Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 4 by Lindsay Dong.
Carbon capture and storage (CCS) is considered to be a promising technology in reducing atmospheric CO2 concentration. Among the CO2 capture technologies, adsorption has grabbed significant attention owing to its advantageous characteristics discovered in recent years. Solid adsorbents have emerged as one of the most versatile CO2 adsorbents.
- porous carbon
- amine functionalization
- physisorption
- chemisorption
- CO2 capture
- activated carbon
- Greenhouse effect
1. Introduction
1.1. Physical and Chemical Properties of CO2
Carbon dioxide (CO2) is a triatomic gas under ambient conditions [1], which is abundant, non-toxic, recyclable, and economical [2]. Moreover, CO2 sublimates from solid-state to gas at −78 °C under atmospheric pressure and is comparatively inert. As a commonly known fact, CO2 gas that naturally occurs in the Earth’s atmosphere is of paramount importance to photosynthesis [1]. From an economic point of view, CO2 can be converted into high-value chemical products such as urea, carbonates, and acrylates [3] through catalytic conversion, mineralization, photochemical, or electrochemical reactions, and supercritical CO2 can be also utilized in various industrial fields, including food beverages, refrigerants, transportation fuels, fire extinguishers, polymer synthesis, medical, and exploitation of heavy oil. Solid-state CO2 can be used in artificial rainfall and concrete production [4][5].
1.2. Trend of Atmospheric CO2 Concentration and Potential CO2 Emissions Sources
Although the natural carbon cycle controls the CO2 concentration level in the Earth’s atmosphere [1], due to both anthropogenic activities and natural emissions, the current atmospheric CO2 concentration reached around 416.5 ppm in mid-2020 [6], which is ~40% greater than the beginning of the industrial revolution (280 ppm) in 1750 [7][8][9], with an average growth rate of 2 ppm per year [9][10]. In other words, the global emission of CO2 was estimated to be more than 36 MT in 2017, which is 18-fold greater than compared to the 1800s [11]. Although it is a consensus that the amount of atmospheric CO2 should not exceed 350 ppm [12], according to the predictions by the International Panel on Climate Change (IPCC), it is expected to reach up to 570 ppm by 2100 [12][13][14]. It is identified that the main causes for the tremendous increase in such atmospheric CO2 concentration are mainly associated with various anthropogenic activities, including vehicular emissions, fossil-fuel power plants, deforestation, chemical processes [15], and waste treatment [16], which have been growing steadily due to rapid industrialization and urban development [15][17]. The natural emission sources, including soil degradation processes and volcanic activities, are also responsible for supplying atmospheric CO2 to some extent [18].
1.3. Significant Outcomes Owing to the Trend of Increasing CO2 Emissions
Unfortunately, the non-controllable anthropogenic activities have negatively affected human beings [19] and the entire ecosystem [3][6] by releasing greenhouse gases, including CO2, into the atmosphere. Among the greenhouse gases, CO2 is considered as one of the primary sources, contributing to roughly 64% of the total greenhouse effect [14][20]. The progressive increase in atmospheric CO2 concentration is responsible for climate change, which might adversely impact the global environmental processes, such as the long-term rise in global temperatures, changes in rainfall patterns, rising sea levels [21][22], ocean acidification [23], species extinction, melting of polar ice [9], shrinkage of snow covers [24], and severe weather events, ranging from flash floods [25], hurricanes, freezing winters, severe droughts [22], heat waves [26], urban smog [17], and cold streaks [27]. According to the predictions made by IPCC, the rise in sea level of 3.8 m [14][28] and rise in mean global temperature by 3.7 °C [29][30] are expected by 2100 [24]. Besides, the increasing trend of CO2 in the air might cause various air-borne diseases, which will increase the risk of health complications [31]. The economic loss due to climate change is expected to be 5–20% of the global domestic production [12][28]. Therefore, extensive research projects are currently underway to reduce and control CO2 emissions from power plants, industries, and transportation [32].1.4. Approaches to Reduce Atmospheric CO2 Concentration
Three feasible strategies to reduce CO2 emissions are exhibited by the modified Kaya identity as expressed in equation (1) [28]. They are namely, (i) improving the energy efficiency of coal-fired plants [33][34], (ii) change of the fossil fuels to renewable and carbon-free energy resources [35], and (iii) utilization of carbon capture and storage (CCS) technologies [28][36][37].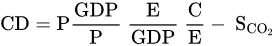 where CD: CO2 emissions, P: Population, GDP: economic development in gross domestic production, E: energy production, C: carbon-based fuels used for energy production, and SCO2: CO2 sinks [28].
Apart from the above-mentioned three strategies, enhancing partial pressure in exhaust gas [36], geoengineering approaches including afforestation and reforestation [38], flue gas separation, and carbon mineralization [39] can also be considered. Among the different CO2 mitigation options, IPCC has suggested CCS as a promising technology for achieving a 19% reduction of global CO2 emissions by 2050 [34]. CCS can reduce CO2 emissions (typically 85–90%) from significant stationary point sources such as power plants, cement kilns, and NG wells [40][41]. Nevertheless, CCS is considered a mid-term solution in reducing global warming, climate change, and simultaneously allowing humans to continue using fossil fuels until a renewable and clean energy source is discovered to replace them [34]. CCS is comprised of three significant steps, namely, (i) capture of emitted CO2 from power plants and industrial processing without releasing them into the atmosphere, (ii) transportation of the captured and compressed CO2, and (iii) underground storage of the captured CO2 [26][42][43]. However, the process of CO2 capture, which accounts for 70–80% of the total cost, has proven to be the major barrier for the deployment of CCS [40][44]. Interestingly, in recent years, carbon capture storage and utilization (CCSU) has grabbed significant attention compared to CCS owing to the convertibility of the captured CO2 into commercial products [45][46]. The success of CCS and CCSU technologies are associated with the CO2 adsorption efficiency, ease of handling, manufacturing cost, and renderability of the associated materials [22].
where CD: CO2 emissions, P: Population, GDP: economic development in gross domestic production, E: energy production, C: carbon-based fuels used for energy production, and SCO2: CO2 sinks [28].
Apart from the above-mentioned three strategies, enhancing partial pressure in exhaust gas [36], geoengineering approaches including afforestation and reforestation [38], flue gas separation, and carbon mineralization [39] can also be considered. Among the different CO2 mitigation options, IPCC has suggested CCS as a promising technology for achieving a 19% reduction of global CO2 emissions by 2050 [34]. CCS can reduce CO2 emissions (typically 85–90%) from significant stationary point sources such as power plants, cement kilns, and NG wells [40][41]. Nevertheless, CCS is considered a mid-term solution in reducing global warming, climate change, and simultaneously allowing humans to continue using fossil fuels until a renewable and clean energy source is discovered to replace them [34]. CCS is comprised of three significant steps, namely, (i) capture of emitted CO2 from power plants and industrial processing without releasing them into the atmosphere, (ii) transportation of the captured and compressed CO2, and (iii) underground storage of the captured CO2 [26][42][43]. However, the process of CO2 capture, which accounts for 70–80% of the total cost, has proven to be the major barrier for the deployment of CCS [40][44]. Interestingly, in recent years, carbon capture storage and utilization (CCSU) has grabbed significant attention compared to CCS owing to the convertibility of the captured CO2 into commercial products [45][46]. The success of CCS and CCSU technologies are associated with the CO2 adsorption efficiency, ease of handling, manufacturing cost, and renderability of the associated materials [22].
1.5. CO2 Emission Sources
The CO2 emission sources are the primary candidates for potential applications of CCS or CCSU technologies. Therefore, from a community and industrial point of view, CO2 capture from typical gas streams, including flue gas, biogas, flare gas, syngas, and ambient air, has grabbed significant interest [47]. Table 1 depicts the summary of the compositions of different gas streams.Table 1.
Compositions of different gas streams which act as potential CO2 capture opportunities (Reprinted with permission from ref. ).
| Component | Cement Rotary Kiln | Dry Atmospheric Air | Biogas Generated from Waste Water Treatment Plant Sludge | Natural Gas Fired Flue Gas | Coal-Fired Flue Gas |
|---|---|---|---|---|---|
| N2 | 59 vol % | 70 vol % | 0–1 vol % | 73–80 vol % | 70–80 vol % |
Table 2.
Comparison of the three main carbon capture technologies.
| CO2 Capture Technology | Advantages | Disadvantages |
|---|---|---|
| Pre-combustion capture |
| |
| CO2 | 19 vol % | 410 ppm |
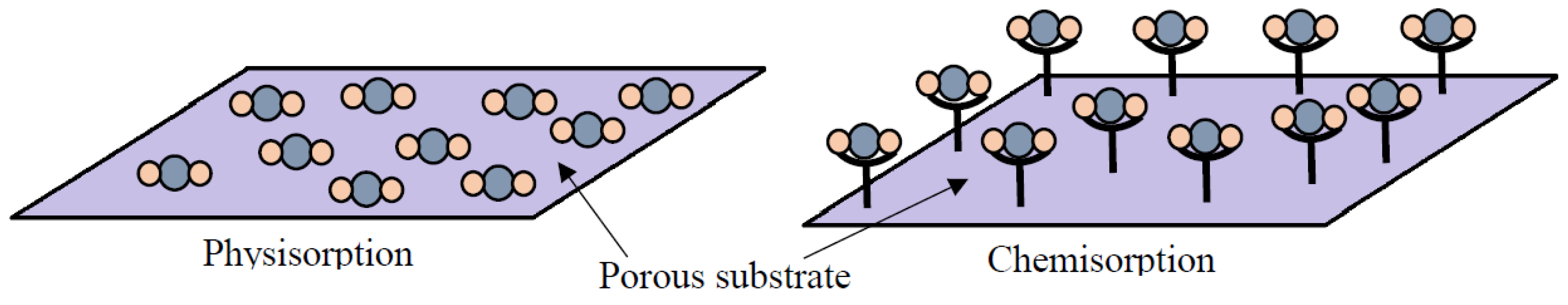
Figure 1. Schematic of the interactions between gas molecules and the adsorbent surface during physisorption and chemisorption (Reprinted with permission from ref. [62]).
Table 3.
Comparison of the CO2 physisorption and chemisorption processes.
| Process | Advantages | Disadvantages | ||
|---|---|---|---|---|
|
||||
| Physisorption |
|
|||
|
Oxy-fuel combustion |
| ||
| Chemisorption |
|
|
2.2. Different Regeneration Strategies
The attached CO2 molecules onto the adsorbent surface could be regenerated through the (i) pressure swing adsorption (PSA), (ii) temperature swing adsorption (TSA), (iii) vacuum swing adsorption (VSA), (iv) pressure and vacuum swing adsorption (PVSA), and (v) electric swing adsorption (ESA) processes [26][28][73]. Table 5 shows the advantages and disadvantages of different regeneration strategies. The regeneration method depends on the chemical and structural properties of a given adsorbent [69].Table 4.
Comparison of different regeneration strategies.
| Regeneration Strategy | Advantages | Disadvantages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature swing adsorption (TSA) | ||||||||||
| 19–33 vol % | 3–8 vol % | 11–15 vol % | ||||||||
|
| |||||||||
| Pressure swing adsorption (PSA) |
|
|
H | |||||||
|
2O | |||||||||
| Electric swing adsorption (ESA) | 13 vol % | - | - | Post-combustion capture |
|
| 7–14.6 vol % | 5–12 vol % | ||
|
O2 | 7 vol % | 21 vol % | <0.5 vol % | 4.5–15 vol % | 3–6 vol % | ||||
| Vacuum swing adsorption (VSA) |
|
SO2 | 5–1200 ppm | - | - | <10 ppm | 200–4000 ppm | |||
| SO3 | - | - | - | - | 0–20 ppm | |||||
| NOX | 100–1500 ppm | - | - | 50–70 ppm | 200–800 ppm | |||||
| CO | - | - | - | - | 50–100 ppm | |||||
| H2 | - | 0.5 vol % | - | 5–300 ppm | 5–20 g/m3 | |||||
| Particulate matter | - | - | - | - | - | |||||
| H2S | - | - | 100–4000 ppm | - | - | |||||
| Ar | - | 0.9 vol % | - | - | - | |||||
| Xe | - | 0.1 vol % | - | - | - | |||||
| Ne | - | 18 ppm | - | - | - | |||||
| He | - | 5.2 ppm | - | - | - | |||||
| CH4 | - | 1.6 vol % | 60–75 vol % | - | - | |||||
| Kr | - | 1.1 vol % | - | - | - | |||||
| N2O | - | 0.3 vol % | - | - | - |
1.6. CO2 Capture Technologies
Table 2 depicts the comparison of the leading carbon capture technologies. According to Table 2, carbon capture from power plants in industries can be classified as (i) pre-combustion capture, (ii) oxy-fuel combustion, and (iii) post-combustion capture [49] depending on the combustion method and composition of the gas stream [502. Solid Adsorbents for CO2 Capture
2.1. Adsorption Process of CO2
Adsorption is a surface phenomenon that highly depends on surface properties and functionalities [50]. Adsorption of CO
|
2.3. Criteria for Selecting CO2 Adsorbents
When synthesizing and selecting an effective CO2 adsorbent, the material should be economical and operational simultaneously [74]. Therefore, a prospective CO2 adsorbent should satisfy the following criteria (Table 5): (i) CO2 adsorption capacity: The adsorption capacity plays a vital role since it determines the amount of adsorbent to be inserted into the adsorption column to attain the desired performance [77][78], (ii) Regenerability: The adsorbent should be fully regenerable and require relatively mild conditions for complete regeneration [78], (iii) CO2 selectivity: The adsorbent should display substantially high selectivity for CO2 in the co-presence of other species (e.g., N2, methane (CH4), sulfur dioxide (SO2), hydrogen sulfide (H2S), and moisture) [74][79][80], (iv) Adsorption/desorption kinetics: A rapid adsorption/desorption is required for swing adsorption to decrease the cycle time [73][74], (v) Thermal, chemical, and mechanical stability: During the cyclic regeneration process, the microstructure and morphology of the adsorbent should be retained. Moreover, the adsorbent should withstand harsh operating conditions, including vibration, high temperatures, pressures, and flow rates. Additionally, the amine-functionalized adsorbents should be resistant against oxidizing agents and contaminants such as sulfur oxides (SOX), nitrogen oxides (NOX), water vapor, and heavy metals [11][81], and (vi) Adsorbent cost: The adsorbent should be synthesized using cheap raw materials while adopting a cost-effective and energy-saving synthesis routes [62].Table 5.
Threshold values of criteria for selecting an effective CO2 adsorbent (Reprinted with permission from refs. ).
| Parameter | Requirement |
|---|---|
| CO2 adsorption capacity | 3–4 mmol/g |
| Regenerability | >1000 cycles |
| CO2 gas selectivity over other gases | >100 |
| Adsorption/desorption kinetics | >1 mmol/g.min |
| Adsorbent cost | $5–15/kg sorbent |
