1. Extinguishing Efficiency
The extinguishing efficiency of C6F12O is evaluated mainly through two ways. One is measuring the flame minimum extinguishing concentration (MEC) at the laboratory scale, and the other is testing the actual fire extinguishing efficiency in a full-scale experiment.
1.1. Laboratory Scale Experiments
MEC is the key parameter to evaluate the efficiency of the fire suppressants and to design the fire extinguishing system. Due to the large amount of preparatory work, high cost, poor repeatability, safety issues and complexity, it is difficult to obtain accurate or even meaningful results of MEC through full-scale experiments
[1][2]. However, the laboratory scale tests overcome these disadvantages and are widely used to test the MEC of various fire suppressants with different fuels.
The cup burner method is widely used to measure the MEC of gaseous fire suppressants. The laminar co-flow diffusion flame of the cup burner resembles real fire. Furthermore, the flame is more stable compared to real fire, leading to the higher MEC measured by the cup burner method. This method has been adopted as the standard procedure listed in ISO 14520 and NFPA 2001, since the first systematic introduction by Hirst
[1]. However, the cup burner method is generally used to test the MEC of gaseous agents at room temperature. As for C6F12O with the high boiling temperature of 49 °C (1 atm), the method is modified so that the liquid agent is measured after vaporization by pre-heating.
Table 1 shows the MEC of C6F12O tested by different researchers through the modified method, from which it can be concluded that the test results of MEC are highly consistent, and the extinguishing efficiency of C6F12O is also very high. In terms of the synergistic effect of C6F12O with nitrogen, carbon dioxide and Halon 1301, the extinguishing efficiency shows a negative synergistic effect of mutual inhibition, while when C6F12O was combined with HFC 125, it shows a positive synergistic effect
[3]. It could be speculated that the fire extinguishing mechanism of C6F12O might be similar with HFC 125.
Table 1.
Measurement of critical fire-extinguishing concentration of C6F12O based on cup burner.
| Researcher |
Fuel |
Test Result of MEC |
Note |
| Carnazza et al. [4] |
n-heptane, alcohol and other liquid fuels |
n-heptane 4.5%, alcohol 5.6% |
|
| Andersson et al. [5][6] |
propane |
6.4% |
lower than HFC 125 and HFC 227ea under the same experimental conditions, higher than Halon 1301 |
| Rivers et al. [7] |
propane |
3.5% |
lower than Halon 1301 and Halon 1211 under the same experimental conditions but the required mass for the same fire extinguishing efficiency is relatively high |
| Takahashi et al. [8] |
propane |
4.17% |
the calculated MEC is 4.12% |
| Li [3] |
n-heptane |
4.5–5% |
under different gasification heating temperature, air temperature, heating coil and environment temperature |
The cup burner method is easily influenced by fuel type, fuel level, burner size, agent temperature, air and agent flow rate and pre-burn time
[9], and the turbulence state in practical fire development is also neglected, leading to consistency problems between the laboratory scale and lager scale experiments
[10]. In order to comprehensively evaluate the fire extinguishing concentration of the fire suppressants, researchers
[11][12] have proposed the tubular burner method, in which the fuel and fire extinguishing mediums are mixed in a hot bath in advance before the fuel burns, and the flow rate of fire suppressants is adjusted until the flame is extinguished. This method determines the amount of needed fire suppressants by measuring the required extinguishing medium portion (REMP) value, which is defined as the ratio of the mass flow rate of the fire suppressant to the mass flow rate of the fuel. Andersson et al.
[5] used this method to measure the REMP value of C6F12O. The results showed that the REMP value of C6F12O (15) was much higher than that of Halon 1301 (1.5), HFC 125 (5.6) and HFC 227ea (6.8), which also verified that the fire extinguishing volume fraction of C6F12O is low, while the required mass concentration is high.
Although there are some differences between the two methods mentioned above in terms of the supply mode of the fire extinguishing agent and fuel and the calculation method of the fire extinguishing concentration and flame combustion state, these two methods have a common character in that when they are applied to measure C6F12O concentration, the fire suppressant vaporizes before reaching the flame, and what they measure is the fire extinguishing concentration of the agent in a gaseous state. For the high boiling point extinguishing agent, C6F12O, partial evaporation occurs in the transport pipe of the fire extinguishing system, and the remaining part of the agent is sprayed in droplets. When the droplets approach the flame, phase change will happen under the high temperature of the fire, which will absorb the heat of the flame and reduce the temperature of the fire. The evaporative heat of this part has effects on the fire extinguishing, especially for water and polar molecules, while these two methods cannot take into account the extinguishing contribution of liquid phase transition of C6F12O
[13].
Yang
[14][15] proposed a dispersed liquid agent fire suppression screen apparatus (DLAFSS), a kind of counterflow cylindrical burner which can form a stable two-dimensional laminar non-premixed flame. It can accurately measure the fire extinguishing efficiency of solid, liquid and gaseous fire extinguishing mediums, and has been applied in when testing the fire extinguishing efficiency of some liquid suppressants
[16]. However, research on the fire extinguishing efficiency of the high boiling point agent C6F12O is still lacking.
C6F12O can be used in a total flooding fire extinguishment system. 3M researchers
[4] tested the minimum inert concentrations for methane and propane air mixtures according to ISO14520 standard and obtained minimum inert concentrations for methane and propane of 8–9%. Andersson et al.
[6] also obtained similar experimental results. The inert concentration of C6F12O (7–9%) is similar to that of Halon 1301 (7.5–8.7%) and lower than that of HFC 125 (14–16%) and HFC 227ea (11–12%). However, in the FAA’s aerosol can test (FAA-ACT)
[17][18][19], aiming at examining the feasibility of applying halon substitutes to aircraft, several halon substitutes including C6F12O were tested with concentrations lower than the minimum inert concentration. The results showed that these halon substitutes all lead to a pressure rise in the can to some degree, and with the addition of a low concentration of C6F12O (4.2%), the pressure increases nearly three times, indicating that C6F12O and other halon substitutes enhance flame combustion under some certain conditions.
1.2. Full-Scale Experiment
According to the problems found in previous studies, researchers carried out various kinds of fire suppression experiments in different fire scenes, as shown in
Table 2. It could be concluded that C6F12O is similar to halon and other substitutes that have high fire extinguishing efficiency, but it also has some problems, such as the large amount of acid gas production, more agents required compared with other gas fire suppressants and combustion enhancement during fire extinguishment. As mentioned in the previous section, the dispersion of C6F12O is relatively poor, and the fire extinguishing effect can be improved under the condition of increasing the charging pressure
[20].
Table 2.
Full-scale fire extinguishing experiments of FK-5112.
| Researcher |
Aim |
Fire Scene |
Result |
| Hodges et al. [21] |
Evaluate the fire extinguishing efficiency of C6F12O and the generation amount of acid gas in specific scenarios |
Military vehicle, 7.36 m2 chamber |
The fire extinguishing efficiency of C6F12O is similar to that of halon and its substitutes, which can extinguish 7.36 m2 fire within 200 ms. However, the mass of fire extinguishing agent and the amount of acid gas in products cannot meet the application standard. |
| Bengtson et al. [22] |
Fire extinguishing efficiency and re-burning of C6F12O in polymer fire ignited by different electric power |
Polymer fire ignited by 192W electric power |
The fire extinguishing concentration is less than Halon 1301 and higher than the n-heptane test result given by NFPA 2001. When the fire suppressant concentration is higher than the test value of cup burner, the fire can be prevented from re-ignition. |
| Kim et al. [23] |
The fire extinguishing efficiency and acid gas generation of C6F12O |
3 kW cable fire, small oil pool and wood stack fire under the design concentration (6.5%) in 58 m3 space |
It takes a long time for the extinguishing agent to reach the extinguishing concentration in the confined space. The cable fire is put out 72 s after combustion, while it is put out in 30 s in the open and ventilated environment. The wood stack and oil pool fire can be put out in 10 s. A large amount of acid gas is produced in a large flame. |
| Liu et al. [24] |
Fire extinguishing efficiency of C6F12O for lithium batteries |
38 Ah prismatic ternary (Li(Ni1/3Co1/3Mn1/3)O2/graphite))battery with the voltage of 4.2 V in 47.5 * 21.5 * 16 cm3 module box |
With the increase in agent concentration, the fire extinguishing effect first decreases and then increases. No obvious cooling effect of C6F12O was found in the experiment |
| FAA et al. [25] |
Feasibility of fire extinguishing application of C6F12O in aviation application |
Jet A fuel in the inclined-plane fire tests,16- and 30-ft pan tests and the simulated engine nacelle fire tests |
It is similar to halotron I (CF3CHCl2/Ar/CF4 mixture), but the volume and mass of C6F12O are larger than halotron I |
2. Fire Extinguishing Mechanism
C6F12O has a high fire extinguishing efficiency in different fire scenes, and its fire extinguishing mechanism can be divided into a physical and chemical mechanism.
2.1. Physical Mechanism
The physical mechanism can be divided into the cooling effect on combustion and the dilution and isolation effect on combustion components.
Due to its high boiling point, C6F12O is stored in liquid phase at room temperature and ejected in the form of a gas–liquid mixture. Liquid C6F12O rapidly vaporizes when it approaches the flame and removes a part of heat from the fire. After vaporization, C6F12O and air will form a gaseous mixture with a high heat capacity which could absorb more heat from the fire. According to the report of 3M Company
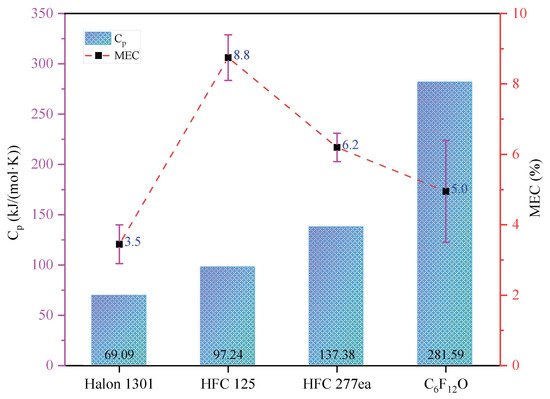
[26], C6F12O mainly absorbs the heat of the fire through this way to cool down and further extinguish the fire. Compared with other commercial halon substitutes, C6F12O has the higher heat capacity, resulting in the lower MEC, as shown in
Figure 1, and it could be found that chemical effect would also play an important role in fire extinguishment for the chemical gas agents, especially for Halon 1301. At the same time, when C6F12O contacts the flame, the heat of the fire will also be taken up by some breaking and formation of chemical bond processes in the thermal decomposition of C6F12O. Moreover, C6F12O is easier to decompose than HFC 227ea, which leads to better cooling effect during pyrolysis. The decrease in temperature will reduce the reaction rate and destroy the conditions of the combustion.
Figure 1.
The comparation between the MEC and the molar specific heat (Cp) of typical fire suppressants.
The dilution and isolation effects of C6F12O play an important role in the total flooding system. These effects can reduce the oxygen concentration in the fire and prevent oxygen from contacting the active radicals, and thus inhibit the free radical chain reactions in the combustion.
2.2. Chemical Mechanism
Chemical Extinguishing Process of C6F12O
The chemical mechanism of the fire suppressant refers to the decomposition products or radicals of C6F12O capturing the combustion radicals generated from the fuel, which would inhibit the combustion chain reactions to extinguish fire.
To obtain the reaction mechanism of C6F12O with the hydrocarbon flame, Linteris et al.
[27] established the reaction kinetics model of C6F12O with hydrocarbon flame by modifying the analogy of similar substances with the existing decomposition model. Four sub-mechanisms were obtained including: (1) hydrocarbon combustion; (2) decomposition products of C6F12O containing fluorinated C1-C3; (3) C3 reactions related to HFC 227ea reaction in the flame; and (4) flame inhibition of C1-C2 fluorocarbon. They concluded that owing to the rapid decomposition of C6F12O, the heat absorption in this process has a limited effect on flame inhibition, and the critical effect on flame inhibition is the reaction of C1–C2 fluorocarbons with free radicals produced in fuel combustion. Moreover, the major breakdown products of C6F12O can form C3F7 and C2F5, and the Fourier transform infrared spectroscopy (FTIR) quantitative analysis of the products from the reaction of C6F12O with the flame shows that the decomposition products have a strong absorption peak at the wave number of 1027.2 cm
−1, which is similar to those of HFC 125 and HFC 227ea
[5]. It can be inferred that the flame inhibition mechanism of C6F12O is the combination of HFC 125 and HFC 227ea. Takahashi et al.
[8] studied the extinguishment of cup burner flames by C6F12O and found that the flame-anchoring reaction kernel weakens as the concentration of C6F12O increases gradually, which leads to the extinguishment of the flame. Xu et al.
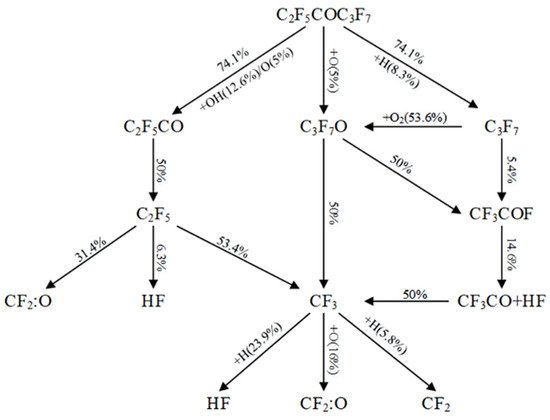
[28] obtained the specific reaction path of C6F12O in methane-air premixed flame through the numerical simulation and counterflow flame experiment, as shown in
Figure 2. In total, 74.1% of C6F12O will directly decompose into C2F5CO and C3F7, and C2F5CO will further decompose into C2F5. The remaining C6F12O will react with free radicals OH (12.6%), H (8.3%) and O (5%). The inhibition mechanism of methane-air premix flame is realized by the direct capture of free radicals in the chain termination reaction to generate stable HF and CF2:O. Because the formation of HF releases heat, the inhibition effect of the agent will be weakened when the exothermic reaction is dominant.
Figure 2.
Reaction path of FK-5112 in methane/air premixed flame.
Influence of H Content in Reaction Environment on Fire Extinguishing Process
Previous studies have speculated that the fire extinguishing mechanism of C6F12O is the combination of HFC 125 and HFC 227ea. However, compared with the latter two agents, C6F12O does not contain H in the molecule, and the level of H in the reaction zone would have a greater impact on fire extinguishment. Linteris et al.
[27] found that when the concentration of C6F12O is high while H is inadequate in the reaction zone, HF cannot be formed and COF2 is formed instead through the simulation method. The heat release rate of the system and the flame temperature would all decrease. Andersson
[5] analyzed the thermal breakdown products of HF and COF2 of C6F12O experimentally and found that the production of HF decreases with the increase in C6F12O concentration, while COF2 demonstrated the contrary. Hence, the relation between the production of HF and COF2 and concentration of C6F12O obtained by Linteris et al.
[27] in the simulation study was verified from the view of decomposition products. However, the presence of water in the reaction zone can provide H and OH. Pagliaro et al.
[29] studied the environmental humidity on the fire extinguishment of C6F12O and concluded that the inhibition effect of the fire suppressant on the combustion depends on the ratio of F/H of the reaction zone. When the concentration of C6F12O is high, that is, the ratio of F/H is large, water vapor can provide H and OH for combustion, resulting in combustion enhancement, and the combustion inhibition will occur provided the ratio is low.
Flame Enhancement Mechanism during the Fire Extinguishment
The aforementioned FAA-ACT showed that C6F12O can cause the overpressure similar to HFCs because of the combustion enhancement by the suppressant. This phenomenon was observed in the changes in flame temperature, speed and system pressure
[27][28][29][30][31] and CO and CO2 production before and after the addition of the agent
[5]. The combustion enhancement of C6F12O is of great concern in its application.
Some researchers have revealed that the inhibition effect of C6F12O on the flame depends on the combustion state of the fuel and the addition amount of the suppressant. Under the condition of the rich combustion, the reaction rate of the system can always be reduced by adding a fire suppressant. Additionally, under the condition of lean combustion, the effect of adding the agent on the reaction rate of the system first increases and then decreases with the increase in the volume fraction of the fire suppressant
[27][28][29][30][31]. In addition, the concentration of C6F12O also has a key effect on combustion enhancement. Liu et al.
[32] found that the inhibition effect of C6F12O on the flame is not sensitive to the type of hydrocarbon fuel. When the volume fraction of C6F12O exceeds a certain value, laminar flame velocity can be inhibited regardless of the chemical equivalent ratio between the fuel and air. Thermodynamic equilibrium and perfectly stirred-reactor calculations showed that the overpressures in FAA test may be caused by high heat release from the reaction of the agent itself
[27][33][34]. It has been considered that the highly exothermic reactions between fluorine-containing groups and combustion radicals is the main reason resulting in combustion enhancement, and when the fire suppressant is insufficient enough to capture the combustion radicals, it would accelerate the release of heat enhancing the combustion
[35][36]. Takahashi et al.
[8] found that exothermic reactions to form HF and CF2O in the two-zone trailing flame results in unwanted combustion enhancement and the total heat release increases up to three times for a large number of carbon and fluorine atoms in the molecules. They further concluded that unwanted combustion enhancement occurs because of the agent reacts exothermically in the air before approaching the main flame zone to inhibit combustion. Physical and chemical effects of C6F12O are coupled in the fire extinguishment, which should be distinguished to study the combustion enhancement and fire extinguishing mechanism. Ren et al.
[37] used the numerical simulation method to decouple the contribution of physical and chemical extinguishing effects. It was concluded that the chemical action of C6F12O can enhance combustion under the poor combustion condition of the fuel, while its physical action always inhibits the combustion. A similar method was adopted by Takahashi et al.
[8], and they found that the blow-off extinguishment occurs at ≈1700 K with the addition of the inert C6F12O, which is identical to that of inert gases. From the perspective of combustion products, the thermal decomposition of C6F12O would produce combustible substances such as C2F4, C4F6 and CO, which can become involved in combustion
[38].
Due to the combustion enhancement of C6F12O, it is necessary to further analyze the occurrence conditions of the flame enhancement phenomenon and the combustible decomposition products of C6F12O in subsequent research to prevent the occurrence of the phenomenon in fire extinguishment.