Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Lung cancer is a disease that involves the accumulation of multiple genetic mutations and epigenetic changes, which results in an out-of-control cell proliferation that disrupts regular cells. Lung cancer is the leading cause of cancer-related fatalities worldwide, accounting for about 1.6 million deaths per year; it is the second most common cancer diagnosis, comprising a total of 13% of new cancer cases each year. Considering the large number of incidences and mortality numbers associated with lung cancer, there is a need for the most accurate clinical procedures.
- lung cancer characterization
- personalized medicine
- clinical biomarkers
1. Introduction
Lung cancer is a disease that involves the accumulation of multiple genetic mutations and epigenetic changes, which results in an out-of-control cell proliferation that disrupts regular cells. Lung cancer is the leading cause of cancer-related fatalities worldwide, accounting for about 1.6 million deaths per year [1][2]; it is the second most common cancer diagnosis, comprising a total of 13% of new cancer cases each year [3]. Age is a risk factor for lung cancer [4], due to biological factors, including DNA damage (over time) and telomere shortening. Smoking is the primary “agent” in the development of lung cancer, responsible for about 80% of lung cancer-related deaths [5]. Men and women who smoke are 23% and 13%, respectively, more likely to develop lung cancer compared to never-smokers [6]. The risk of being diagnosed with lung cancer, due to tobacco consumption, varies in ethnic groups, e.g., compared to white people, African Americans and Native Hawaiian smokers are shown to be at a greater risk of developing lung cancer, with the highest incidences and death rates. Latino and Japanese American smokers are less likely to develop the disease and present the lowest cancer-specific mortality [1][6]. Accumulating evidence supports that genetic factors are also risk factors for lung cancer [7]. Recently, several novel lung cancer susceptibility genes, including those on chromosomes 6q23-25 and 13q31.3, were identified by large-scale genome-wide association studies as being associated with lung cancer risk, particularly in never-smokers, who account for 25% of lung cancer patients worldwide [8][9]. Furthermore, an individual who has a positive family history of lung cancer has a 1.7-fold increased risk of developing lung cancer [9][10][11]. Lung cancer in never-smokers has been associated with genetic factors, as well as occupational exposures to lung carcinogens, exposure to ionizing radiation, and a poor diet [1][5][9][12].
Lung cancer can be classified into two major histological subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for about 85% of all lung cancer cases and presents a 25% chance of a 5-year-survival [13]. Adenocarcinoma and squamous cell carcinoma are the two major histologic types, accounting for about 40% and 25% of lung cancers, respectively [14]. SCLC is the lung cancer type that tends to spread the fastest, accounting for 10% to 15% of all lung cancers [15]. Individuals who have this type of lung cancer present a 7% chance of a 5-year-survival [13]. Figure 1 represents the distribution of the main histological subtypes.
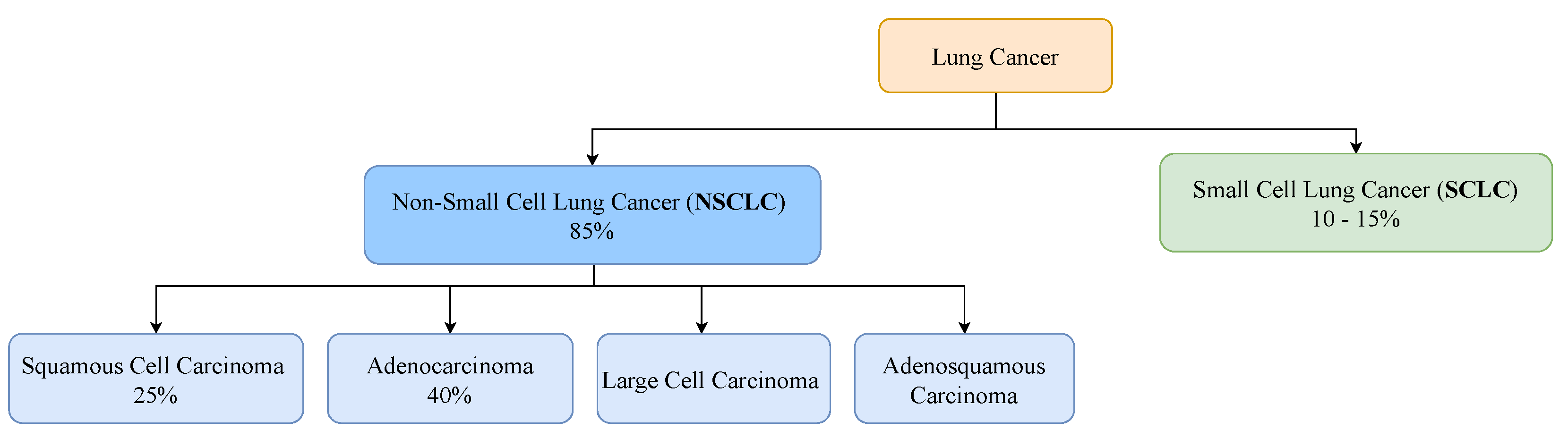
Figure 1.
Prevalence of the major histological subtypes of lung cancer: non-small cell lung cancer and small cell lung cancer.
Computed tomography (CT) is the most useful imaging modality used for lung cancer management, including diagnosis, staging, treatment planning, and treatment response evaluation [16][17][18][19]. CT is the recommended screening test for lung cancer; but confirmation of the malignancy and characterization of the nodule is traditionally conducted via a biopsy, which is an invasive and risky procedure for the patient that can lead to some clinical complications. Recently, non-invasive, fast, and easy-to-use techniques, such as computer-aided diagnosis (CAD) based on CT scans, have been developed for lung cancer characterization, to improve the accuracy of diagnosis, determine the most appropriate treatment for each subject and, consequently, decrease the mortality rate of patients battling lung cancer [20][21][22]. Since imaging is already regularly repeated during treatment, it has the potential to continuously supervise therapy and monitor the rise and growth of the disease or its response to therapy.
2. Clinical Pathway for Lung Cancer
The clinical pathway for lung cancer consists of the following main steps: screening, diagnosis, and treatment plan development [23]. The process of diagnosis begins with an initial evaluation, and it is followed by an analysis of tissues collected in the biopsy for the cancer confirmation, characterization, and staging. The treatment plan will consider the diagnosis and the patient’s functional status.
2.1. Screening
Screening involves testing an asymptomatic individual for a disease. Lung cancer typically does not cause signs or symptoms in its earliest stages; these only occur when the disease is advanced. Thus, screening exams are the most powerful tools for early detection. A total of 65% of patients are diagnosed when the disease has already reached the metastatic stage; these individuals have a 6% chance of 5-year-survival [24]. Only 17% of cases are diagnosed in a local state; in these cases, the 5-year survival rate increases to 59% [13]. The US national lung screening trial (NLST) and NELSON (two randomized controlled trials of low-dose CT (LDCT)-based lung cancer screenings in high-risk populations) showed evidence of a statistically significant mortality reduction in patients [19][25]. CT exams are recommended for adults aged 50 to 80 who have 20 packs-a-year smoking histories and who currently smoke or have quit within the past 15 years [26].
2.2. Diagnosis
2.2.1. Initial Evaluation
The most common lung cancer symptoms are chronic cough, repeated respiratory infections, fatigue, hemoptysis, shortness of breath, hoarseness, and chest pain [27]. The initial evaluation should include a careful analysis of risk factors for lung cancer, prior history of cancer, evaluation of comorbidities, functional status, and overall health status [23]. All patients suspected of having lung cancer, who are undergoing initial evaluations, will require imaging studies. CT is the primary imaging exam performed to assess the existence of nodules and eventually lung cancer. The nodule size, nodule growth rate, and spiculation of the nodule anatomical margins are the main radiological predictors of malignancy risk [28]. The tissue biopsy will confirm the disease.
2.2.2. Tissue Biopsy
Tissue biopsy is the current standard procedure for lung cancer classification, consisting of an assessment technique that includes several methods, such as fine-needle aspiration, bronchoscopy, endobronchial ultrasound, mediastinoscopy, thoracentesis, thoracoscopy, and electromagnetic navigation. Bronchoscopy is the main diagnosis procedure, with flexible bronchoscopy being more useful for central lesions and navigational bronchoscopy displaying higher sensitivities for peripheral lesions [29][30]. Percutaneous approaches include transthoracic needle aspiration (TTNA) or needle/core biopsy (TTNB) of the primary tumor. The samples collected will be analysed in immunohistochemical stains and molecular tests in order to assess the mutation status of predominant oncogenes and identify the main biomarkers of the tumor, supporting precision medicine [31].
Despite a tissue biopsy being considered a relatively safe procedure, it is not free of complications since the invasive nature of a tissue biopsy limits its use, particularly in patients with inaccessible tumor sites or when repeated biopsies are needed [32]. Moreover, tissue biopsies have some limitations related to tumor heterogeneity, since a single biopsy may not represent the complexity of the entire tumor and its genetic alteration. Thus, there is an inability to carry out a complete therapeutic decision and prognosis, which are the main issue in clinical practices [29][30]. Therefore, although recommendations are clear about the need for a biopsy, its invasive nature limits repetition for treatment response evaluations [33].
2.2.3. Liquid Biopsy
A liquid biopsy is a non-invasive, safe, and accessible technique that allows the detection of tumor cells or tumor-derived products in body fluids. Liquid biopsies consist of the analysis of circulating tumor cells and/or circulating tumor DNA (ctDNA) molecules employing simple tests on body fluid samples. A liquid biopsy may be viewed as a key strategy to improve lung cancer early diagnosis—either alone or as complementary data for imaging findings. Other clinical applications consist of patient stratification, therapeutic decisions, and disease monitoring [29][30]. Liquid biopsies are shown to be useful in the management of NSCLC in clinical practices. This approach overcomes both spatial and temporal tumor heterogeneity problems and enables repeatable evaluations of cancer patients while reducing the inherent risks and discomfort of tissue biopsies [34].
Several liquid biopsy-derived biomarkers have been identified, such as circulating tumor cells, circulating cell-free DNA, circulating micro-RNAs, tumor-derived exosomes, and tumor-educated platelets [29][30]. Evidence indicates that a liquid biopsy can be applied to dynamically evaluate resistance mutations during treatment with epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) inhibitors [35]. However, there are limitations, i.e., related to low sensitivity for the detection in early-stage tumors and, consequently, less utility in clinical practices [36][37]. In the early stages of lung cancer, when the cancer biomarkers have very low values, the available biomarkers display significant proportions of false negatives or a need for a confirmatory tissue biopsy. Thus, the development of more sensitive and specific assays must occur in the following years to allow its standard use in clinical practice. There is also the possibility of combining different biomarkers and other diagnostic techniques, such as imaging techniques, although more robust studies are required to define the best combinations and to validate the clinical role of liquid biopsy in the screening or diagnosis of lung cancer [29][30].
2.2.4. Staging
For confirmed malignant cases, it is important to determine the extension of cancer (cancer staging) and identify additional pathologies that can influence the treatment plan of the patient. The type of cancer is characterized in a triple form, using cytology and histology, immunohistochemical stains, and molecular testing, allowing to identify the type and subtype of cancer, to assess the PD-L1 expression, and define the genomic profile. Staging tests may include imaging procedures that allow the clinician to search for evidence that cancer has spread beyond the lungs. These tests include CT, magnetic imaging resonance (MRI), positron emission tomography (PET), and bone scans. The characterization and staging of cancer will help define a treatment plan, combining one or more types: surgery, radiotherapy, and drug therapy (chemo- and immunotherapy); as well as the order that they are applied, taking into account the specific conditions of the patient. In cases where there is a progress of cancer after the line of treatment, the process of diagnosis will restart, using an imaging analysis, and if the tumor is so different from the expected, a biopsy will be performed.
2.3. Treatment Plan
Depending on the staging of lung cancer, patients are eligible for treatments that may be local, such as surgery and radiation therapy; systemic, such as chemotherapy and targeted therapy; or combined, meaning the merging of two or more types of treatments [38]. Early-stage NSCLC patients can be treated surgically with a 5-year-survival rate of 77% [39]. If stage I–II patients are unable to tolerate surgery (due, for example, to other associated health problems or inaccessible tumor location), they usually receive stereotactic body radiation therapy. If the tumor is found to be resectable from imaging studies and biopsies and the patient is able to tolerate surgery, surgery is typically performed to remove the tumor. However, most cases are identified in the late stages, when surgery is no longer an option as a result of distant metastases. Treatment for stage III NSCLC patients includes a combination of radiation therapy and chemotherapy. Immunotherapy is a targeted therapy that attacks immune checkpoint pathways, which includes the blockade of the inhibitory receptors cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1), and its ligand, PD-L1, and has altered the management of NSCLC over the last 10 years [40]. For stage IV NSCLC patients, who constitute 57% of newly diagnosed lung cancer patients [41], there are several lines of treatment, depending on whether the cancer cells have certain genetic or protein alterations, and the overall health of the patient. Personalized medicine by targeting appropriate genomic biomarkers with small-molecule tyrosine kinase inhibitors (TKIs) has helped improve survival in stage IV NSCLC patients, while decreasing multiple undesirable side effects associated with cancer treatment [42][43][44][45]. In lung cancer, one of the most relevant oncogenes and a predictive biomarker with clinically approved therapies is EGFR [46]. EGFR-dedicated therapies with TKIs, such as afatinib and erlotinib, are currently used as first- and second-line lung cancer treatments [47], improving objective response rates and progression-free survival compared to cytotoxic therapy for patients with mutated EGFR [48][49][50]. Furthermore, for patients whose tumor expresses the PDL-1 protein on at least 50% of the cells, the treatment options might include the administration of an immunotherapy drug, such as pembrolizumab, which is a human immune checkpoint inhibitor that can inhibit the PD-1 or PD-L1 and improve antitumor immunity [51][52][53]. However, immunotherapy is only effective for a small percentage of cancer patients (20%), due to the low performance of the current predictive biomarkers of the response to the immune checkpoint blockade therapy, which relies on the detection of PD- L1 in cancer tissue [54]. On the other hand, if the PD-L1 levels are lower than 50%, the treatment often consists of chemo- and immunotherapy combination.
2.4. Main Biomarkers for Target Therapies
Only a small portion of NSCLC patients are diagnosed at an early stage (I or II) when surgical resection is an optimal treatment option [55][56]. Researchers are focusing on developing targeted therapies because of the ability to deliver drugs effectively with high specificity while being less toxic. Target therapies identify and block specific enzymes, proteins, or other molecules involved in cancer development. Thus, understanding the pathophysiology of cancer is a crucial step to identify molecular targets that favor the promotion of cancer cell growth, interfere with the regulation of cell cycle, and/or induce cell death, so as to interfere within the tumor microenvironment and activate the immune system [57]. In NSCLC, one-third of the patients have an oncogenic driver mutation that is druggable, another third show excessive inflammation in the tumor micro-environment that can be targeted with an immune checkpoint, and the last third of patients are treated with combined chemotherapy [58]. In NSCLC, some specific targets that have already been studied are oncogenes, such as EGFR, KRAS, or ALK, and immune checkpoints, such as PD-1/PD-L1 and CTLA-4.
2.4.1. Oncogenes
EGFR is a receptor found on the surface of cells and is a member of the EGFR-family of extracellular protein ligands that cannot penetrate the cell membrane; thus, they function via targeted signal transduction pathways that carry cellular information [59][60]. Ultimately, its function as cell proliferation, differentiation, motility, and survival factor allows cancer cell growth and development, as well its metastasis [59]. It is a tyrosine kinase receptor that is frequently overexpressed in tumors, and so it is considered a predictor of survival [59]. The EGFR mutation occurs in 10–20% of patients with lung cancer (80–85% of NSCLC) and is mostly adenocarcinoma in younger women and never-smokers [56]. Within the EGFR mutation, the most common are the exon 19 deletion and exon 21 L858R point mutation—almost 90% of them—and they are also the ones with the better response to EGFR-targeted therapies [58]. For EGFR, there are two types of drugs: (1) monoclonal antibodies, which bind to the extracellular domain of EGFR, preventing its dimerization; (2) tyrosine kinase inhibitors, which block the intracellular part of the receptor [59][61].
The rat sarcoma proto-oncogene (RAS) family mutations are the most frequent cause of cancer, including three different oncogenes: the Kirsten rat sarcoma oncogene KRAS, the neuroblastoma rat sarcoma oncogene (NRAS) and the Harvey rat sarcoma oncogene (H-RAS) [62]. The KRAS isoform expresses the most alterations, accounting for 86% of RAS mutations, mainly in lung, pancreatic, and colon cancers [62][63][64]. KRAS mutations are responsible for about 30% of lung adenocarcinomas, with higher prevalence in Western countries and in smoking patients, showing worse outcomes in early and advanced stages of lung cancer [62][63][64].
KRAS is a guanosine triphosphate protein (GTPase) encoded by the KRAS oncogene and activated by cell surface receptors, such as EGFR, fibroblast growth factor receptor (FGFR), and human epidermal growth factor receptors 2–4 (HER2-4), which through downstream pathways will induce cell proliferation, differentiation, or cell death. Although the molecular processes involving the RAS family are quite known, there is no potent anti-RAS therapy as yet, since, for the last four decades, every targeted therapy to this molecular pathway has not shown good clinical results [62][63][64][65]. Reasons for that likely include heterogeneity involving the RAS mutations, mainly the ones in the KRAS oncogene, besides the co-occurring genetic events and the diverse KRAS allelic content that contributes to direct clinical implications [62].
ALK is a member of the transmembrane insulin receptor superfamily of receptor tyrosine kinases [59][66]. Although its mutations have been known for more than 10 years, the ALK role, as well as ligands, are still being debated [66]. In NSCLC, ALK mutations represent 2–7% mostly in never- to light-smokers, men, with 50 being the median age of diagnosis [56]. ALK tyrosine kinase inhibitors are ATP-competitive antagonists, preventing ALK kinase activity and promoting tumor reduction [59]. Crizotinib is an example of an ALK inhibitor, which reduces 50–60% of tumor sizes in patients with this mutation and provides greater improvement in one’s quality of life, although most of the patients had previous chemotherapy [59][66].
2.4.2. Immunobiomarkers
Inhibitory checkpoint molecules generated upon T cell activation, such as those that regulate the immunological synapses between T cells and dendritic cells in lymph nodes (CTLA-4 and B7.1), or between T cells and tumor cells (PD-1 and PDL-1/2), are currently the most relevant targets for immunotherapy (see Figure 2) [56].
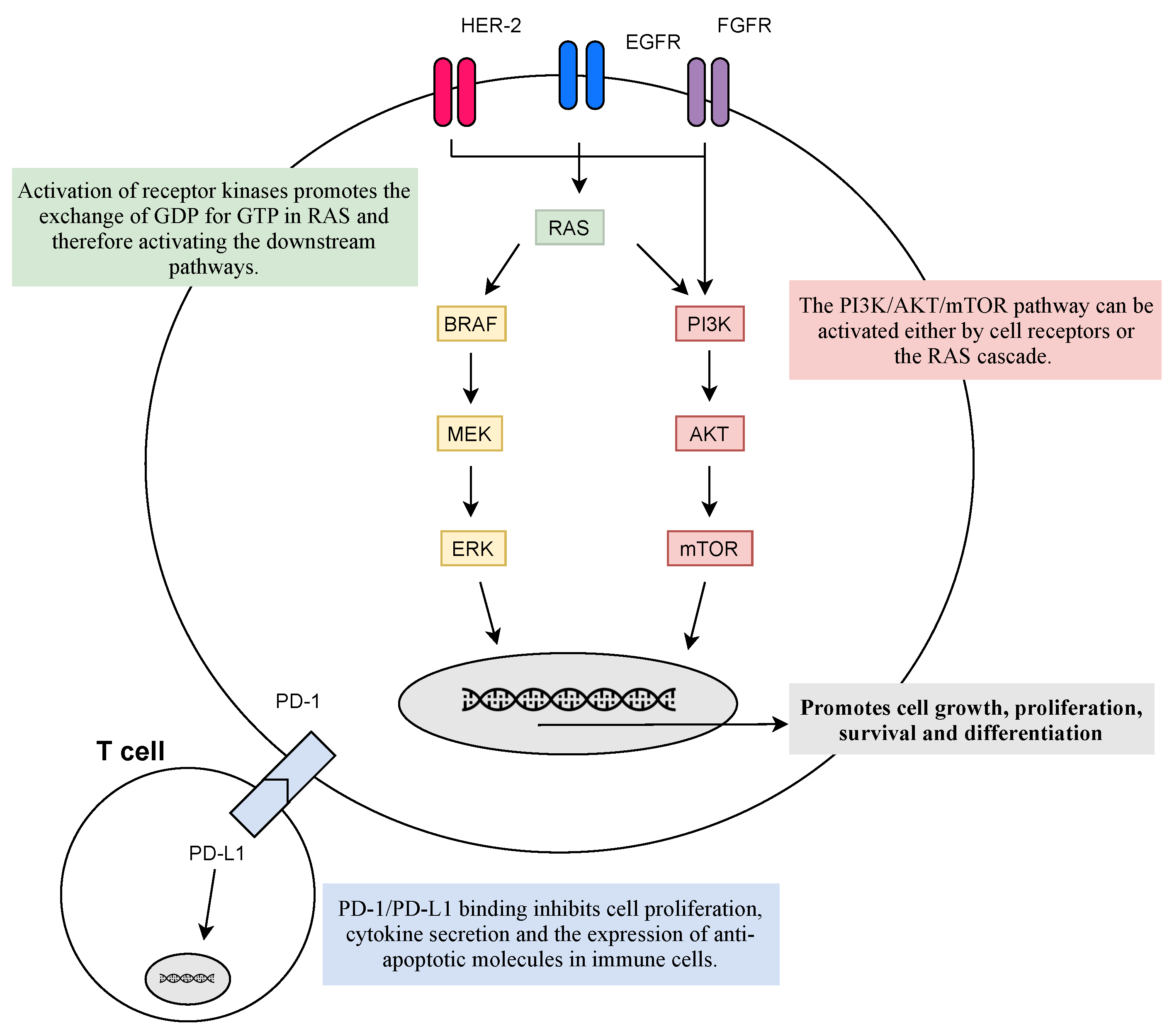

Figure 2. Physiological response to the activation of membrane receptors and immune receptors. Activation of receptor kinases, such as EGFR, FGFR, or HER-2, promotes the activation of RAS and its downstream pathways that facilitate cell growth, proliferation, cell survival, and differentiation. However, mutations in the RAS family lead to its constitutive activation and the hyperactivation of the downstream pathways—leading to uncontrolled cell survival. On the other hand, PD-L1 is present on the cell surface of immune cells and its binding to tumor cells PD-1 inhibits cell proliferation, cytokine secretion, and the expression of anti-apoptotic molecules in immune cells culminating in the escape of cancer from immunosurveillance. The goal of target therapies is to diminish the activation of abnormal signalling pathways, which can be inhibited at every step.
PD-1 presents on the cell surface as a co-inhibitory receptor, expressed in T cells, B-cells, monocytes, and natural killer T cells after activation. Binding of PD-L1 to PD-1 inhibits cell proliferation, cytokine secretion, and the expression of anti-apoptotic molecules in immune cells [55][59][67]. In various malignancies, including lung cancer, PD-L1 is overexpressed, allowing the activation of PD-1 signalling pathways and ultimately the escape of cancer from immunosurveillance [67]. Blockage of PD-1/PD-L1 pathways has been the most successful strategy as it promotes the programmed death of tumor cells, with various anti-PD-1/PD-L1 antibodies approved for first- and second-line settings with manageable toxicity profiles, improved efficacy, and longer durations of response compared to standard chemotherapy [59][67][68].
CTLA-4 (or CD152) is a known receptor of an immune checkpoint pathway that downregulates T cell proliferation, mainly in lymph nodes, and promotes immune self-tolerance [59][67]. CTLA-4 is often overexpressed in a chronic inflammatory status, such as in cancer, implying that its presence in the tumor microenvironment may be involved in the dysregulation of the immune response [67]. Therefore, targeting CTLA-4 allows for enhancing T cell-mediated anti-tumor activity [59][67]. Monoclonal antibodies, such as ipilimumab, prevent CTLA-4 binding to its ligands (CD80/CD86) and, thus, augmenting T cell activation [59][68].
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Glob. Health 2019, 85, 8.
- World Health Organization. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. In International Agency for Research on Cancer; World Health Organization: Geneva: Switzerland, 2018.
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. Lung Cancer Pers. Med. 2016, 893, 1–19.
- Galvez-Nino, M.; Ruiz, R.; Pinto, J.A.; Roque, K.; Mantilla, R.; Raez, L.E.; Mas, L. Lung cancer in the young. Lung 2020, 198, 195–200.
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288.
- Kanwal, M.; Ding, X.J.; Cao, Y. Familial risk for lung cancer. Oncol. Lett. 2017, 13, 535–542.
- Ji, X.; Bossé, Y.; Landi, M.T.; Gui, J.; Xiao, X.; Qian, D.; Joubert, P.; Lamontagne, M.; Li, Y.; Gorlov, I.; et al. Identification of susceptibility pathways for the role of chromosome 15q25. 1 in modifying lung cancer risk. Nat. Commun. 2018, 9, 3221.
- Yokota, J.; Shiraishi, K.; Kohno, T. Genetic basis for susceptibility to lung cancer: Recent progress and future directions. Adv. Cancer Res. 2010, 109, 51–72.
- Okazaki, I.; Ishikawa, S.; Ando, W.; Sohara, Y. Lung adenocarcinoma in never smokers: Problems of primary prevention from aspects of susceptible genes and carcinogens. Anticancer. Res. 2016, 36, 6207–6224.
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220.
- Rivera, G.A.; Wakelee, H. Lung cancer in never smokers. Lung Cancer Pers. Med. 2016, 893, 43–57.
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902.
- Society, A.C. Cancer Facts & Figures. 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021 (accessed on 15 April 2021).
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454.
- Society, A.C. Lung Cancer. 2019. Available online: https://www.cancer.org/cancer/lung-cancer.html (accessed on 15 April 2021).
- Zhang, H.; Cai, W.; Wang, Y.; Liao, M.; Tian, S. CT and clinical characteristics that predict risk of EGFR mutation in non-small cell lung cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2019, 24, 649–659.
- Chaudhry, A.; Gul, M.; Chaudhry, A. Utility of computed tomography lung cancer screening and the management of computed tomography screen-detected findings. J. Thorac. Dis. 2018, 10, 1352.
- Al Mohammad, B.; Brennan, P.C.; Mello-Thoms, C. A review of lung cancer screening and the role of computer-aided detection. Clin. Radiol. 2017, 72, 433–442.
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513.
- Junior, J.R.F.; Koenigkam-Santos, M.; Cipriano, F.E.G.; Fabro, A.T.; de Azevedo-Marques, P.M. Radiomics-based features for pattern recognition of lung cancer histopathology and metastases. Comput. Methods Programs Biomed. 2018, 159, 23–30.
- Ostridge, K.; Wilkinson, T.M. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur. Respir. J. 2016, 48, 216–228.
- Bakr, S.; Gevaert, O.; Echegaray, S.; Ayers, K.; Zhou, M.; Shafiq, M.; Zheng, H.; Benson, J.A.; Zhang, W.; Leung, A.N.; et al. A radiogenomic dataset of non-small cell lung cancer. Sci. Data 2018, 5, 180202.
- Ost, D.E.; Jim Yeung, S.C.; Tanoue, L.T.; Gould, M.K. Clinical and Organizational Factors in the Initial Evaluation of Patients With Lung Cancer. Chest 2013, 143, e121S–e141S.
- Aggarwal, A.; Lewison, G.; Idir, S.; Peters, M.; Aldige, C.; Boerckel, W.; Boyle, P.; Trimble, E.L.; Roe, P.; Sethi, T.; et al. The state of lung cancer research: A global analysis. J. Thorac. Oncol. 2016, 11, 1040–1050.
- Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409.
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970.
- Latimer, K.M.; Mott, T.F. Lung cancer: Diagnosis, treatment principles, and screening. Am. Fam. Physician 2015, 91, 250–256.
- Loverdos, K.; Fotiadis, A.; Kontogianni, C.; Iliopoulou, M.; Gaga, M. Lung nodules: A comprehensive review on current approach and management. Ann. Thorac. Med. 2019, 14, 226–238.
- Freitas, C.; Sousa, C.; Machado, F.; Serino, M.; Santos, V.; Cruz-Martins, N.; Teixeira, A.; Cunha, A.; Pereira, T.; Oliveira, H.P.; et al. The Role of Liquid Biopsy in Early Diagnosis of Lung Cancer. Front. Oncol. 2021, 11, 1130–1156.
- Ganesha, D.V.; Naik, R.; Mufti, S.S.; Varayathu, H. Molecular Therapeutics of Non-Small Cell Lung Cancer (NSCLC) and Challenges in Repeat Tissue Biopsy. Adv. Lung Cancer 2021, 10, 21–39.
- Tuzi, A.; Bolzacchini, E.; Suter, M.B.; Giaquinto, A.; Passaro, A.; Gobba, S.; Vallini, I.; Pinotti, G. Biopsy and re-biopsy in lung cancer: The oncologist requests and the role of endobronchial ultrasounds transbronchial needle aspiration. J. Thorac. Dis. 2017, 9, S405.
- Scrivener, M.; de Jong, E.E.; van Timmeren, J.E.; Pieters, T.; Ghaye, B.; Geets, X. Radiomics applied to lung cancer: A review. Transl. Cancer Res. 2016, 5, 398–409.
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346.
- Wu, Z.; Yang, Z.; Dai, Y.; Zhu, Q.; Chen, L.A. Update on liquid biopsy in clinical management of non-small cell lung cancer. Oncotargets Ther. 2019, 12, 5097.
- Rijavec, E.; Coco, S.; Genova, C.; Rossi, G.; Longo, L.; Grossi, F. Liquid biopsy in non-small cell lung cancer: Highlights and challenges. Cancers 2020, 12, 17.
- Bai, Y.; Zhao, H. Liquid biopsy in tumors: Opportunities and challenges. Ann. Transl. Med. 2018, 6, S89.
- Pisapia, P.; Malapelle, U.; Troncone, G. Liquid biopsy and lung cancer. Acta Cytol. 2019, 63, 489–496.
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266.
- Carnio, S.; Novello, S.; Papotti, M.; Loiacono, M.; Scagliotti, G.V. Prognostic and predictive biomarkers in early stage non-small cell lung cancer: Tumor based approaches including gene signatures. Transl. Lung Cancer Res. 2013, 2, 372.
- Dine, J.; Gordon, R.; Shames, Y.; Kasler, M.K.; Barton-Burke, M. Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pac. J. Oncol. Nurs. 2017, 4, 127.
- N.I.H. National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer; 2020. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 1 May 2021).
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61.
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.; Faivre-Finn, C.; Mok, T.; Reck, M.; Van Schil, P.; Hellmann, M.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237.
- Schrank, Z.; Chhabra, G.; Lin, L.; Iderzorig, T.; Osude, C.; Khan, N.; Kuckovic, A.; Singh, S.; Miller, R.J.; Puri, N. Current molecular-targeted therapies in NSCLC and their mechanism of resistance. Cancers 2018, 10, 224.
- Jiang, W.; Cai, G.; Hu, P.C.; Wang, Y. Personalized medicine in non-small cell lung cancer: A review from a pharmacogenomics perspective. Acta Pharm. Sin. B 2018, 8, 530–538.
- Nan, X.; Xie, C.; Yu, X.; Liu, J. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget 2017, 8, 75712.
- Holleman, M.S.; Al, M.J.; Zaim, R.; Groen, H.J.; Uyl-de Groot, C.A. Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations. Eur. J. Health Econ. 2020, 21, 153–164.
- Zhang, C.; Leighl, N.B.; Wu, Y.L.; Zhong, W.Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 45.
- Liu, Y.; Kim, J.; Qu, F.; Liu, S.; Wang, H.; Balagurunathan, Y.; Ye, Z.; Gillies, R.J. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology 2016, 280, 271–280.
- Pinheiro, G.; Pereira, T.; Dias, C.; Freitas, C.; Hespanhol, V.; Costa, J.L.; Cunha, A.; Oliveira, H.P. Identifying relationships between imaging phenotypes and lung cancer-related mutation status: EGFR and KRAS. Sci. Rep. 2020, 10, 3625.
- Ma, W.; Gilligan, B.M.; Yuan, J.; Li, T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J. Hematol. Oncol. 2016, 9, 47.
- Kerr, K.M.; Nicolson, M.C. Non–small cell lung cancer, PD-L1, and the pathologist. Arch. Pathol. Lab. Med. 2016, 140, 249–254.
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in non–small cell lung cancer: Facts and hopes. Clin. Cancer Res. 2019, 25, 4592–4602.
- Sharma, P. Immune checkpoint therapy and the search for predictive biomarkers. Cancer J. 2016, 22, 68.
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52, 103–109.
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancte 2017, 389, 299–311.
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196.
- Pakkala, S.; Ramalingam, S.S. Personalized therapy for lung cancer: Striking a moving target. JCI Insight 2018, 3, e120858.
- Silva, A.P.; Coelho, P.V.; Anazetti, M.; Simioni, P.U. Targeted therapies for the treatment of non-small-cell lung cancer: Monoclonal antibodies and biological inhibitors. Hum. Vaccines Immunother. 2017, 13, 843–853.
- Liu, T.C.; Jin, X.; Wang, Y.; Wang, K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Cancer Res. 2017, 7, 187–202.
- Dassonville, O.; Bozec, A.; Fischel, J.L.; Milano, G. EGFR targeting therapies: Monoclonal antibodies versus tyrosine kinase inhibitors. Similarities and differences. Crit. Rev. Oncol. 2007, 61, 53–61.
- Uras, I.Z.; Moll, H.P.; Casanova, E. Targeting KRAS mutant non-small-cell lung cancer: Past, present and future. Int. J. Mol. Sci. 2020, 21, 4325.
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716.
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177.
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552.
- Golding, B.; Luu, A.; Jones, R.; Viloria-Petit, A.M. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol. Cancer 2018, 17, 52.
- Paulsen, E.E.; Kilvaer, T.K.; Rakaee, M.; Richardsen, E.; Hald, S.M.; Andersen, S.; Busund, L.T.; Bremnes, R.M.; Donnem, T. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: Diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol. Immunother. 2017, 66, 1449–1461.
- Moya-Horno, I.; Viteri, S.; Karachaliou, N.; Rosell, R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Ther. Adv. Med. Oncol. 2018, 10, 1–12.
More
