Chimeric antigen receptor (CAR) T cell therapy has been approved to treat patients with various B cell-related tumors, including B-cell precursor acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), and high-grade B-cell lymphoma. T cell receptor (TCR) knockout is a critical step in producing universal CAR T cells. A promising approach to achieving the knockout is to deliver the CRISPR/Cas9 system into T cells using electrotransfer technology.
- CAR-T
- Electrotransfer
- Caspase-3
- Apoptosis
- Cell Viability
1. Introduction
CAR T cells can be generated from the patient’s own T cells or those from healthy donors. The autologous CAR T cells are patient-specific, but it is a challenge to produce them for a subpopulation of cancer patients, and the production platform is currently inefficient for large-scale clinical applications[1]. To avoid these problems, multiplexed genome editing strategies have been used to knock out certain endogenous genes, such as αβ T-cell receptor (TCR), in donor T cells to generate allogeneic universal CAR T cells[2][3], which can be produced massively and applied to treat a large number of patients[2]. The elimination of the αβ TCR is critical for avoiding the graft-versus-host-disease (GVHD) risk in cancer patients[4]. Previous studies have shown that the elimination can happen in cells with T cell receptor-α constant (TRAC) mutation or be achieved through TRAC knockout[3][5]. One of the promising approaches to gene knockout is to deliver the CRISPR/Cas9 system into cells using electrotransfer technology[6].
The technology has been used for the delivery of various molecular cargo into cells, such as DNA, RNA, protein, and ribonucleoprotein (RNP)[7][8]. It can be applied to all cell types and has few restrictions on the type of molecular cargo being delivered[7]. Moreover, electrotransfer is easy to operate and can be readily scaled up for producing a large amount of cells needed for cell therapy in the clinic[7]. Recently, electrotransfer has been employed in the production of CAR T cells in a clinical trial[9]. Despite these advantages, electrotransfer may result in severe cell death[9][10]. The viability of human primary T cells in gene electrotransfer experiments is highly dependent on the donors, varying from 20% to 40% under optimized experimental conditions for achieving adequate electrotransfer efficiency (e.g., 40%)[10][11][12]. The low viability is a major issue that needs to be tackled before the technology can be applied successfully to manufacturing CAR-T cells.
2. Inhibition of Caspases in Human Primary T Cells Improves Gene Editing Efficiency
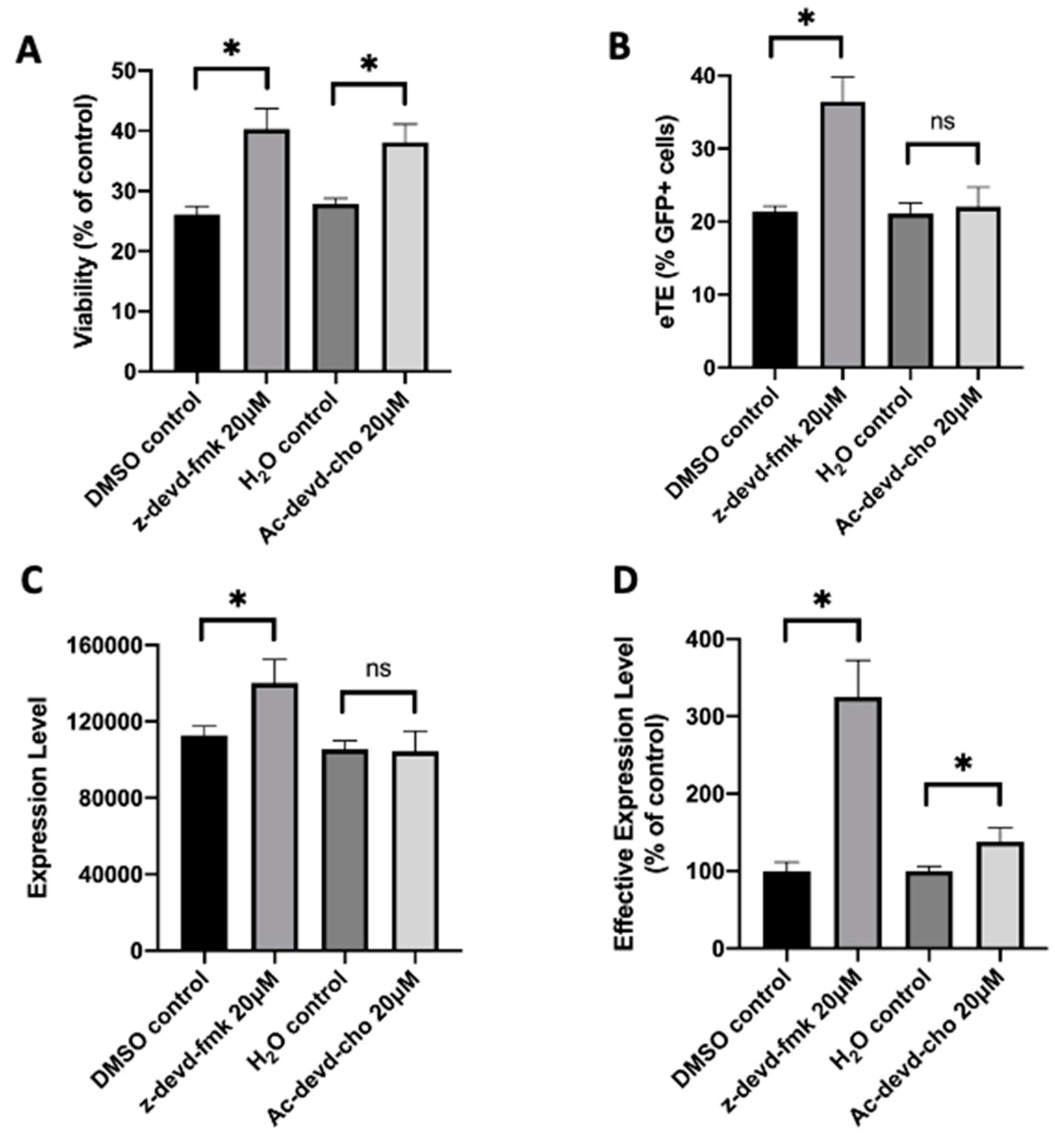
To demonstrate the capability of caspase inhibition for improving CAR T cell production, we treated human primary T cells with a pan-caspase inhibitor, z-vad-fmk, after pDNA or RNP electrotransfer. Our data showed that although the compound was nontoxic to T cells, it became highly toxic when the treatment is combined with electrotransfer. At the concentrations that worked for improving electrotransfer in Jurkat cells, the treatment with z-vad-fmk killed the majority of human T cells. Even at 0.5 µM, which was 100 times lower than the optimal concentration for Jurkat cells, the inhibitor treatment still killed approximately half of the T cell population, compared to the untreated controls. To reduce the toxicity caused presumably by non-specific inhibition of all caspases, we tested inhibitors more specific to caspase 3, such as z-devd-fmk and Ac-devd-cho. We observed that both inhibitors could increase the T cell viability by ~30% at the optimal treatment concentrations, compared to the matched controls (Figure 1A). Inhibition of caspase 3 in human T cells with z-devd-fmk treatment at 20 µM could effectively enhance the electrotransfer efficiency (Figure 1B–D). The treatment of T cells with Ac-devd-cho resulted in insignificant or minor changes in the electrotransfer efficiency (Figure 1B–D).
Figure 1. Effects of caspase inhibitors on cell viability and EGFP expression in human primary T cells. Human primary T cells were treated with z-devd-fmk and Ac-devd-cho for 24 hours post electrotransfer to inhibit cell apoptosis. (A) Cell viability; (B) eTE; (C) expression level; (D) effective expression level. Pulsing condition: 650 V/0.2 cm, 400 µs, 1 pulse. ns, non-significant. Error bars, SEM; * p < 0.05, Student’s t-test. N = 4.
Further, we tested the effect of caspase 3 inhibition on improving TRAC knockout efficiency in human primary T cells. We electrotransferred an RNP, a complex of Cas9 protein and TRAC-targeting sgRNA, into human T cells, followed by the treatment of the cells with either inhibitor (z-devd-fmk or Ac-devd-cho) at 20 µM or equal amount of solvent (DMSO or H2O). The TRAC editing in T cells was confirmed using ICE analysis (Figure 2A,D), and the indel was determined using the TIDE analysis. At 24 h post electrotransfer, the inhibition of caspase 3 with z-devd-fmk or Ac-devd-cho treatment increased T cell viability from 57% to 71%, or from 57% to 68%, respectively, compared to the matched controls (Figure 2B,E). The same treatments insignificantly alter the indel quantified at 48 h post electrotransfer (Figure 2C,F). These data demonstrated that inhibition of caspase 3 could effectively increase the total number of gene-edited human T cells by means of increasing cell viability without decreasing the percent of gene-edited cells.
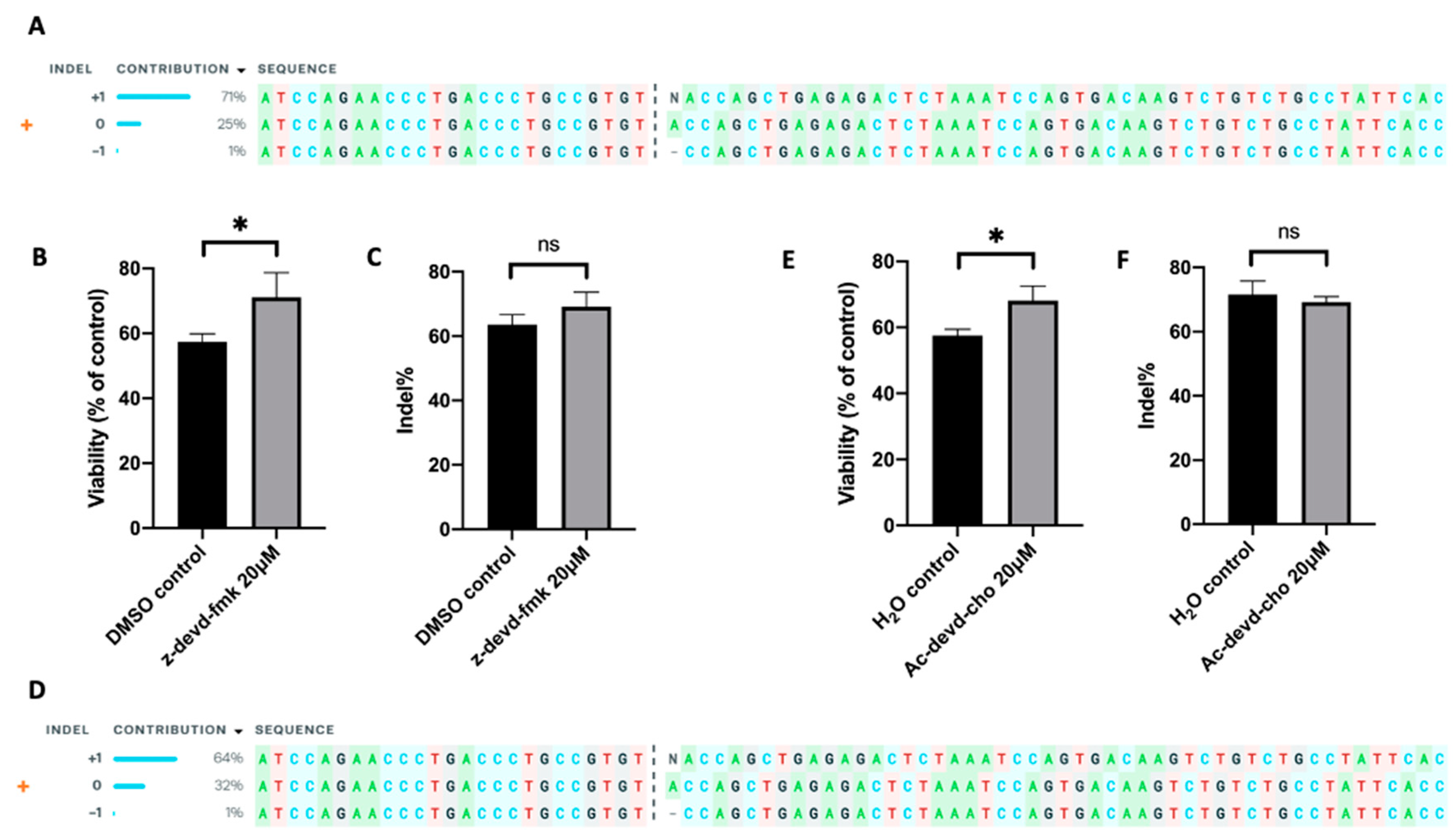 Figure 2. Effects of apoptosis inhibition on cell viability and indel frequency in human primary T cells. The gene-editing was achieved through electrotransfer of an RNP targeting the TRAC gene. (A–C) Apoptosis was inhibited with z-devd-fmk treatment; (D–F) apoptosis was inhibited with Ac-devd-cho treatment. (A,D) ICE analysis, showing the outcome of TRAC editing in T cells; (B,E) cell viability measured at 24 h post pulsing; (C,F) Indel within TRAC gene determined at 48 h post pulsing using TIDE analysis. Pulsing condition: 650 V/0.2 cm, 300 µs, 2 pulses, 10 Hz. ns, non-significant. Error bars, SEM; * p < 0.05, Student’s t-test, N = 4.
Figure 2. Effects of apoptosis inhibition on cell viability and indel frequency in human primary T cells. The gene-editing was achieved through electrotransfer of an RNP targeting the TRAC gene. (A–C) Apoptosis was inhibited with z-devd-fmk treatment; (D–F) apoptosis was inhibited with Ac-devd-cho treatment. (A,D) ICE analysis, showing the outcome of TRAC editing in T cells; (B,E) cell viability measured at 24 h post pulsing; (C,F) Indel within TRAC gene determined at 48 h post pulsing using TIDE analysis. Pulsing condition: 650 V/0.2 cm, 300 µs, 2 pulses, 10 Hz. ns, non-significant. Error bars, SEM; * p < 0.05, Student’s t-test, N = 4.
The auhtors' data show that specific inhibitors for caspase 3, such as z-devd-fmk and Ac-devd-cho, are non-toxic to human primary T cells even when combined with electrotransfer. Thus, they can be used to improve the efficiency of gene-editing in T cells. Results from the study suggest that inhibition of caspases is a promising strategy for improving CAR T cell production, and more generally, electrotransfer of molecular cargo in cell engineering applications.
References
- Zhao, J.; Lin, Q.; Song, Y.; Liu, D. Universal CARs, universal T cells, and universal CAR T cells. J. Hematol. Oncol. 2018, 11, 132. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Cheng, C.; Cheng, A.W.; Zhang, X.; Li, N.; Xia, C.; Wei, X.; Liu, X.; Wang, H. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017, 27, 154–157. [Google Scholar] [CrossRef]
- Torikai, H.; Reik, A.; Liu, P.-Q.; Zhou, Y.; Zhang, L.; Maiti, S.; Huls, H.; Miller, J.C.; Kebriaei, P.; Rabinovitch, B. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood J. Am. Soc. Hematol. 2012, 119, 5697–5705. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, G.; Zhang, L.; Zhao, Q. Building potent chimeric antigen receptor T cells with CRISPR genome editing. Front. Immunol. 2019, 10, 456. [Google Scholar] [CrossRef]
- Morgan, N.V.; Goddard, S.; Cardno, T.S.; McDonald, D.; Rahman, F.; Barge, D.; Ciupek, A.; Straatman-Iwanowska, A.; Pasha, S.; Guckian, M. Mutation in the TCRα subunit constant gene (TRAC) leads to a human immunodeficiency disorder characterized by a lack of TCRαβ+ T cells. J. Clin. Investig. 2011, 121, 695–702. [Google Scholar] [CrossRef]
- Chen, S.; Lee, B.; Lee, A.Y.-F.; Modzelewski, A.J.; He, L. Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. J. Biol. Chem. 2016, 291, 14457–14467. [Google Scholar] [CrossRef]
- Cervia, L.D.; Yuan, F. Current progress in electrotransfection as a nonviral method for gene delivery. Mol. Pharm. 2018, 15, 3617–3624. [Google Scholar] [CrossRef]
- Kebriaei, P.; Singh, H.; Huls, M.H.; Figliola, M.J.; Bassett, R.; Olivares, S.; Jena, B.; Dawson, M.J.; Kumaresan, P.R.; Su, S. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Investig. 2016, 126, 3363–3376. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.T.; Collins, M.; Terefe, J.; Ugozzoli, L.; Rubio, T. Optimizing electroporation conditions in primary and other difficult-to-transfect cells. J. Biomol. Tech. JBT 2008, 19, 328. [Google Scholar] [PubMed]
- Zhang, Z.; Qiu, S.; Zhang, X.; Chen, W. Optimized DNA electroporation for primary human T cell engineering. BMC Biotechnol. 2018, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.P.; Huntoon, C.J.; Graham, D.; McKean, D.J. The analysis of costimulatory receptor signaling cascades in normal T lymphocytes using in vitro gene transfer and reporter gene analysis. Nat. Med. 2001, 7, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, P.; Aksoy, B.A.; Czech, E.; Hammerbacher, J. Viable and efficient electroporation-based genetic manipulation of unstimulated human T cells. BioRxiv 2019, 466243.