Lectins are natural proteins with the ability to bind specific carbohydrates related to various microorganisms, including viruses, bacteria, fungi and parasites. Lectins have the ability to agglutinate and neutralize these pathogeneses. The delivery of the encapsulated antiviral agents or vaccines across the cell membrane can be possible by functionalized micellar and liposomal formulations.
- coronaviruses,COVID-19 pneumonia,lectins,nanoformulations
- coronaviruses
- COVID-19 pneumonia
- lectins
- nanoformulations
1. Introduction
Coronaviruses, a large family of RNA viruses (a positive sense, single-stranded RNA in the range size of 27–32 kb), belong to the subfamily Orthocoronavirinae in the family of Coronaviridae in the order Nidovirales[1]. The genome of the coronavirus is composed of open reading frames (ORFs), the first of which comprises two-thirds of the genome and encodes the replicase proteins. The last third contains the structural protein genes in a fixed order[2]. These viruses may cause diseases in birds and mammals, such as the infectious bronchitis virus (IBV) and feline coronavirus (FCoV), respectively[3][4][5]. The 1930s brought about the discovery of the first member of the family of the coronavirus[6]. The severe acute respiratory syndrome (SARS) outbreak in 2002–2003 shook the world and brought this to the forefront of research, and thus the discovery of more of the members of this family of viruses after the epidemic[2]. As illustrated in Figure 1, a complete viral particle is composed of several proteins including nucleocapsid (RNA + nucleoprotein), spike, envelope and the membrane proteins[7]. It is important to note that some viruses may contain hemagglutinin esterase (Figure 1). The membrane and envelope proteins are involved in virus assembly, whereas the spike protein is the leading mediator of viral entry and the principal player in determining host range[2]. The 3C-like protease and the papain-like protease (PLP) are the main viral proteases related to coronaviruses[5]. In humans, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019 (COVID-19) can lead to health-threatening diseases. The novel virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to COVID-19. The outbreak of this novel strain of coronavirus as the causative agent of COVID-19 pneumonia, first identified in Wuhan, China in December 2019, has resulted in considerable focus being placed on coronavirus virulence properties[8]. The most severe form of this novel virus has been reported to lead to hypoxia and acute respiratory distress syndrome (ARDS), which can lead to the requirement of invasive mechanical ventilation[9]. Many countries in the Middle East, Europe and the United States have been affected by this infection. Based on recent reports, COVID-19 can be classified as a mild, moderate and severe disease. The fatality rate of 2% up to 2.5% and the acute disease are as a result of severe alveolar damage and respiratory failure[8][10].
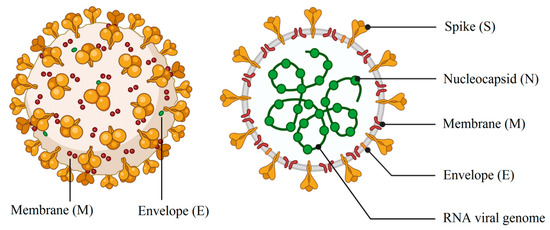
Figure 1.
Schematic drawing of main components of spherical or pleiomorphic coronaviruses (credit:
).
A viral infection is dependent on the interaction of viral particles with specific receptors on the cell membrane. The fusion of the virus envelope with the cell membrane is mediated by the spike glycoproteins[2]. Furin is one of the proteases with high expression in lung cells which can be responsible for the proteolytic cleavage of the envelope proteins of viruses such as SARS-CoV and human immunodeficiency virus (HIV). In SARS-CoV-2, the furin-like cleavage site in the spike glycoprotein may contribute to [11][12]the pathogenicity and the viral life cycle[13].
2. Lectins
Antitumor, antiviral and antimicrobial activities of lectins have been reported by various studies [14][15][16]. Before the initiation of an antibody’s activity in the body, the mannose-binding lectin (MBL) as a humoral protein can block the entry of coronavirus into the target cells [11][17]. Moreover, MBL increases viral neutralization by the activation of the complement system and the influx of innate immune cells[18]. The innate immune system employs this mechanism to hinder viral and bacterial infections in alveolar cells. Surface-active phospholipoprotein complexes containing phospholipids (80%), cholesterol (10%) and proteins (10%) with the names of SP-D, SP-C, SP-B, and SP-A are secreted by type II alveolar cells. SP-A and SP-D collectins having C-type lectins can opsonize bacterial and viral pathogens and facilitate the phagocytosis action by monocytes and macrophages. Interaction of collectins with carbohydrate moieties of spike protein on the surface of the SARS-CoV can lead to inactivate viral uptake and reducing pulmonary inflammation, wherein a mild and moderate illness in smokers may be resulted from increased levels of SP-A protein levels in sputum, plasma and the serum[19].
Natural carbohydrate-binding proteins such as lectins have demonstrated antiviral properties in the case of HIV and coronaviruses[20][21]. In this case, the spike glycoprotein related to SARS coronavirus and the envelope glycoprotein GP120 of HIV is the main targets for a red algae-derived lectin (griffithsin)[22][23]. In addition, griffithsin has antiviral activities against the hepatitis C virus[24]. Plants express twelve different families of lectins with binding ability to mono- or oligo-saccharides related to glycolipids or glycoproteins[25][26]. As illustrated in Figure 2, two functional subunits including S1 and S2 subunits are recognized for spike protein, which facilitates binding to the receptors of the host cell and fusion of viruses with the cellular membrane. The spike protein with a trimer structure is decorated with N-linked glycans that are critical components for appropriate folding and neutralizing by specific antibodies and host proteases[27]. The high-mannose oligosaccharides related to spike and membrane glycoproteins can be targeted by herbal lectins, wherein two coronaviruses, namely, feline infectious peritonitis virus and mouse hepatitis virus, were blocked by Galanthus nivalis agglutinin (GNA), Urtica dioica agglutinin (UDA), and Hippeastrum hybrid agglutinin (HHA) via the inhibition of virus entry at a post-binding stage[28].
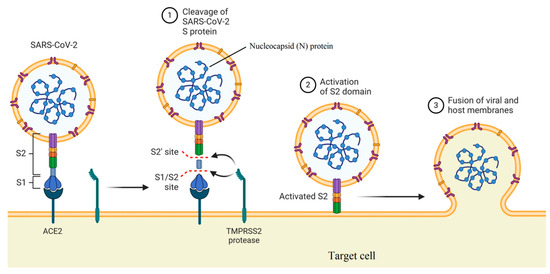
Figure 2.
Mechanism of viral entry by function of two subunits of the spike protein (created in
).
According to structure type, there are four lectins, namely, super lectins (with dissimilar carbohydrate-binding domains), chimero lectins (hybrid proteins composed of one or more carbohydrate-binding sites), hololectins (at least two carbohydrate-binding domains) and merolectins (single carbohydrate-binding site). Almost all agglutinating lectins are located in the hololectins group[27]. A reduction in the intracellular loading of RNA associated with SARS-CoV was observed for the MBL of the amaryllis plant species after 8 h of infection. Red, brown, and particularly green alga (nearly 500 types of lectin) are the main sources of algal lectins. Self-assembly of viruses may be disrupted by the effects of MBL[28]. More antiviral activities of MBL (extracted from red algae Grateloupia chiangii) toward the herpes simplex virus was observed compared to the influenza virus. In this case, MBL had a significant ability to bind maltoheptaose-β-Sp1 and maltohexaose-β-Sp1[29].
3. Micellar, Liposomal, and Lipid NP Formulations
Mixed micelles are formed by the self-assembly of two different di- or tri-block copolymers of surfactants in a colloidal medium[30]. The bioavailability of poorly water-soluble drugs can be augmented by polymeric mixed micelles. Efavirenz (EFV) is a non-nucleoside reverse transcriptase inhibitor which is recommended as an oral administration with other antiviral agents against HIV. Recent information suggests that this drug may inhibit SARS-CoV-2 by a combination with other antiretroviral drugs[31]. The high hydrophobic property of this drug leads to a lower bioavailability and dose-dependent side effects on the central nervous system (CNS). Loading of EFV by mixed micelles prepared by the use of Pluronic® F127 and Tetronic® T904 showed the enhanced bioavailability of this drug by four folds[32]. Viral RNA replication and translation are inhibited by a camptothecin drug as a topoisomerase inhibitor[33]. A polymethacrylate block containing 2,6-diacylaminopyridine pendant (DAP) units and a PEG (at 2000 (PEG2) and 10,000 (PEG10) molar masses) were utilized to encapsulate this hydrophobic drug in a spherical micelle with a maximum diameter size of 25 nm. Improved stability under acidic conditions and a controlled drug release was observed for the prepared micelle by PEG10 compared to the PEG2 unit[34]. Nelfinavir mesylate (NFM), an antiretroviral drug, is a HIV-1 protease inhibitor which also has reported antiviral activities toward SARS-CoV and SARS-CoV-2[35]. A short half-life of 3.5 to 5 h and its lipophilic nature are two major disadvantages for oral administration consideration. Loading NFM by mixed micelles (with an average diameter size of 104.1 nm) composed of pluronic F127 and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) improved drug loading, entrapment efficiency, bioavailability, sustained drug release and biodegradability[36].
The use of essential oils or various extracts of plants to treat microbial infections has a long history in traditional and modern medicine. These properties have resulted from the existence of active metabolites in several parts of medicinal plants. For example, artemisinin and its derivative, dihydroartemisinin, are two sesquiterpene lactone metabolites extracted from the Artemisia annua plant species with anti-parasitic effects[37][38]. Moreover, there are several reports of anti-infection properties in the case of other species of the Artemisia plant genus[39]. Potential antiviral activities of essential oils extracted from Artemisia arborescens toward herpes simplex virus type 1 (HSV-1) was evaluated by a liposomal formulation[40]. In the general sense, one strategy towards controlling and managing the pandemic is to design and formulate efficient vaccines against the virus. In the case of mRNA vaccines, a carrier system is strictly required in order to protect the sensitive structure of mRNA molecules and deliver the active agent to certain cells of the immune system (drug targeting) to induce the expected immunization. Amongst the available strategies for the encapsulation and targeting drugs and active agents are liposomes, nanoliposomes, solid lipid nanoparticles and tocosomes[41]. Using an antiviral sample, compound and derived lipids, as well as immuno-modulator lipids, are other strategies to synthesize novel antiviral carriers or vaccine-based liposomes. As a viral mimic, a double-stranded RNA of polyinosinic–polycytidylic acid and poly-L-lysine (poly-ICLC) is utilized as a prophylactic and antiviral agent for viral infections[42]. Upregulation of the expression of β-, γ- and α-interferon has resulted from the poly-ICLC effect. Further antiviral activities against Dengue virus (DENV) were shown for the liposomal formulation of poly-ICLC compared to poly-ICLC alone by the higher expression of IFN-γ and the promotion of innate immunity[43]. As previously mentioned, loading antibody fragments on the surface of liposomes may be another way to functionalize actively targeted carriers. Coupling llama heavy-chain antibody fragments (Vhh) onto the PEGylated liposome surface via covalent and non-covalent bonds having dapivirine, a non-nucleoside reverse transcriptase inhibitor, resulted in a reduction of HIV replication in vitro. A strong binding affinity to HIV-1 envelope glycoprotein gp120 and reduced neutralization potency were observed for covalently linked Vhh compared with a non-covalent one. In this regard, free antibodies with non-covalent bonds demonstrated a higher number of Vhh to bind to gp120[44]. The thin-film hydration technique was utilized to prepare EFV-loaded liposomes with average diameter sizes and significant encapsulation efficiencies of 411.1 nm and 98.86%, respectively. The high encapsulation efficiency of this formulation was obtained using the soybean lecithin in the bilayer of the liposome, which had a solubilizing effect on EFV[45]. Lipid compounds may be employed to coat other organic or inorganic NPs such as polymeric NPs, metal NPs (MNPs), metal oxide NPs (MONPs) and mesoporous silica NPs (MSNs). For instance, ML336, a benzamidine antiviral medicine, was encapsulated via liposome-coated MSNs against the Venezuelan equine encephalitis virus (VEEV) (Figure 3). In vitro inhibition of VEEV resulted from improved circulation time and biocompatibility, as well as with a sustained drug release (6.6 ± 1.3 μg/mg) over 24 h[46].
Figure 3. (a) Schematic and (b) TEM images of liposome-coated mesoporous silica nanoparticles (MSNs) with loading ML336 antiviral drug (scale bar = 50 nm) reproduced with permission from[46].
Adefovir dipivoxil, a nucleotide analogue to decrease DNA level of hepatitis B virus (HBV) in serum, was loaded on SLNs towards the HepG2.2.15 cell line derived from the chronic HBV infection. Prepared drug–SLN formulations with a mean size of 389.4 ± 166.5 nm displayed sustained drug release. These formulations also displayed values of 15.32 ± 2.58% and 3.06 ± 0.51% for drug entrapment efficiency and drug loading, respectively[47]. The LDC form (PEGylated lipid–indinavir NPs) was activated by binding peptides of CD4-BP2 and CD4-BP4 to target CD4+ cells. Efficient drug delivery and indinavir release at acidic conditions (pH = 5) of endosome were explained as a pH-dependent drug release model for these carriers[48].
4. Application of Lectins in Micelle and Liposome Formulation
In this section, the functionalization of micelles and liposomes by several types of lectin is presented for non-viral infections because of the lack of this modification for antiviral purposes. Therefore, these examples can be useful to prepare novel formulations of vaccines or antiviral agents by lectin-modified micelles and liposomes in future investigations. To date, there is a lack of studies regarding modification of micelles by lectin. As one promising example, concanavalin A (Con A) lectin was conjugated to poly(ε-caprolactone)-block-glycopolymer micelles to improve mucoadhesiveness[49]. However, lectin modification of liposomes is more efficient owing to the stability of their bilayer structure compared to a micelle. As illustrated in Table 1, several interactions between liposomal components and lectins were used to prepare lectin-modified liposomes.
Table 1. Applications of several types of lectin in liposome formulations.
|
Sources of Lectin |
Application in Liposome |
References |
|
Wheat germ agglutinin (WGA) lectin of Triticum vulgare plant |
Incorporation of the lectin in the bilayer liposome for potential oral vaccine carriers. [50] |
|
|
WGA lectin of T. vulgare plant |
WGA-modified liposome with the ability to bind the N-acetylglucosamine was used as an aerosol formulation for drug delivery to human alveolar epithelial cells. |
[51] |
|
Con A, WGA, and soybean agglutinin (SBA) extracted from Canavalia ensifomis, T. vulgare, and Glycine max plant species, respectively |
Coupling lectins with liposomes with avidin/biotin technology. Con A, WGA, and SBA have carbohydrates specificity for α-D-mannose or α-D-glucose, N-acetyl-glucosamine oligomers and N-acetyl I-galactosamine, respectively. |
[52] |
|
Cratylia mollis plant species |
Encapsulation of the lectin in the liposome. |
[53] |
|
WGA lectin of T. vulgare plant |
Phosphatidylethanolamine in the bilayer was covalently bound to the lectin to prepare the WGA-modified liposome. |
[54] |
|
Lectin extracted from Lotus tetragonolobus plant species |
Formation of oleic acid–lectin conjugation in the phospholipid bilayer of liposomes with the ability to bind glycans having alpha-1,2-linked fucose. [55] |
|
|
Tarin lectin of Colocasia esculenta plant species |
The lectin was encapsulated in the aqueous phase of a liposome. Tarin demonstrated a promising binding site for complex and high-mannose N-glycan chains related to viral surface antigens. This lectin can help a host to recover from infections by the stimulation of innate and adaptive immune responses. |
|
|
Lectin extracted from Bauhinia variegate plant species |
Based on FTIR and NMR analyses, there was a strong interaction between the lectin and phosphatidylcholine of the liposome in the outer and inner polar surface of the liposome. The rotational motion of the lipid group was restricted by this interaction. |
[58] |
|
Lectin of T. vulgare plant, WGA |
Interaction of the lectin with the bilayer of the liposome resulted in cytoadhesive and cytoinvasive effects ,as well as increased permeability in the cell membrane and cellular uptake compared with the non-lectin liposomes for oral epithelial cells |
[59] |
|
WGA-N-glutaryl-phosphatidylethanolamine |
Protection of the drug against enzyme degradation in vitro as well as higher stabilization of WGA-modified SLNs compared to bare SLNs. |
[60] |
References
- Banerjee, A.; Kulcsar, K.; Misra, V.; Frieman, M.; Mossman, K. Bats and Coronaviruses. Viruses 2019, 11, 41.
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033.
- Jiang, Y.; Cheng, X.; Zhao, X.; Yu, Y.; Gao, M.; Zhou, S. Recombinant infectious bronchitis coronavirus H120 with the spike protein S1 gene of the nephropathogenic IBYZ strain remains attenuated but induces protective immunity. Vaccine 2020, 38, 3157–3168.
- Guan, X.; Li, H.; Han, M.; Jia, S.; Feng, B.; Gao, X.; Wang, Z.; Jiang, Y.; Cui, W.; Wang, L.; et al. Epidemiological investigation of feline infectious peritonitis in cats living in Harbin, Northeast China from 2017 to 2019 using a combination of an EvaGreen-based real-time RT-PCR and serum chemistry assays. Mol. Cell. Probes 2020, 49, 101495.
- Deng, X.; StJohn, S.E.; Osswald, H.L.; O’Brien, A.; Banach, B.S.; Sleeman, K.; Ghosh, A.K.; Mesecar, A.D.; Baker, S.C. Coronaviruses Resistant to a 3C-Like Protease Inhibitor Are Attenuated for Replication and Pathogenesis, Revealing a Low Genetic Barrier but High Fitness Cost of Resistance. J. Virol. 2014, 88, 11886.
- Hudson, C.B.; Beaudette, F.R. Infection of the cloaca with the virus of infectious bronchitis. Science 1932, 76, 34.
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 1–22.
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422.
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581.
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924.
- Zhou, Y.; Lu, K.; Pfefferle, S.; Bertram, S.; Glowacka, I.; Drosten, C.; Pöhlmann, S.; Simmons, G. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010, 84, 8753–8764.
- Auriti, C.; Prencipe, G.; Moriondo, M.; Bersani, I.; Bertaina, C.; Mondi, V.; Inglese, R. Mannose-binding lectin: Biologic characteristics and role in the susceptibility to infections and ischemia-reperfusion related injury in critically ill neonates. J. Immunol. Res. 2017, 2017, 7045630.
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020, 176, 104742.
- Hwang, H.-J.; Han, J.-W.; Jeon, H.; Cho, k.; Kim, J.-H.; Lee, D.-S.; Han, J.W. Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga, Grateloupia chiangii. Biomolecules 2020, 10, 333.
- Breitenbach Barroso Coelho, L.C.; Marcelino dos Santos Silva, P.; Felix de Oliveira, W.; De Moura, M.C.; Viana Pontual, E.; Soares Gomes, F.; Guedes Paiva, P.M.; Napoleão, T.H.; Dos Santos Correia, M.T. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018, 125, 1238–1252.
- Liang, Z.; Yang, L.; Zheng, J.; Zuo, H.; Weng, S.; He, J.; Xu, X. A low-density lipoprotein receptor (LDLR) class A domain-containing C-type lectin from Litopenaeus vannamei plays opposite roles in antibacterial and antiviral responses. Dev. Comp. Immunol. 2019, 92, 29–34.
- Ip, W.K.E.; Chan, K.H.; Law, H.K.W.; Tso, G.H.W.; Kong, E.K.P.; Wong, W.H.S.; To, Y.F.; Yung, R.W.H.; Chow, E.Y.; Au, K.L.; et al. Mannose-Binding Lectin in Severe Acute Respiratory Syndrome Coronavirus Infection. J. Infect. Dis. 2005, 191, 1697–1704.
- Zhang, W.; Bouwman, K.M.; van Beurden, S.J.; Ordonez, S.R.; van Eijk, M.; Haagsman, H.P.; Hélène Verheige, M.; Veldhuizen, E.J.A. Chicken mannose binding lectin has antiviral activity towards infectious bronchitis virus. Virology 2017, 509, 252–259. [Google Scholar] [CrossRef]
- Weiskirchen, R. Severity of Coronavirus Disease 2019 (COVID-19): Does surfactant matter? Front. Microbiol. 2020, 11, 1905. [Google Scholar] [CrossRef]
- Mazalovska, M.; Kouokam, J.C. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. Biomed. Res. Int. 2018, 8, 3750646. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug. Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.J.; Shirakura, M.; et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef]
- Gautam, A.K.; Sharma, D.; Sharma, J.; Saini, K.C. Legume lectins: Potential use as a diagnostics and therapeutics against the Cancer. Int. J. Biol. Macromol. 2019, 142, 474–483. [Google Scholar] [CrossRef]
- Cavada, B.S.; Osterne, V.J.S.; Oliveira, M.V.; Pinto-Junior, V.R.; Silva, M.T.L.; Bari, A.U.; Lima, L.D.; Lossio, C.F.; Nascimento, K.S. Reviewing Mimosoideae lectins: A group of under explored legume lectins. Int. J. Biol. Macromol. 2020, 154, 159–165. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Van der Meer, F.; de Haan, C.A.M.; Schuurman, N.M.P.; Haijema, B.J.; Verheije, M.H.; Bosch, B.J.; Egberink, H.F. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J. Antimicrob. Chemother. 2007, 60, 741–749. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Panda, P.K.; Sinha, N.; Praharaj, P.P.; Bhol, C.S.; Panigrahi, D.P.; Mahapatra, K.K.; Saha, S.; Patra, S.; Mishra, S.R.; et al. Plant lectins in cancer therapeutics: Targeting apoptosis and autophagy-dependent cell death. Pharmacol. Res. 2019, 144, 8–18. [Google Scholar] [CrossRef]
- Gondim, A.C.S.; da Silva, S.R.; Mathys, L.; Noppen, S.; Liekens, S.; Sampaio, A.H.; Nagano, C.S.; Rocha, C.R.C.; Nascimento, K.S.; Cavada, B.S.; et al. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. MedChemComm 2019, 10, 390–398.
- Hwang, H.-J.; Han, J.-W.; Jeon, H.; Cho, k.; Kim, J.-H.; Lee, D.-S.; Han, J.W. Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga, Grateloupia chiangii. Biomolecules 2020, 10, 333.
- Singh, V.; Khullar, P.; Dave, P.N.; Kaur, N. Micelles, mixed micelles, and applications of polyoxypropylene (PPO)-polyoxyethylene (PEO)-polyoxypropylene (PPO) triblock polymers. IJIC 2013, 4, 1–18.
- Tang, B.; Li, S.; Xiong, Y.; Tian, M.; Yu, J.; Xu, L.; Zhang, L.; Li, Z.; Ma, J.; Wen, F.; et al. Coronavirus Disease 2019 (COVID-19) Pneumonia in a Hemodialysis Patient. Kidney Med. 2020, 2, 354–358.
- Chiappetta, D.A.; Hocht, C.; Opezzo, J.A.W.; Sosnik, A. Intranasal administration of antiretroviral-loaded micelles for anatomical targeting to the brain in HIV. Nanomedicine 2013, 8, 223–237.
- Wu, K.X.; Chu, J.J.-H. Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor. Antivir. Res. 2017, 143, 122–133.
- Concellón, A.; Clavería-Gimeno, R.; Velázquez-Campoy, A.; Abian, O.; Piñol, M.; Oriol, L. Polymeric micelles from block copolymers containing 2, 6-diacylaminopyridine units for encapsulation of hydrophobic drugs. RSC Adv. 2016, 6, 24066–24075.
- Yamamoto, N.; Yang, R.; Yoshinaka, Y.; Amari, S.; Nakano, T.; Cinatl, J.; Rabenau, H.; Doerr, H.W.; Hunsmann, G.; Otaka, A.; et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004, 318, 719–725.
- Patil, P.H.; Mahajan, H.S. Mixed micelles for bioavailability enhancement of nelfinavir mesylate: In vitro characterisation and In vivo pharmacokinetic study. Mater. Technol. 2018, 33, 793–802.
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Hoeijmakers, W.A.M.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 2020, 367, 51–59.
- van der Pluijm, R.W.; Imwong, M.; Chau, N.H.; Hoa, N.T.; Thuy-Nhien, N.T.; Thanh, N.V.; Jittamala, P.; Hanboonkunupakarn, B.; Chutasmit, K.; Saelow, C.; et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: A prospective clinical, pharmacological, and genetic study. Lancet Infect. Dis. 2019, 19, 952–961.
- Alavi, M.; Karimi, N.; Salimikia, I. Phytosynthesis of zinc oxide nanoparticles and its antibacterial, antiquorum sensing, antimotility, and antioxidant capacities against multidrug resistant bacteria. J. Ind. Eng. Chem. 2019, 72, 457–473.
- Sinico, C.; De Logu, A.; Lai, F.; Valenti, D.; Manconi, M.; Loy, G.; Bonsignore, L.; Fadda, A.M. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur. J. Pharm. Biopharm. 2005, 59, 161–168.
- Zarrabi, A.; Alipoor Amro Abadi, M.; Khorasani, S.; Mohammadabadi, M.R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and tocosomes as multifunctional nanocarriers for the encapsulation of nutraceutical and Dietary Molecules. Molecules 2020, 25, 638.
- Saxena, M.; Sabado, R.L.; La Mar, M.; Mohri, H.; Salazar, A.M.; Dong, H.; Da Rosa, J.C.; Markowitz, M.; Bhardwaj, N.; Miller, E. Poly-ICLC, a TLR3 Agonist, induces transient innate immune responses in patients with treated HIV-Infection: A randomized double-blinded placebo controlled trial [Clinical Trial]. Front. Immunol. 2019, 10, 1–12.
- Hu, Y.; Hu, Y.; Sun, L.; Wong, J.; Wang, M. Antiviral effects of liposome-encapsulated PolyICLC against Dengue virus in a mouse model. Biochem. Biophys. Res. Commun. 2016, 478, 913–918.
- Wang, S.X.; Michiels, J.; Ariën, K.K.; New, R.; Vanham, G.; Roitt, I. Inhibition of HIV virus by neutralizing Vhh attached to dual functional liposomes encapsulating dapivirine. Nanoscale Res. Lett. 2016, 11, 350.
- Okafor, N.I.; Nkanga, C.I.; Walker, R.B.; Noundou, X.S.; Krause, R.W.M. Encapsulation and physicochemical evaluation of efavirenz in liposomes. J. Pharm. Investig. 2020, 50, 201–208.
- LaBauve, A.E.; Rinker, T.E.; Noureddine, A.; Serda, R.E.; Howe, J.Y.; Sherman, M.B.; Rasley, A.; Brinker, C.J.; Sasaki, D.Y.; Negrete, O.A. Lipid-Coated mesoporous silica nanoparticles for the delivery of the ML336 antiviral to inhibit Encephalitic Alphavirus infection. Sci. Rep. 2018, 8, 13990.
- Zhang, X.-G.; Miao, J.; Li, M.-W.; Jiang, S.-P.; Hu, F.-Q.; Du, Y.-Z. Solid lipid nanoparticles loading adefovir dipivoxil for antiviral therapy. J. Zhejiang Univ. Sci. B 2008, 9, 506–510.
- Endsley, A.N.; Ho, R.J.Y. Enhanced anti-HIV efficacy of indinavir after inclusion in CD4-targeted lipid nanoparticles. J. Acquir. Immune Defic. Syndr. 2012, 61, 417–424.
- Li, N.N.; Cai, X.Y.; Chen, J.C.; Hu, X.F.; Xu, L.Q. Conjugation of lectin to poly (ε-caprolactone)-block-glycopolymer micelles for In Vitro intravesical drug delivery. Polymers 2016, 8, 379.
- Chen, H.; Torchilin, V.; Langer, R. Lectin-bearing Polymerized Liposomes as Potential Oral Vaccine Carriers. Pharm. Res. 1996, 13, 1378–1383.
- Abu-Dahab, R.; Schäfer, U.F.; Lehr, C.-M. Lectin-functionalized liposomes for pulmonary drug delivery: Effect of nebulization on stability and bioadhesion. Eur. J. Pharm. Sci. 2001, 14, 37–46.
- Brück, A.; Abu-Dahab, R.; Borchard, G.; Schäfer, U.F.; Lehr, C.-M. Lectin-functionalized liposomes for pulmonary drug delivery: Interaction with human alveolar epithelial cells. J. Drug Target. 2001, 9, 241–251.
- Andrade, C.A.S.; Correia, M.T.S.; Coelho, L.C.B.B.; Nascimento, S.C.; Santos-Magalhāes, N.S. Antitumor activity of Cratylia mollis lectin encapsulated into liposomes. Int. J. Pharm. 2004, 278, 435–445.
- Zhang, N.; Ping, Q.N.; Huang, G.H.; Xu, W.F. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int. J. Pharm. 2005, 294, 247–259.
- Della Giovampaola, C.; Capone, A.; Ermini, L.; Lupetti, P.; Vannuccini, E.; Finetti, F.; Donnini, S.; Ziche, M.; Magnani, A.; Leone, G.; et al. Formulation of liposomes functionalized with Lotus lectin and effective in targeting highly proliferative cells. BBA-Gen Subj. 2017, 1861, 860–870.
- Pereira, P.R.; Corrêa, A.C.N.T.F.; Vericimo, M.A.; Paschoalin, V.M.F. Tarin, a potential immunomodulator and COX-Inhibitor lectin found in Taro (Colocasia esculenta). Compr. Rev. Food Sci. Food 2018, 17, 878–891.
- Corrêa, A.C.; Vericimo, M.A.; Dashevskiy, A.; Pereira, P.R.; Paschoalin, V.M.F. Liposomal taro lectin nanocapsules control Human glioblastoma and mammary adenocarcinoma cell proliferation. Molecules 2019, 24, 471.
- dos Santos, M.C.; Kroetz, T.; Dora, C.L.; Giacomelli, F.C.; Frizon, T.E.A.; Pich, C.T.; da Silva Pinto, L.; Soares, A.S.; Rodembusch, S.F.; de Lima, V.R.; et al. Elucidating Bauhinia variegata lectin/phosphatidylcholine interactions in lectin-containing liposomes. J. Colloid Interface Sci. 2018, 519, 232–241.
- Wijetunge, S.S.; Wen, J.; Yeh, C.-K.; Sun, Y. Lectin-Conjugated liposomes as biocompatible, bioadhesive drug carriers for the management of oral ulcerative lesions. ACS Appl. Biol. Mater. 2018, 1, 1487–1495.
- Zhang, N.; Ping, Q.; Huang, G.; Xu, W.; Cheng, Y.; Han, X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 2006, 327, 153–159.
- Zhang, N.; Ping, Q.; Huang, G.; Xu, W.; Cheng, Y.; Han, X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 2006, 327, 153–159.
