 | Several WWTPs, Quebec (Canada). | [42] | ||||||
| (2) | <0.04 | <0.01–<0.53 | <0.01–0.95 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (1) | <0.05 | n.e. | <0.08 | Several WWTPs and rivers (Germany). | [33] | ||||||
| MPA | (5) | <0.21–0.31 | n.e. | n.e. | Freshwater aquaculture (China). | [47] | |||||
| (1) | <0.1 | <0.8 | <0.2 | Basel and canton Zürich WWTPs (Switzerland). | [31] | ||||||
| (2) | <0.1 | <0.15–4.4 | <0.09–0.58 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (2) | <0.01 | <0.04–8.1 | <0.04–0.38 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (3) | <0.1 | <0.8–5.3 | <0.2 | Jona River and several WWTPs (Switzerland). | [36] | ||||||
| (1) | <0.05–0.1 | n.e. | <0.08–<0.3 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (4) | n.e. | 3.09 | 0.23 | 21 WWTPs (China). | [34] | ||||||
| (4) | n.e. | n.e. | <0.6 | Several WWTPs effluents (Germany). | [44] | ||||||
| MGA | (4) | n.e. | n.e. | <60 | Gran Canaria (Spain). | [45] | |||||
| (1) | <0.1 | <1 | <0.6 | Basel and canton Zürich WWTPs (Switzerland). | [31] | ||||||
| (2) | <0.01 | 0.52–13.0 | 0.13–1.0 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (1) | <0.05–<0.2 | n.e. | <0.06–<0.3 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (2) | <0.07 | <0.03–<6.3 | <0.06–0.4 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (7) | <6–<20 | n.e. | n.e. | Water bodies in Santa Maria (Brazil). | [50] | ||||||
| (4) | n.e. | 0.84 | 0.29 | 21 WWTPs (China). | [34] | ||||||
| Spironolactone | derivative | DSP | (6) | 0.26–4.30 | n.e. | n.e. | Lake Balaton (Hungry). | [40] | |||
| (1) | <0.3 | <4 | <1 | Basel and canton Zürich WWTPs (Switzerland). | [31] | ||||||
| (2) | <0.85 | 0.64–0.77 | <0.18–<0.62 | Blanice River and WWTPs (Czech Republic). | [32] | ||||||
| (2) | <0.04 | 0.34–6.7 | <0.07–<0.29 | Several WWTPs (Czech and Slovak Republics). | [35] | ||||||
| (3) | <0.3 | <4 | <1 | Jona River and several WWTPs (Switzerland). | [36] | ||||||
| (1) | <0.3 | n.e. | <0.05 | Several WWTPs and rivers (Germany). | [33] | ||||||
| (4) | n.e. | 0.69 | 0.39 | 21 WWTPs (China). | [34] | ||||||
| (4) | n.e. | n.e. | <0.8 | Several WWTPs effluents (Germany). | [44] |