The small molecule macrocyclic lactone ivermectin, approved by the US Food and Drug Administration for parasitic infections, has received attention in the last eight years due to its exciting potential as an antiviral. It was identified in a high-throughput chemical screen as inhibiting recognition of the nuclear localizing Human Immunodeficiency Virus 1 (HIV-1) integrase protein by the host heterodimeric importin (IMP) α/β1 complex, and has since been shown to bind directly to IMPα to induce conformational changes that prevent its normal function in mediating nuclear import of key viral and host proteins. Excitingly, cell culture experiments show robust antiviral action towards HIV-1, dengue virus (DENV), Zika virus, West Nile virus, Venezuelan equine encephalitis virus, Chikungunya virus, Pseudorabies virus, adenovirus, and SARS-CoV-2 (COVID-19). Phase III human clinical trials have been completed for DENV, with >60 trials currently in progress worldwide for SARS-CoV-2.
- ivermectin
- antiviral
- SARS-CoV-2
- COVID-19
- flavivirus
- dengue virus
- Zika virus
1. Introduction
The 2015 Nobel Prize for medicine recognizes the seminal contribution of Campbell and Ōmura in terms of the “wonder drug” ivermectin, a macrocyclic lactone 22,23-dihydroavermectin B produced by the bacterium Streptomyces avermitilis [1][2][7][8][9][10][11][12][13][14][15][16][17][18][19][20], as a novel therapeutic against “infections caused by roundworm parasites”. Discovered in 1975, ivermectin was marketed successfully from 1981 for parasitic infection indications in animals, and then approved for human use for activity against onchocerciasis (river blindness) in 1987. It has since been used successfully to treat a number of human parasitic worm infestations causing river blindness/filariasis, strongyloidiasis/ascariasis, ectoparasites causing scabies, pediculosis and rosacea [3]. More recent applications include to control insect mediators of infection, such as malaria [1[3][4][5]. Ivermectin is on the WHO List of Essential Medicines [6].
From 2012 onwards, there have multiple reports of ivermectin’s antiviral activity towards RNA viruses [7][8][9][10][11][12][13][14][15][16][17], including human immunodeficiency virus (HIV)-1, influenza, flaviruses such as dengue virus (DENV) and Zika virus (ZIKV) and, most recently, SARS-CoV-2 (COVID-19) [17]. Evidence for activity against DNA viruses is more limited, but encompasses Pseudorabies, polyoma and adenoviruses [18][19][20]. The basis of ivermectin’s broadspectrum antiviral activity appears to relate to the fact that ivermectin binds to, and inhibits, the nuclear transport role of the host importin α (IMPα) protein [11[18][20],18,20], which is known to mediate nuclear import of various viral proteins and key host factors, but other possible antiviral actions of ivermectin have been proposed (e.g., [12]), including in the case of SARS-CoV-2 (e.g., [21,22][21][22]). This mini-review summarises the evidence for ivermectin’s broad-spectrum antiviral activity and the basis of its IMPα-directed activity in light of the possibility that ivermectin could be critically useful in the current SARS-CoV-2 crisis [6,17][6][17].
2. Ivermectin as an FDA-Approved Anti-Parasitic Agent
It is difficult to overestimate the impact of ivermectin as a therapeutic agent to control various parasitic diseases [1–6][1][2][3][4][5][6]. It is administered as a single oral yearly dose (e.g., 150 or 200 μg/kg, respectively) to treat onchocerciasis and strongyloidiasis. Lymphatic filariasis is similarly treated in endemic areas with a once-yearly dose (300–400 μg/kg), or alternatively bi-yearly dosing (150–200 μg/kg) [23]. Ivermectin’s documented antiparasitic mode of action is through potentiating GABA-mediated neurotransmission, and by binding to invertebrate glutamate-gated Cl−channels to effect parasite paralysis and death [24]. Selectivity comes from the fact that ivermectin does not readily penetrate the central nervous system of mammals [24].
Doses up to 2000 µg/kg are well tolerated in patients with parasitic infections [23[23][25],25], with analysis of the first 11 years of mass global ivermectin (Mectizan) administration indicating a cumulative incidence of one serious adverse side effect case per million [4,26][26]. Similarly, although drug resistance can occur in animals, no resistance in humans has yet been confirmed in over 25 years. Based on this, ivermectin is unquestionably a safe, potent antiparasitic agent likely to be used as such long into the future [1,4][4].
3. Ivermectin as an IMPα Targeting Agent with Antiviral Activity
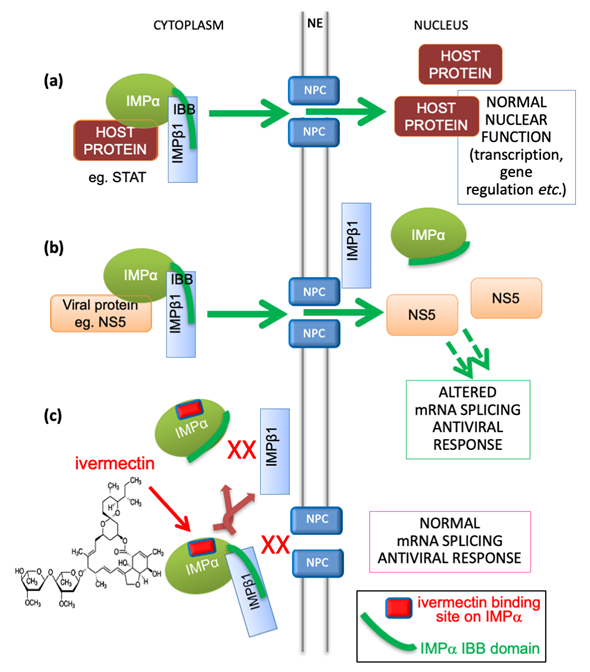
Transport into and out of the nucleus is central to eukaryotic cell and tissue function, with a key role to play in viral infection, where a common strategy used by viruses is to antagonize the cellular antiviral response [14,27][27]. The targeting signal-dependent mediators of this transport are the members of the IMP superfamily of proteins, of which there are multiple α and β forms [14,27][27]. The pathway mediated by the IMPα/β1 heterodimer is the best characterized pathway by which host proteins, including members of the signal transducers and activators of transcription (STATs) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor families, enter the nucleus through nuclear envelope-embedded nuclear pores. A large number of viral proteins (e.g. [27,28][27][28]) also use this pathway (see Figure 1), where IMPα within the IMPα/β1 heterodimer performs the adaptor role of specific targeting signal recognition, while IMPβ1 performs the main nuclear roles of binding to/translocation through the nuclear pores, and release of the nuclear import cargo within the nucleus (Figure 1) [27].
Figure 1. Schematic showing IMPα’s role in nuclear transport of host and viral proteins, and mechanism of inhibition by ivermectin. (a) Host proteins, such as members of the STAT or NF-kB transcription factor families, localize in the nucleus through the action of the IMPα/β1 heterodimer, where the “IBB” (IMPβ-binding) region of IMPα (green curved line) is bound by IMPβ1 to enable cargo recognition by IMPα within the heterodimer; IMPβ1 subsequently mediates transport of the trimeric complex through the nuclear pore (NPC, nuclear pore complex) embedded within the nuclear envelope (NE) into the nucleus. This is followed by release within the nucleus to enable the transcription factors to carry out normal function in transcriptional regulation, including in the antiviral response. IMPα can only mediate nuclear import within the heterodimer with IMPβ1. (b) In viral infection, specific viral proteins (e.g., NS5 in the case of DENV, ZIKV, WNV) able to interact with IMPα utilize the IMPα/β1 heterodimer to access the nucleus and antagonize the antiviral response [14,27,28][27][28]. This is critical to enable optimal virus production as shown by mutagenic and inhibitor studies. (c) The IMPα targeting compound ivermectin binds to IMPα (binding site shown as red lozenge) both within the IMPα/β heterodimer to dissociate it, and to free IMPα to prevent it binding to IMPβ1, thereby blocking NS5 nuclear import [11]. GW5074 has been shown to exhibit a similar mechanism [29].
The importance of nuclear targeting of viral proteins to the nucleus in the infectious cycle has been demonstrated for a number of viruses. Mutagenic analyses, for example, show that specific recognition by IMPα is critical to nuclear localization of various viral proteins, such as DENV non-structural protein (NS) 5 [30]; significantly, DENV, which shows the same reduced interaction of NS5 with IMPα is severely attenuated, underlining the importance of the NS5-IMPα interaction for dengue infection. As has since been shown using a range of different small molecules, the critical importance of this interaction to dengue infection is the basis for the fact that multiple distinct small molecules that disrupt IMPαrecognition of dengue NS5 are able to limit dengue infection [7,8,11,29,31][31]. In the case of ivermectin, this activity extends to a large number of different viruses (see below) [7–17][7][8][9][10][11][12][13][14][15][16][17], including SARS-CoV-2 [18]. Which SARS-CoV-2 proteins may access the nucleus in infected cells has not been examined in detail but, in terms of related coronaviruses, ORF6 (Open Reading Frame 6) protein from SARS-CoV-1 has been shown to bind IMPα [32], and ORF4b from MERS-CoV (Middle Eastern Respiratory Syndrome Coronavirus) is known to access the nucleus in NLS-dependent fashion [33]. Ongoing research will establish which of the SARS-CoV-2 ORFs may play comparable roles, and be potential targets of the impact of ivermectin on IMPα.
We identified ivermectin in 2011 in a high-throughput chemical compound library screen for inhibitors of HIV-1 Integrase (IN) recognition by IMPα/β1 [34]; specific inhibitors targeting IMPα/β1 directly (such as ivermectin) and not IN (such as budenoside) were identified using a nested counterscreen strategy [34,35][34][35]. Of several compounds subsequently confirmed to be active against IMPα/β1 and possess antiviral activity as a consequence [7,14,29,36][36], ivermectin has been the best characterized in this regard, and shown to have broad-spectrum activities, a number of which are summarized in Table 1. It was initially shown to inhibit nuclear import not only of IN, but also of simian virus SV40 large tumour antigen (T-ag) and other IMPα/β1-dependent (but not IMPβ1-dependent) cargoes, consistent with the idea that IMPα (not IN) is the direct target [34,35][34][35]. Subsequent work has confirmed this, with ivermectin’s ability to inhibit the nuclear accumulation of various different host, including NF-kB p65 [37] and viral proteins demonstrated in transfected and infected cell systems (see Table 1) [14,34][34]. Ivermectin’s ability to inhibit binding of IMPα to the viral proteins NS5 and T-ag has also been confirmed in a cellular context using the biomolecular fluorescence complementation technique [11].
Table 1.
In vitro properties of ivermectin.
|
Documented Action on IMPa |
Virus1 |
Inhibitory Concentration (Assay)/ Fold reduction2 |
|
· Inhibits interaction in vitro of IMPawith HIV-IN [34], DENV2 NS5 (1 μM) [7,11], T-ag [31], Hendra V (15 uM) [13], IMPb1 (7 μM) [11]
· Inhibits interaction of IMPa with T-ag and NS5 in a cell context as visualised by quantitative BiFc [11]
· Inhibits CoIP from cell lysates of IMPa with T-ag, Adenovirus EIA [20]
· Inhibits nuclear accumulation in a cellular context of IMPα/β1- but not β1-recognised viral proteins such as T-ag [7,16], DENV2 NS5 [7], VEEV Capsid [16], adenovirus E1A [20], PSV UL42 [18] as well as host cargoes [see 15,30]
· Reduces nuclear localisation in infected cells of VEEV Capsid [9] and adenovirus E1A [20]
|
Coronavirus SARS-CoV-2
HIV-1 (VSV-G-pseudotyped NL4-3.Luc.R-E-HIV)
Influenza VLPs (avian influenza A/ MxA escape mutants)
Flavivirus: YFV (17D)
DENV1 (EDEN-1) DENV2 (NGC)
DENV2 (EDEN-2) DENV3 (EDEN-3) DENV4 (EDEN-4) WNV (NY99) WNV (MRM61C) ZIKV (Asian/Cook Islands/ 2014)
Alphavirus: Chikungunya virus (CHIKV-Rluc) Sindbis (HR) Semliki forest virus VEEV (TC83)
Hendra (Hendra virus/Australia/ Horse/1994)
DNA viruses Adenovirus (HAdV-C5) Adenovirus (HAdV-B3) BK polyomavirus (BKPyV)
Pseudorabies |
EC50 = 2.2/2.8 μM (qPCR/released /cell-associated virus) [17] 5000-fold
50 μM > 2-fold (luciferase) [7]
10 μM total inhibition (luciferase) [10]
EC50 = 5/0.5 nM (CPE/qPCR) [12] 3 μM > 50,000-fold (pfu) [15] EC50 = 2.3/3 μM (CFI, 2 hosts) [8] EC50 = 0.7 μM (qPCR) [12] EC50 = 0.4/0.6 μM (pfu/qPCR) [11] 50 μM total inhibition (pfu) [7] EC50 = 2.1/1.7 μM (CFI, 2 hosts) [8] EC50 = 1.7 μM (CFI) [8] EC50 = 1.9 μM (CFI) [8] EC50 = 4 μM (qPCR) [12] EC50 = 1/0.5 μM (pfu/qPCR) [11] EC50 = 1.3/1.6 μM (pfu/qPCR) [11]
EC50 = 1.9/0.6 μM (luciferase, 2 hosts) 3 μM > 5000-fold (pfu) [15] 3 μM > 1000-fold (pfu) [15] 3 μM > 200-fold (pfu) [15] 1 μM c. 20-fold (pfu) [9]
est. EC50 = 2 μM (TCID/luciferase) [13]
EC50 = c. 2.5 μM; 10 μM 20-fold (qPCR) [20] 10 μM c. 8-fold (qPCR) [20]
Est. EC50 1.5 μM (PFU/CPE/qPCR) [19]
Est. EC50 c. 0.8 μM 1000-fold [18] |
1 Entries in brackets indicate virus strains/constructs used 2 Est. estimated.
Although targeting of IMPα by ivermectin was clearly supported by many years of research (see also below), direct binding to IMPα was only recently formally demonstrated using a set of biophysical techniques, including thermostability, analytical ultracentrifugation, and circular dichroism (CD) [11]. Importantly, the CD/thermostability studies indicate that binding of ivermectin by IMPα induces a structural change, which is likely the basis of IMPα’s inability to bind viral nuclear import cargoes. Strikingly, the structural change also appears to impair heterodimerisation of IMPα with IMPβ1 [11]1; IMPα alone cannot mediate nuclear import, only within the heterodimer with IMPβ1. Thus, ivermectin inhibits nuclear import not only by preventing signal recognition by IMPα, but also by ensuring that the IMPα/β1 complex essential to mediate subsequent transport through the nuclear pore is prevented from forming. Interestingly, GW5074 (see Table 1) appears to have a close to identical mechanism to that for ivermectin in binding to IMPα to induce structural changes that prevent both cargo recognition and specific interaction with IMPβ1 [29]; there is no such data for GSP (see Table 1), which is believed to impact the NLS-binding site of IMPα and prevent cargo recognition more directly (manuscript in preparation).
4. Ivermectin as an Antiviral
Consistent with the fact that many viruses are known to rely on IMPα/β1-dependent nuclear import of specific viral proteins for robust infection [14,27[14][27][28],28], ivermectin has been confirmed in a body of in vitro studies to be active in limiting infection by a range of different RNA viruses [10[10][14],14], including HIV-1 [7], DENV (all four serotypes) and related flaviviruses [8[8][11][12],11,12], influenza, and alphaviruses such as Venezuelan equine encephalitis virus (VEEV) and chikungunya [9,15,16][9][15][16] (see Table 1); it is also active against DNA viruses [18–20][18][19][20]. Recent studies indicate it is a potent inhibitor of SARS-CoV-2 [17].
A striking aspect of this antiviral activity is that, where determined, the EC50 for viral inhibition as assessed by a range of different techniques is in the low μM range (see right column, Table 1), interestingly aligning perfectly with its activity in inhibiting recognition of viral nuclear import cargoes by IMPα (see top of left column, Table 1). The clear implication is that the mechanism of inhibition of infectious virus production in the case of all of the viruses listed in Table 1 is largely through targeting IMPα to prevent its role in nuclear import, and of viral proteins in particular (see Figure 1). Significantly, two other small molecules (GW5074 and gossypol) that appear to target IMPα in a very similar way to prevent its nuclear import function [29] have comparable antiviral properties [13,29[36],36], consistent with the idea that the host protein IMPα is a key contributor to infection by a number of medically important viruses.
5. Ivermectin as an Antiviral in the Clinic
One of the challenges in antiviral research is to transition from laboratory experiments to preclinical/clinical studies, especially reagrding the question of dosing [6]. However, it is important to stress that the antiviral activities of ivermectin in Table 1 have been derived from laboratory experiments that largely involve high, generally non-physiological, multiplicities of infection, and cell monolayer cultures, often of cell lines such as Vero cells (African green monkey kidney, impaired in interferon α/β production) that are not clinically relevant. Clearly, the results in Table 1 for low μM EC50 values should not be interpreted beyond the fact that they reveal robust, dose-dependent antiviral activity in the cell model system used, and it would be naïve to strive for μM concentrations of ivermectin in the clinic based on them.
A key consideration in any clinical intervention using ivermectin is its host-directed (IMPα-directed) mechanism of action. Host-directed agents that impact cellular activities that are essential to healthy function must be tested with caution; although ivermectin has an established safety profile in humans [23[23][25],25], and is FDA-approved for a number of parasitic infections [1[1[3][5],3,5], it targets a host function that is unquestionably important in the antiviral response, and titration of a large proportion of the IMPα repertoire of a cell/tissue/organ likely to lead to toxicity. With this in mind, where a host-directed agent can be a “game-changer” in treating viral infection may well be in the initial stages of infection or even prophylactically (see Section 6) to keep the viral load low so that the body’s immune system has an opportunity to mount a full antiviral response [11,17][11].
Ivermectin’s real potential as an antiviral to treat infection can only be demonstrated in preclinical/clinical studies. Preclinical studies include a lethal Pseudorabies (PRV) mouse challenge model which showed that dosing (0.2 mg/kg) 12 h post-infection protected 50% of mice, which could be increased to 60% by administering ivermectin at the time of infection [18]. Apart from the many clinical trials currently running for SARS-CoV-2 (see Section 6), the only study thus far reported relates to a phase III trial for DENV infection [40][38][49][50][51]. Almost 70% of the world’s population in over 120 countries is currently threatened by mosquito-borne flaviviral infections, with an estimated 100 million symptomatic DENV infections and up to 25,000 deaths each year from dengue haemorrhagic fever [41,42][39][40], despite sophisticated large-scale vector control programs. As for the closely related ZIKV (cause of large outbreaks in the Americas in 2015/2016), the dearth of antiviral treatments and challenges in developing efficacious vaccines hamper disease control. Clinical data published in preliminary form for the phase III trial in Thailand [40][38] indicate antiviral activity; daily dosing (0.4 mg/kg) was concluded to be safe, and have virological efficacy, but clear clinical benefit was not reported, potentially due to the timing of the intervention. The authors concluded that dosing regimen modification was required to ensure clinical benefit [40][38]. This study both underlines ivermectin’s potential to reduce viral load in a clinical context, and highlights the complexities of timely intervention and effective dosing regimens to achieve real clinical benefit in the field.
6. A Viable Treatment for SARS-CoV-2?
Despite efforts in multiple domains, the current SARS-CoV-2 pandemic has now eclipsed the porcine flu epidemic in terms of numbers of infections (> 33 million) and deaths (close to 1 million) worldwide. The search for antivirals for SARS-CoV-2 through repurposing existing drugs has proved challenging (e.g., see [43–47][41][42][43][44][45]), one important aspect of repurposing being the perceived need to achieve therapeutic levels in the lung. Published pharmacokinetic modelling based on both the levels of ivermectin achievable in human serum from standard parasitic treatment dosing and robust large animal experiments where lung levels of ivermectin can be measured, indicates that concentrations of ivermectin 10 times higher than the c. 2.5 μM EC50 indicated by in vitro experiments (Table 1) are likely achievable in the lung in the case of SARS-CoV-2 [48][46]; modelling based on different assumptions predicts lower values, but stresses the long-term stability of ivermectin in the lung (for over 30 days) based on data from animals [49][47].
There are currently more than 60 trials worldwide testing the clinical benefit of ivermectin to treat or prevent SARS-CoV-2. These include variations on combination therapies (see [51,52][49][50]), dosing regimens, and prophylactic protocols. With respect to the latter, preliminary results from recently completed study NCT04422561 (Table 2, no. 22), that examines asymptomatic family close contacts of confirmed COVID patients, show that two doses of ivermectin 72 h apart result in only 7.4% of 203 subjects reporting symptoms of SARS-CoV-2 infection, in stark contrast to control untreated subjects, of whom 58.4% reported symptoms, underlining ivermectin’s potential as a prophylactic. It is to be hoped that the results from rigorous randomised clinical trials will emerge in the next few months to document ivermectin’s credentials as “the real deal” for COVID-19 infection or otherwise. In this context, it is noteworthy that ivermectin has already been approved for the treatment of SARS-CoV-2 in humans by the Republic of Peru[48] [53] and in the Northeastern Beni region of Bolivia [54][50].
References
- Crump, A.; Omura, S. Ivermectin, wonder drug from Japan: The human use perspective. Proc. Jpn. Acad. 2011, 87, 13–28.
- Nobel Foundation. The Nobel Prize in Medicine or Physiology, 2015. Available online: https://www.nobelprize.org/prizes/medicine/2015/press-release (accessed 11 September 2020).
- González Canga, A.; Sahagún Prieto, A.M.; Diez Liébana, M.J.; Fernández Martínez, N.; Sierra Vega, M.; García Vieitez, J.J. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPSJ 2008, 10, 42–46.
- Crump, A.; Omura, S. Ivermectin: Panacea for resource-poor communities? Trends in Parasitol. 2014, 30, 445–455.
- World Health Organization. World Health Organization’s List of Essential Medicines 21st List 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf (accessed 11 September 2020).
- Kumar, B.S.; Jeyaraman, M.; Jain, R.; Anudeep, T.C. A Wonder Drug in the Arsenal against COVID—19: Medication Evidence from Ivermectin. J. Adv. Med. Med. Res. 2020, 32, 30–37.
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856.
- Tay, M.Y.; Fraser, J.E.; Chan, W.K.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306.
- Lundberg, L.; Pinkhan, C.; Baer, A.; Amaya, M.; Narayan, A.; Wagstaff, K.M.; Jans, D.A.; Kehn-Hall, K. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antivir. Res. 2013, 100, 662–672.
- Gotz, V.; Magar, L.; Dornfeld, D.; Giese, S.; Pohlmann, A.; Höper, D.; Kong, B-W.; Jans, D.A.; Beer, M.; Haller, O.; Schwemmle, M. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci. Rep. 2016, 6, 23138.
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760.
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.: Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894.
- Atkinson, S.; Audsley, M.; Lieu, K.; Marsh, G.; Thomas, D.R.; Heaton, S.; Paxman, J.; Wagstaff, K.M.; Buckle, A.G.; Moseley, G.; et al. Recognition by host nuclear transport proteins drives disorder-to-order transition in Hendra virus V. Sci. Rep. 2018, 8, 23.
- Jans, D.A.; Martin, A.J.; Wagstaff, K.M. Inhibitors of nuclear transport. Curr. Opin. Cell Biol. 2019, 58, 50–60.
- Varghese, F.S.; Kaukinen, P.; Glaesker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124.
- Thomas, D.R.; Lundberg, L.; Pinkhan, C.; Shechter, S.; Debono, A.; Baell, J.; Wagstaff, K.M.; Hick, C.A.; Kehn-Hall, K.; Jans, D.A. Identification of novel antivirals inhibiting recognition of Venezuelan equine encephalitis virus capsid protein by the Importin α/β1 heterodimer through high-throughput screening. Antivir. Res. 2018, 151, 8–19.
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787.
- Lv, C.; Liu, W.; Wang, B.; Dang, R.; Qiu, L.; Ren, J.; Yan, C.; Yang, Z.;. Wang, X. Ivermectin inhibits DNA polymerase UL42 of Pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antivir. Res. 2018, 159, 55–62.
- Bennett, S.M.; Zhao, L.; Bosard, C.; Imperiale, M.J. Role of a nuclear localization signal on the minor capsid proteins VP2 and VP3 in BKPyV nuclear entry. Virology 2015, 474, 110–116.
- King, C.R.; Tessier, T.M.; Dodge, M.J.; Weinberg, J.B.; Mymryk, J.S. Inhibition of human adenovirus replication by the importin α/β1 nuclear import inhibitor ivermectin. J. Virol. 2020, in press, d.o.i. 10.1128/JVI.00710-20.
- Changeux, J-P.; Amoura, Z.; Rey, F.A.; Miyara, M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Comptes Rendus Biol. 2020, 343, 33–39.
- Krause, R.M.; Buisson, B.; Bertrand, S.; Corringer, P.J.; Galzi, J.L.; Changeux. J.P.; Bertrand, D. Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 1998, 53, 283–294.
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G.; Chen, C.; Tipping, R.; Clineschmidt, C.M.; Sciberras, D.G.; Hsieh, J.Y.K.; Lasseter, K.C. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 2002, 42, 1122–1133.
- Loukas, A.; Hotez, P.J. Chemotherapy of helminth infections. In: Brunton, L.L.; Lazo, J.S.; Parker, K.L. editors; Goodman & Gilman’s The pharmacological basis of therapeutics. 11th ed. New York (NY): McGraw Hill 2006 1073–1093.
- Navarro, M.; Camprubí, D.; Requena-Méndez, A.; Buonfrate, D.; Giorli, G.; Kamgno, J.; Gardon, J.; Muñoz, J.; Krolewiecki, A. Safety of high-dose ivermectin: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2020, 75, 827–834.
- Twum-Danso, N.A.Y.; Meredith, S.E.O. Variation in incidence of serious adverse events after onchocerciasis treatment with ivermectin in areas of Cameroon co-endemic for loiasis. Trop. Med. Int. Health 2003, 8, 820–831.
- Fulcher, A.; Jans, D.A. Regulation of nucleocytoplasmic trafficking of viral proteins; an integral role in pathogenesis ? Biochem. Biophys. Acta Mol. Cell Res. 2011, 1813, 2176–2190.
- Caly, L.; Wagstaff, K.M.; Jans, D.A. Nuclear trafficking of proteins from RNA viruses: Potential target for anti-virals? Antivir. Res. 2012, 95, 202–206.
- Yang, S.N.Y.; Atkinson, S.C.; Fraser, J.E.; Wang, C.; Maher, B.;. Roman, N.; Forwood, J.K.; Wagstaff, K.M.; Borg, N.A.; Jans, D.A. Novel Flavivirus Antiviral That Targets The Host Nuclear Transport Importin α/β1 Heterodimer. Cells 2019, 8, 281, doi:10.3390/cells8030281.
- Pryor, M.J.; Rawlinson, S.M.; Butcher, R.E.; Barton, C.L.; Waterhouse, T.A.; Vasudevan, S.G.; Bardin, P.G.; Wright, P.J.; Jans, D.A.; Davidson, A.D. Nuclear localization of dengue virus nonstructural protein 5 through its importin alpha/beta-recognized nuclear localization sequences is integral to viral infection. Traffic 2007, 8, 795–807, http://dx.doi.org/10.1111/j.1600-0854.2007.00579.x.
- Fraser, J.E.; Watanabe, S.; Wang, C.; Chan, W.K.; Maher, B.; Lopez-Denman, A.; Hick, C.; Wagstaff, K.M.; Mackenzie, J.M.; Sexton, P.M.; et al. A nuclear transport inhibitor that modulates the unfolded protein response and provides in vivo protection against lethal dengue virus infection. J. Infect. Dis. 2014, 210, 1780–1791, http://dx.doi.org/10.1093/infdis/jiu319.
- Frieman, M.; Yount, B.; Heise, M.; Kopecky-Bromberg, S.A.; Palese, P.; Baric, R.S. Severe Acute Respiratory Syndrome Coronavirus ORF6 Antagonizes STAT1 Function by Sequestering Nuclear Import Factors on the Rough Endoplasmic Reticulum/Golgi Membrane. J. Virol. 2007, 81, 9812–9824.
- Yang, Y.; Ye1, F.; Zhu, N.; Wang, W.; Deng, Y.; Zhao, Z.; Tan, W. Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets. Sci. Rep. 2015, 5, 17554, doi:10.1038/srep17554.
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An AlphaScreen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen. 2011, 16, 192–200, http://dx.doi.org/10.1177/1087057110390360.
- Wagstaff, K.M.; Headey, S.; Telwatte, S.; Tyssen, D.; Hearps, A.C.; Thomas, D.R.; Tachedjian, G.; Jans, D.A. Molecular dissection of an inhibitor targeting the HIV integrase dependent preintegration complex nuclear import. Cell. Microbiol. 2018, e12953, http://dx.doi.org/10.1111/cmi.12953.
- Lopez-Denman, A.J.; Russo, A.; Wagstaff, K.M.; White, P.A.; Jans, D.A.; Mackenzie, J.M. Nucleocytoplasmic shuttling of the West Nile virus RNA-dependent RNA polymerase NS5 is critical to infection. Cell. Microbiol. 2018, 20, e12848, http://dx.doi.org/10.1111/cmi.12848.
- Ci, X.; Li, H.; Yu, Q.; Zhang, X.; Yu, L.; Chen, N.; Song, Y.; Deng, X. Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam. Clin. Pharmacol. 2009, 23, 449–455, https://doi.org/10.1111/j.1472-8206.2009.00684.x.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507.
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760, doi:10.1371/journal.pntd.0001760.
- Boulware, D.R.; Pullen, M.F.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.: Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M.; et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. New England J. Med. 2020, 383, 517–525, doi:10.1056/ NEJMoa2016638.
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. June 15, 2020. Available online: https://www.fda.gov/media/138945 (accessed 11 September 2020).
- US Food and Drug Administration. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. July 1, 2020, Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/OSE%20Review_Hydroxychloroquine-Cholorquine%20-%2019May2020_Redacted.pdf (accessed 11 September 2020).
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, R.: Song, B.; Cai, C.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. New England J. Med. 2020, 382, 1787–1799, doi:10.1056/NEJMoa2001282.
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. The Lancet 2020, 395, 1569–1578, https://doi.org/10.1016/S0140-6736(20)31022-9.
- Arshad, U.; Pertinez, H.; Box, H.; Tatham, L.; Rajoli, R.K.R.; Curley, P.; Neary, M.; Sharp, J.; Liptrott, N.J.; Valentijn, A.; et al. Prioritisation of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived From Their Established Human Pharmacokinetics. Clin. Pharm. Ther. 2020, doi:10.1002/cpt.1909.
- Schmith, V.D.; Zhou, J.J.; Lohmer, L.R. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin. Pharm. Ther. 2020, doi:10.1002/cpt.1889.
- Trial Site News. Eurnekian Public Hospital Argentina’s IVERCAR Ivermectin & Carrageenan Study Shows Positive Results Targeting COVID-19. September 3, 2020; Available online: https://www.trialsitenews.com/argentinas-ivercar-ivermectin-carrageenan-study-shows-positive-results-targeting-covid-19/ (accessed 11 September 2020).
- Alam, M.; Murshed, R.; Bhiuyan, E.; Saber, S.; Alam, R.F.; Robin, R.C. A Case Series of 100 COVID-19 Positive Patients Treated with Combination of Ivermectin and Doxycycline. Bangladesh Coll. Phys. Surg. 2020, 38, 10–15; doi:https://doi.org/10.3329/jbcps.v38i0.47512.
- Rahman, M.A.; Iqbal, S.A.; Islam, M.A.; Niaz, M.K.; Hussain, T.; Siddiquee, T.H. Comparison of Viral Clearance between Ivermectin with Doxycycline and Hydroxychloroquine with Azithromycin in COVID-19 Patients. Bangladesh Coll. Phys. Surg. 2020, 38, 5–9; doi:https://doi.org/10.3329/jbcps.v38i0. 47514.
- Republica Del Peru Ministerio De Salud. RM_270-2020-MINSA, May 8, 2020, Available online: https://cdn.www.gob.pe/uploads/document/file/694719/RM_270-2020-MINSA.PDF (accessed 11 September 2020).
- The Gobierno del Estate Plurinacional de Bolivia Misterio de Salud. Ministerial Resolution No 259 from the Gobierno del Estate Plurinacional de Bolivia Misterio de Salud; May 20, 2020, Available online: https://www.minsalud.gob.bo/component/jdownloads/send/27-comunicado-oficial/425-resolucion-ministerial-n-0259 (accessed 11 September 2020).