Enantiomers share the same chemical formula but have different chemical structures, i.e., type of isomers. Enantiomers are present in several drugs, perfumes, food, and are a fundamental part of biomolecules. This subject is highly important for pharmaceutical companies. Enantiomeric drugs present different actuation in the human body; depending on the compound, one might combat the symptom, whereas its pair might cause damage. The separation of pairs of enantiomers requires a chiral environment that provokes a structural imbalance that conventional methods cannot provide. Enantioresolution is one of the most promissory studies that benefit several areas, such as pharmaceutical, biotechnology, food industry, and fine chemistry. Its resolution is of great importance, therefore, its main mechanisms of resolution will be explained herein.
- enantiomer
- chirality
- nomenclature
- market
- enantioresolution
| Isomerism | Chemical Formula | Structural Formula | |||
|---|---|---|---|---|---|
| Structural | Function | C3H6O |  |
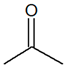 |
|
| Propanal | Propanone | ||||
| Chain | C4H10 |  |
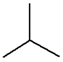 |
||
| n-Butane | Isobutane | ||||
| Position | C5H10O | 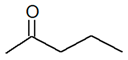 |
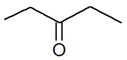 |
||
| 2-Pentanone | 3-Pentanone | ||||
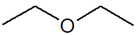
| Metamerism | C4H10O | 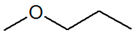 |
 |
||
| 1-Methoxypropane | Ethoxyethane | ||||
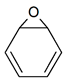
| Tautomerism | C6H6O | 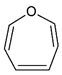 |
 |
 |
|
| Oxepin | Benzene oxide | ||||
| Stereo | Diastereomer (Geometric) |
C2H2Cl2 | 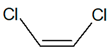 |
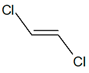 |
|
| cis-1,2-dichloroethene | trans-1,2-dichloroethene | ||||
| Enantiomer (Optical) |
C3H6O3 | 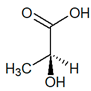 |
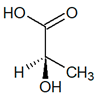 |
||
| (S)-Lactic acid | (R)-Lactic acid | ||||
Optical activity is the phenomenon of shifting in the direction of the light plane when it passes through a compound. Light is an electromagnetic radiation that, when interacting with electrons in a molecule, slightly diverts its direction. Some molecules, though, do not present optical activity, i.e., for any light shift, either to the left or right, there is a molecule that shifts it in the opposite direction, nulling the optical activity. On the other hand, when light passes through pure there is a shift of the light; when light shifts to left, the enantiomer is called levorotatory (receives the prefix or (−)) and to the right it is called dextrorotatory (receives the prefix or (+)).
A polarimeter is the instrument responsible for reading the light rotation in degrees of a certain enantiomer concentration. This rotation must be converted into a specific rotation to take in account the polarimeter length and sample concentration, as given by: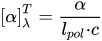 where is the specific rotation, superscript designates the temperature (usually 20 °C), superscript the light wavelength (usually at 589.6 nm of Na D-lines), is the polarimeter length in dm, is the enantiomer concentration in g·cm−3, and is the observed rotation in degrees in the polarimeter. It means that an enantiomer has a negative specific rotation, a enantiomer a positive one, and a racemate has a
where is the specific rotation, superscript designates the temperature (usually 20 °C), superscript the light wavelength (usually at 589.6 nm of Na D-lines), is the polarimeter length in dm, is the enantiomer concentration in g·cm−3, and is the observed rotation in degrees in the polarimeter. It means that an enantiomer has a negative specific rotation, a enantiomer a positive one, and a racemate has a 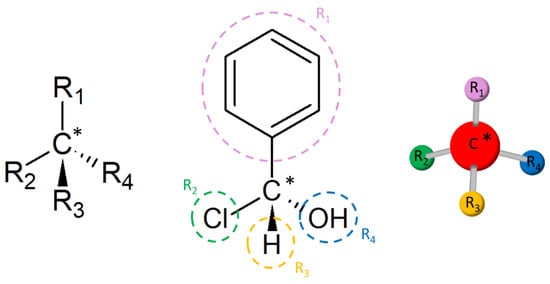
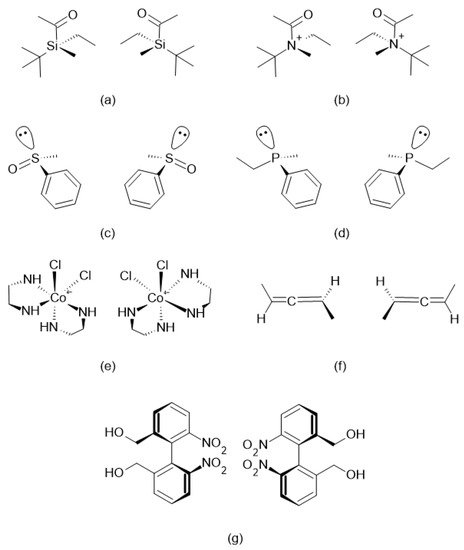

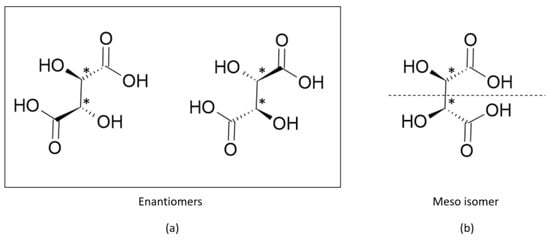
where and are the number of enantiomers and chiral carbons, respectively, as exemplified in Figure 3. However, this correlation is not always true; molecules with at least two chiral carbons that present a plane of symmetry are not chiral molecules. One of its carbons shifts the light plan to left, whereas the other nulls its effect, an internal compensation. An example is illustrated in Figure 4, although the tartaric acid has two chiral carbons, it has only two enantiomers and one meso isomer, a diastereomer and a non-optically active stereoisomer (see Table 1).
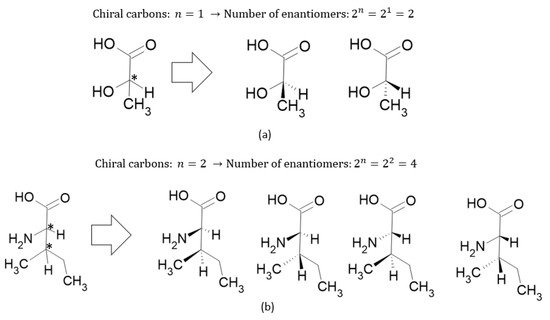
References
- Johnson, M. Integrating Health Information: A Case Study of a Health Information Service for Thalidomide Survivors. Inform. Health Soc. Care 2007, 32, 27–33.
- Maier, N.; Pilar, F.; Lindner, W. Separation of Enantiomers: Needs, Challenges, Perspectives. J. Chromatogr. A 2001, 906, 3–33.
- Molbase. Available online: http://www.molbase.com/ (accessed on 28 January 2019).
- Pais, L.M.S. Chiral Separation by Simulated Moving Bed Chromatography; Universidade do Porto: Campo Alegre, Porto, 1999.
- Lough, W.J. Chiral Liquid Chromatography; Springer: New York, NY, USA, 1989.
