Therapeutic enzymes are biocatalyst drugs that bind to target substrates with a high affinity and specificity, catalyzing their conversion into their relevant products. The past five decades have seen the development of therapeutic enzymes for treating a wide range of medical conditions, including inherited enzyme deficiency disorders, acute poisoning, digestive disorders, cancer and cardiovascular diseases. Chemical modifications of the native enzyme (e.g., conjugation with polyethylene glycol) are often employed in the manufacturing process to increase protein stability, decrease immunogenicity, reduce renal ultrafiltration and in some cases, to enable targeting of the enzyme to the appropriate cellular compartment. The erythrocyte carrier has been extensively studied as a strategy for overcoming these limitations and increasing therapeutic efficacy. For a majority of the therapeutic applications investigated, the ability of the cell to reseal after creating pores in the membrane has been exploited for the purpose of introducing therapeutic agents.
1. Introduction
Therapeutic enzymes are biocatalyst drugs that bind to target substrates with a high affinity and specificity, catalyzing their conversion into their relevant products. The past five decades have seen the development of therapeutic enzymes for treating a wide range of medical conditions, including inherited enzyme deficiency disorders, acute poisoning, digestive disorders, cancer and cardiovascular diseases. Chemical modifications of the native enzyme (e.g., conjugation with polyethylene glycol) are often employed in the manufacturing process to increase protein stability, decrease immunogenicity, reduce renal ultrafiltration and in some cases, to enable targeting of the enzyme to the appropriate cellular compartment [1]. However, despite these strategies, the metabolic and clinical efficacy of parenterally or intramuscularly administered therapeutic enzymes is still limited. This is principally due to short circulatory half-lives, hypersensitivity reactions and immunogenicity. Immune responses are influenced by several factors including the biophysical characteristics of the protein, the route of delivery, the degree of exposure and the use of immunosuppressive agents during administration. Host factors may also play a part, for example patients with congenital enzyme deficiencies may fail to recognize a therapeutic protein as “self” and may be more likely to mount an immune response during the administration of therapeutic replacement enzyme [2]. The consequences of an immune reaction to a therapeutic enzyme range from a transient appearance of antibodies, without any clinical sequel, to severe life-threatening conditions.
The erythrocyte carrier has been extensively studied as a strategy for overcoming these limitations and increasing therapeutic efficacy. For a majority of the therapeutic applications investigated, the ability of the cell to reseal after creating pores in the membrane has been exploited for the purpose of introducing therapeutic agents [3,4,5]. The resealed erythrocyte is biocompatible and, in the human, has a normal in vivo circulating half-life of 19–29 days, and thus raises the potential to extend the half-life of encapsulated enzymes through the avoidance of plasma clearance due to the action of proteases, anti-enzyme antibodies and renal clearance, and through minimizing immune reactions.
2. Therapeutic Strategies
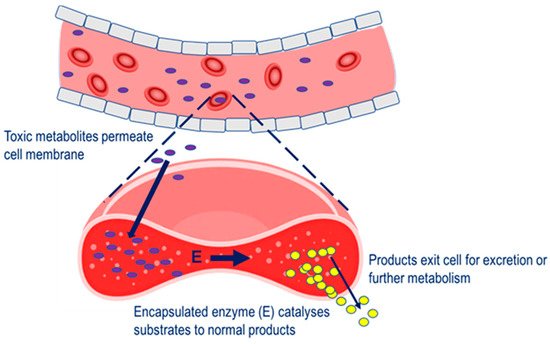
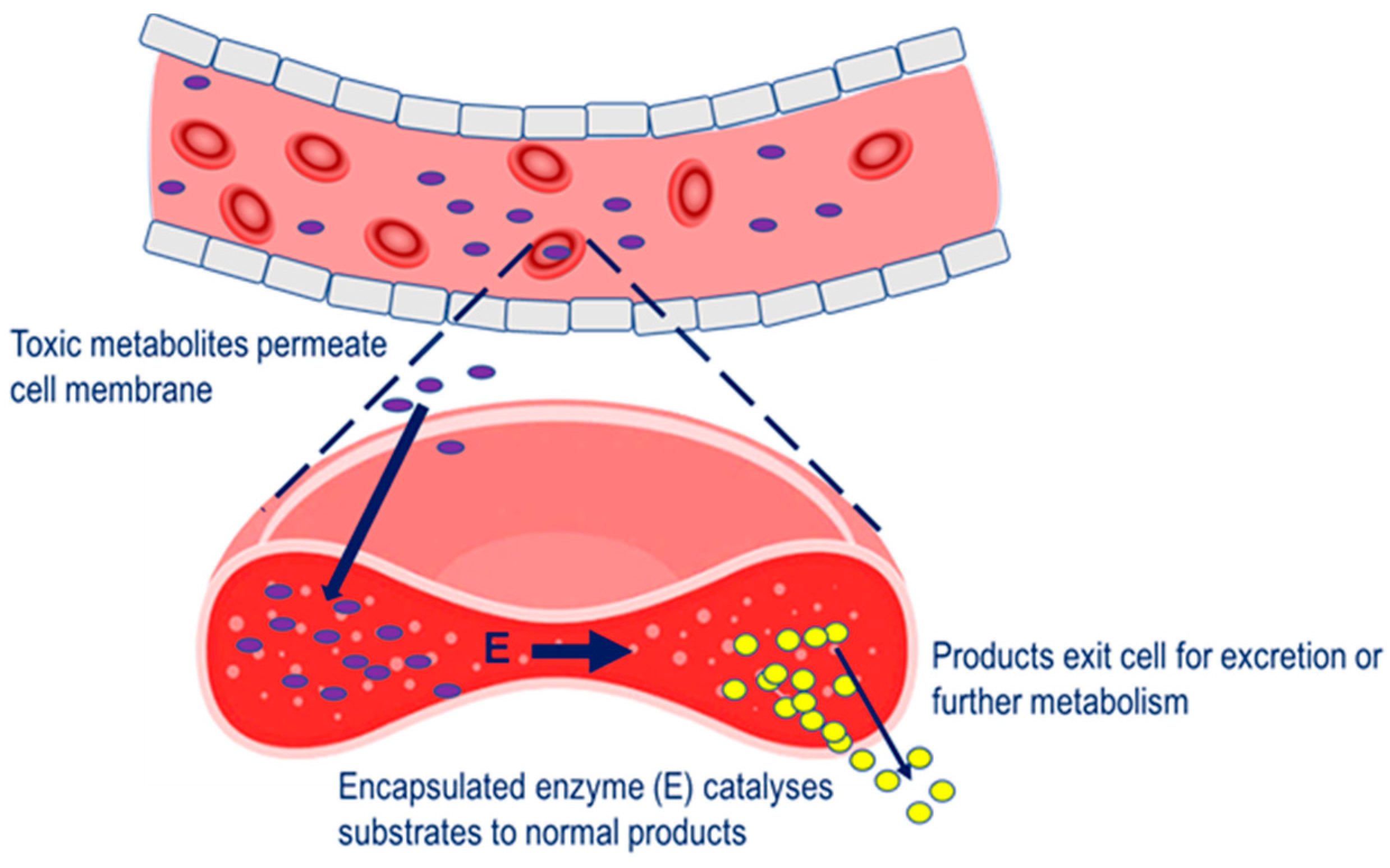
Four main therapeutic strategies have been applied to the erythrocyte carrier in terms of a vehicle for therapeutic enzymes. In the first, enzyme-loaded erythrocytes can be employed as circulating bioreactors whereby blood-based molecules diffuse into the erythrocyte and are degraded. This strategy is relevant to the treatment of congenital enzyme-deficiencies where a pathologically elevated plasma metabolite is able to permeate erythrocyte membrane and undergo metabolism to the normal product [6]. Also relevant to this therapeutic strategy is the depletion of plasma metabolites for the treatment of auxotrophic tumors and detoxification after exposure to toxic chemicals, The erythrocyte carrier has been extensively studied as a strategy for overcoming these limitations and increasing therapeutic efficacy. For a majority of the therapeutic applications investigated, the ability of the cell to reseal after creating pores in the membrane has been exploited for the purpose of introducing therapeutic agents [3][4][5]. The resealed erythrocyte is biocompatible and, in the human, has a normal in vivo circulating half-life of 19–29 days, and thus raises the potential to extend the half-life of encapsulated enzymes through the avoidance of plasma clearance due to the action of proteases, anti-enzyme antibodies and renal clearance, and through minimizing immune reactions.
2. Therapeutic Strategies
Four main therapeutic strategies have been applied to the erythrocyte carrier in terms of a vehicle for therapeutic enzymes. In the first, enzyme-loaded erythrocytes can be employed as circulating bioreactors whereby blood-based molecules diffuse into the erythrocyte and are degraded. This strategy is relevant to the treatment of congenital enzyme-deficiencies where a pathologically elevated plasma metabolite is able to permeate erythrocyte membrane and undergo metabolism to the normal product [6]. Also relevant to this therapeutic strategy is the depletion of plasma metabolites for the treatment of auxotrophic tumors and detoxification after exposure to toxic chemicals, Strategy for the removal of pathological metabolites from the circulation. Pathological metabolites in the blood enter the erythrocyte carrier, to undergo metabolism by the encapsulated enzyme (E).
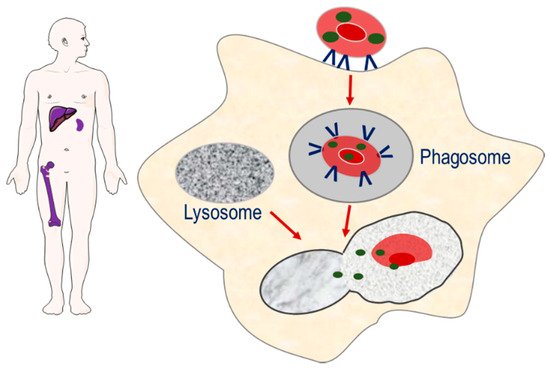
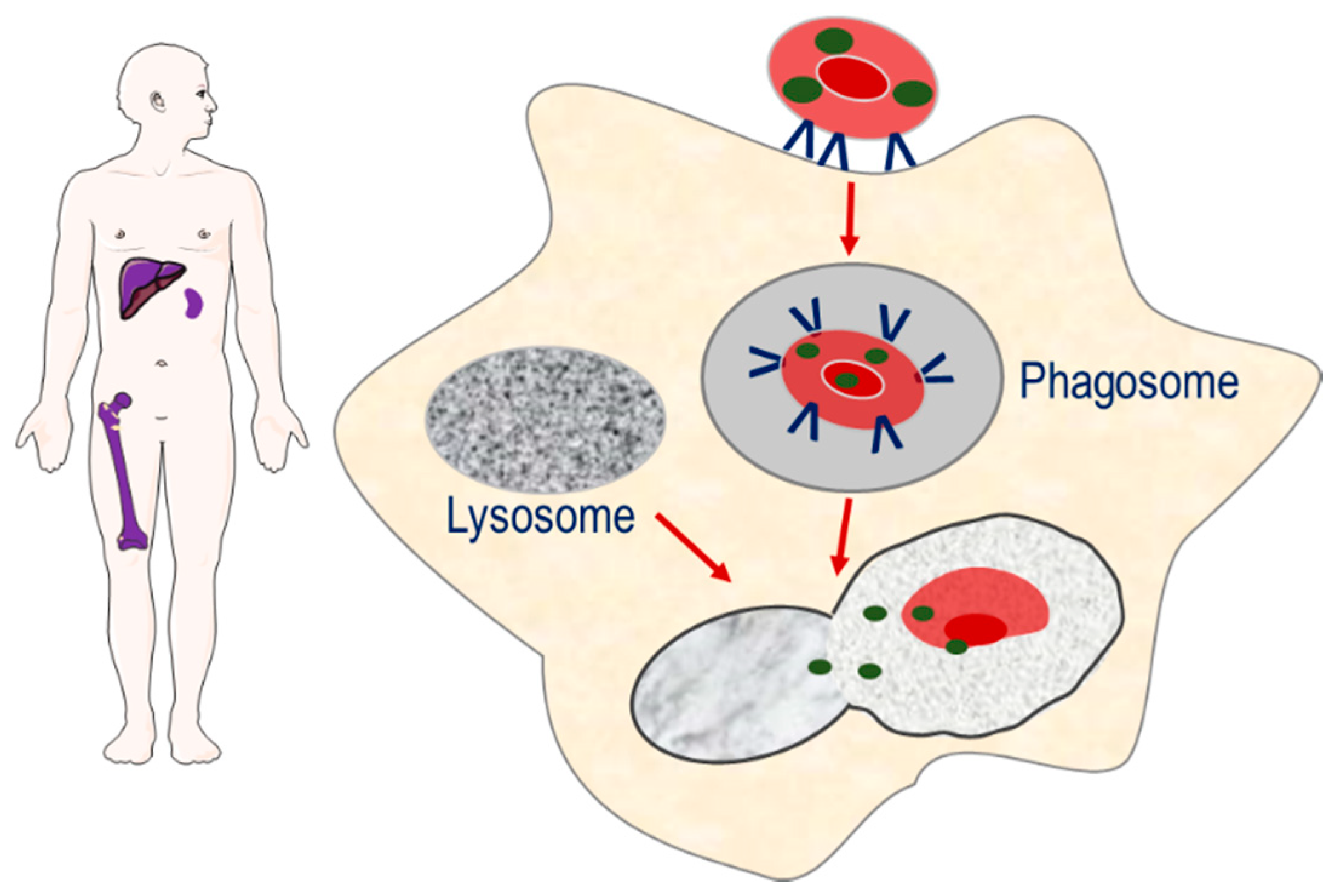
The second strategy involves the targeting of encapsulated enzymes to the monocyte–macrophage system of the spleen, liver and bone marrow, through exploiting the natural sites of erythrophagocytosis [7]. The loss of phospholipid asymmetry and the exposure of phosphatidylserine on the surface of the plasma membrane of senescence erythrocytes induces erythrocyte sequestration by macrophages. As erythrocyte degradation occurs in the lysosomal compartment of macrophages, it is proposed that erythrocyte encapsulated enzymes can potentially target macromolecules that accumulate in the lysosomal compartment as a result of congenital enzyme-deficiencies, Strategy for targeting enzymes to the monocyte–macrophage system of the spleen, liver and bone marrow. Senescent erythrocyte carriers loaded with enzymes are removed from the circulation by macrophages. The resulting phagosome fuses with macromolecule-laden lysosomes, resulting in the release of enzyme and breakdown of macromolecules.
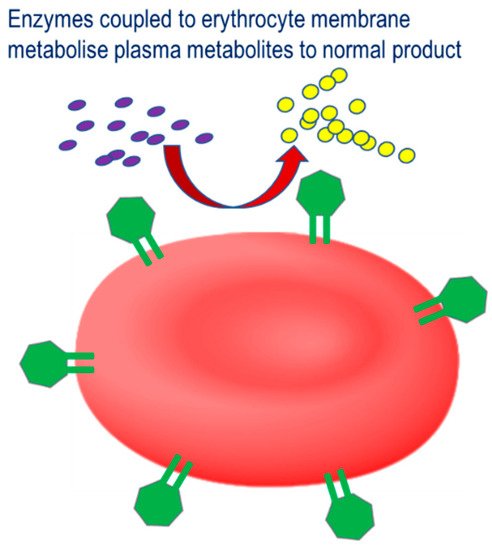
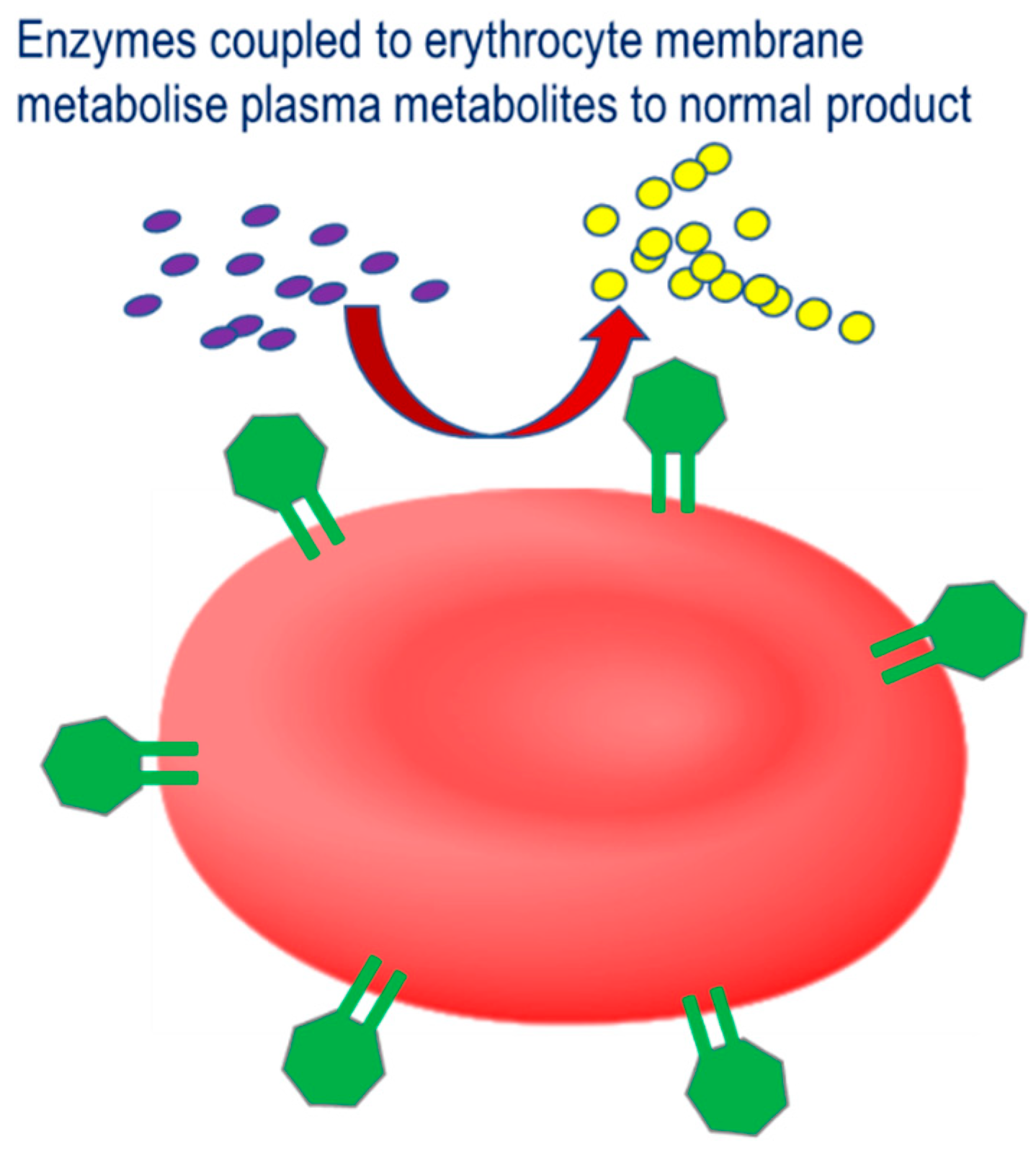
The third strategy involves the coupling of therapeutic enzymes to the erythrocyte surface with the aim of improving the therapeutic profile, Therapeutic strategy for therapeutic enzymes coupled to the erythrocyte membrane. Pathological plasma metabolites are catalyzed by the conjugated enzyme to their corresponding non-pathological product.
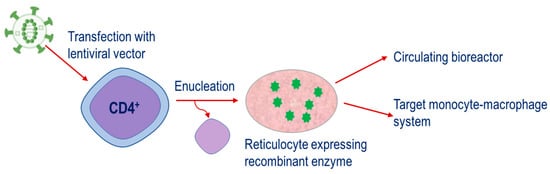
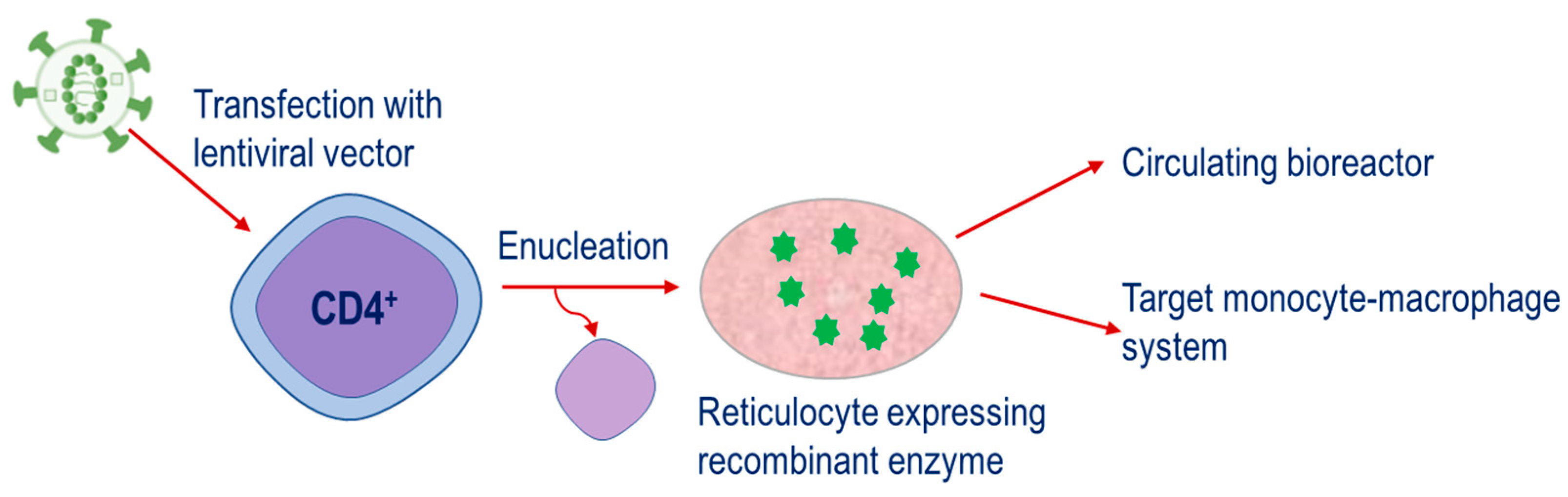
The fourth strategy is a relatively new approach. This is based on the engineering of CD34 hematopoietic precursor cells to express therapeutic enzymes and then their subsequent differentiation until the nucleus is ejected, resulting in the mature reticulocyte,
Figure 4. This strategy has potential applications for both the metabolism of plasma metabolites and targeting of the monocyte–macrophage system. . This strategy has potential applications for both the metabolism of plasma metabolites and targeting of the monocyte–macrophage system.
Strategy for therapeutic enzymes produced through the transfection of CD34
+ cells with lentiviral vectors containing constructs encoding for the enzyme of interest. Cells are expanded and differentiated until the nucleus is ejected, resulting in the mature reticulocyte expressing the recombinant enzyme.
3. Therapeutic Applications
Over the past five decades, the erythrocyte carrier has been extensively investigated as a potential modality for targeting a wide range of therapeutic applications. In relation to carriers of enzymes, these applications can broadly be divided into four key areas of research interest: exogeneous chemical detoxification, thrombolytic therapy, treatments for metabolic diseases and antitumour therapies. Although a majority of these studies have not translated beyond the pre-clinical stage, a few are currently undergoing clinical development. The following provides a discussion of the applications investigated under these four research areas.
3.1. Detoxification of Exogenous Chemicals
The utility of the erythrocyte as a carrier of specific enzymatic antidotes against chemical intoxicants has been investigated by a number of groups. Investigations into this therapeutic application have focused on encapsulating the relevant enzyme within the erythrocyte. Way et al. first established the potential of the erythrocyte carrier as antidote candidate for cyanide intoxication [8,9]. Cyanide is a rapidly lethal poison and exerts its toxic effect by blocking the mitochondrial respiration chain and the formation of intracellular adenosine triphosphate through binding to cytochrome-c oxidase, the terminal enzyme complex of the respiratory chain in complex IV. Sodium thiosulfate is one of the antidotes used to combat intoxication and works by acting as a sulfur donor for the mitochondrial enzyme, thiosulfate sulfotransferase (rhodanase, EC 2.8.1.1) [10]. Sodium thiosulfate does not readily permeate cell membranes and therefore is not able to distribute to sites of thiosulfate sulfotransferase or cyanide localization; this provided the rationale for investigating the erythrocyte carrier as an alternative approach to cyanide antagonism. In vivo studies in mice demonstrated that erythrocytes containing sodium thiosulfate and rhodanase could rapidly metabolize cyanide to the less toxic thiocyanate and antagonize the effects of a lethal dose of potassium cyanide [11,12,13]. Moreover, by replacing sodium thiosulfate with butanethiosulfonate, a more reactive sulfur donor substrate, an enhanced protective effect against cyanide was found [14].
The application of the erythrocyte carrier as an antagonist of the lethal effects of parathion, a once widely used agricultural organophosphorous insecticide was also investigated as an alternative antidote approach to paraoxon intoxication. The toxicity of parathion is attributed to its in vivo metabolism to paraoxon which inhibits acetylcholinesterase, leading to an accumulation of acetylcholine and ultimately altering cholinergic synaptic transmission at neuroeffector junctions, at skeletal myoneural junctions and autonomic ganglia in the central nervous system [15]. Two antidotes for parathion poisoning are atropine, a competitive antagonist of acetylcholine at the muscarinic receptor and pralidoxime, which regenerates acetylcholinesterase [16]. However, neither of these antidotes are able to degrade parathion. In vivo studies in mice investigated the efficacy of erythrocyte encapsulated phosphotriesterase (EC 3.1.8.1) in antagonizing the lethal effects of paraoxon through its hydrolysis to the less toxic 4-nitrophenol and diethylphosphate [17]. The results indicated that although the phosphotriesterase-loaded erythrocytes were more effective than the classic antidotal combination of atropine and pralidoxime, a combination of the loaded erythrocytes with the classic antidot, provided a 1000-fold protection against paraoxon. The same group also investigated the application of erythrocyte encapsulated recombinant paraoxonase as an approach to directly hydrolyze paraoxon; treated mice showed no signs of intoxication at paraoxon dose levels that were lethal when using the classical antidotal combination of atropine and pralidoxime. Moreover, erythrocyte encapsulated paraoxonase, in combination with the classic antidotal combination, provided the highest antidotal efficacy ever reported against any chemical toxicant [18].
Another category of detoxifying enzymes that have been investigated are those associated with the metabolism of ethanol and methanol. Ethanol detoxification requires two enzymic reactions: the oxidation of ethanol to acetaldehyde by alcohol dehydrogenase (EC. 1.1.1.1), followed by the oxidation acetaldehyde to acetate by aldehyde dehydrogenase (EC 1.2.1.5). Chronic alcohol consumption decreases acetaldehyde oxidation, either due to decreased aldehyde dehydrogenase activity or impaired mitochondrial function. The application of the erythrocyte carrier as an alcohol detoxifier was first proposed by Magnani et al. They demonstrated that mice administered acetaldehyde dehydrogenase-loaded erythrocytes intraperitoneally had 35% less blood acetaldehyde compared to controls, one hour after receiving an acute dose of ethanol [19]. Lizano et al. investigated the co-encapsulation of alcohol dehydrogenase and aldehyde dehydrogenase in human erythrocytes, demonstrating their superior ability to metabolize ethanol in vitro, compared to erythrocytes loaded with alcohol dehydrogenase alone [20]. In vivo studies in mice receiving acute doses of ethanol and treated with co-encapsulated enzymes showed blood ethanol to be eliminated at rates of 1.7 mmol/L to 4 mmol/L loaded erythrocytes/hour [21,22]. However, these rates of plasma ethanol clearance were lower by an order of magnitude than those expected from the activities of encapsulated enzymes. By employing a mathematical modeling approach and then conducting a subsequent in vitro study, Alexandrovich et al. were able to theorize and then demonstrate the rate limiting step of external ethanol oxidation. They found this was due to the rate of nicotinamide–adenine dinucleotide (NAD+) generation in erythrocyte glycolysis, rather than the activities of the loaded enzymes. By supplementing the erythrocytes with NAD+ and pyruvate they were able to demonstrate an elimination of 17 mmol ethanol/L loaded erythrocytes/hour [23].
In mammalian species, methanol is metabolized to formaldehyde via alcohol dehydrogenase, followed by the conversion of formaldehyde into formic acid via aldehyde dehydrogenase. Formic acid metabolism is mediated through a tetrahydrofolate-dependent pathway by folate-dependent enzymes. Humans have 60% less liver folate concentrations compared to mice and rats, and for this reason humans are more sensitive to methanol poisoning [24]. Specifically, formic acid inhibits mitochondrial cytochrome c oxidase, leading to cellular hypoxia and metabolic acidosis. Magnani et al. investigated the application of erythrocyte-encapsulated methylotrophic yeast alcohol oxidase (EC 1.1.3.13) as an approach to the detoxification of methanol. In vivo studies showed that two hours following an acute dose of methanol (0.7 g/kg), mice that had received enzyme-loaded erythrocytes had 50% less blood methanol compared to controls, with antagonism persisting for at least one week [25]. On the basis that formate dehydrogenase (EC 1.2.1.2) converts formate into CO2 in the presence of NAD, Muthuvel et al. administered formate dehydrogenase-loaded erythrocytes to methanol-intoxicated folate-deficient rats, which were pre-treated with carbicarb to correct the metabolic acidosis, and demonstrated a marked elimination of formate [26].
A detoxifying enzyme that has been investigated in relation to lead poisoning is δ-aminolevulinic acid dehydratase (E.C. 4.2.1.24). Lead is a potent inhibitor of δ-aminolevulinic acid dehydratase through its displacement of zinc from the enzyme’s active site. The resulting inactivation leads to an accumulation of its substrate, aminolevulinic acid, and this has been shown to have a neuropathogenic effect [27]. The in vitro and in vivo mouse studies of Bustos et al. showed that it was possible to correct the defective δ-aminolevulinic acid dehydratase activity in erythrocytes by encapsulating exogenous human enzyme, thus providing a rationale for this approach for treating lead intoxication [28,29]. In the first clinical application, 100 mL autologous erythrocytes loaded with 25,600 units of human δ-aminolevulinic acid dehydratase were administered to a single patient with a history of chronic lead intoxication, producing a reduction in blood lead concentration and decrease in urinary porphyrin excretion, which was sustained for a period of four years [30].
Biochemical decompression has been proposed as a method for reducing the amount of time required for deep-sea divers to return to the surface. Divers breathing H2/O2 mixtures would be administered hydrogenase (EC 1.12.1.4), thus accelerating decompression through the removal of excess hydrogen from the tissues. The application of erythrocyte encapsulated hydrogenase as an approach to converting dissolved hydrogen gas into non-gaseous forms was investigated by Axley et al. Hydrogenase activities of 7-µmol H2/minute/mL packed human erythrocytes were successfully encapsulated, however the cells were not able to consume hydrogen due to the enzyme’s dependence on NAD for activity. The co-encapsulation of FAD with hydrogenase was shown to overcome this [31].
3.2. Thrombolytic Therapy
The application of erythrocytes as a strategy for thrombolytic therapy has been explored by several groups. Thrombolytics (or fibrinolytics), also known as plasminogen activators, facilitate the removal of pre-existing thrombotic clots through degrading the fibrin meshwork into plasmin. However, their application is limited by their rapid clearance and inactivation by plasminogen activator inhibitor-1, the risk of bleeding caused by dissolution of hemostatic clots and filtration into extravascular tissues, such as the central nervous system, causing toxicity [32]. The rationale for the erythrocyte encapsulation of fibrinolytics was based on addressing these issues with the study of Delahousse et al. who successfully demonstrated the encapsulation of the fibrinolytic enzymes urokinase (EC 3.4.21.31), streptokinase (EC 3.4.99.0) and recombinant tissue plasminogen activator (tPA; EC 3.4.21.68) in human erythrocytes. However, in vitro stability studies using human erythrocytes and in vivo studies in the mouse were not able to support the objective of a sustained half-life and demonstrated a rapid destruction of the encapsulated enzymes [33]. Flynn and coworkers reported that Aspergillus oryzae brinase (EC. 3.4.99) encapsulated into rabbit erythrocytes was able to lyse clotted blood in vitro [34]. The coupling of fibrinolytics to the surface of the erythrocyte carrier was proposed as a preferable alternative to encapsulation [35]. Murciano et al. investigated the conjugation of tPA to erythrocytes as a strategy for thromboprophylaxis, by employing mouse and rat models of venous and arterial thrombosis. Following intravenous injection, the fibrinolytic activity of tPA conjugated to erythrocytes was shown to persist in the circulation at least tenfold longer than that of free tPA. Free tPA was able to lyse pulmonary clots that had lodged before administration, but not those that lodged after injection. Erythrocyte conjugated-tPA, however was more selective in lysing nascent over preexisting pulmonary emboli and arterial clots, an effect that is most likely a result of restricting the diffusion of tPA into fibrin [36]. The utility of this approach in preventing cerebrovascular thrombosis was also examined using mouse and rat models of cerebrovascular thromboembolism and ischemia, with the results indicating that erythrocyte-conjugated tPA was an effective thromboprophylaxis, whereas free tPA was ineffective up to a 10-fold higher dose [37].
3.3. Enzyme Replacement for Metabolic Diseases
The application of the erythrocyte as a carrier of enzymes for the treatment of inherited metabolic diseases and other metabolic disturbances has generated a large amount of interest from those working in a field where there are enormous unmet needs. With the exception of phenylketonuria, these therapeutic applications have focused on the use of encapsulated enzyme.
cells with lentiviral vectors containing constructs encoding for the enzyme of interest. Cells are expanded and differentiated until the nucleus is ejected, resulting in the mature reticulocyte expressing the recombinant enzyme.
3. Therapeutic Applications
Over the past five decades, the erythrocyte carrier has been extensively investigated as a potential modality for targeting a wide range of therapeutic applications. In relation to carriers of enzymes, these applications can broadly be divided into four key areas of research interest: exogeneous chemical detoxification, thrombolytic therapy, treatments for metabolic diseases and antitumour therapies. Although a majority of these studies have not translated beyond the pre-clinical stage, a few are currently undergoing clinical development. The following provides a discussion of the applications investigated under these four research areas.
3.1. Detoxification of Exogenous Chemicals
The utility of the erythrocyte as a carrier of specific enzymatic antidotes against chemical intoxicants has been investigated by a number of groups. Investigations into this therapeutic application have focused on encapsulating the relevant enzyme within the erythrocyte. Way et al. first established the potential of the erythrocyte carrier as antidote candidate for cyanide intoxication [8][9]. Cyanide is a rapidly lethal poison and exerts its toxic effect by blocking the mitochondrial respiration chain and the formation of intracellular adenosine triphosphate through binding to cytochrome-c oxidase, the terminal enzyme complex of the respiratory chain in complex IV. Sodium thiosulfate is one of the antidotes used to combat intoxication and works by acting as a sulfur donor for the mitochondrial enzyme, thiosulfate sulfotransferase (rhodanase, EC 2.8.1.1) [10]. Sodium thiosulfate does not readily permeate cell membranes and therefore is not able to distribute to sites of thiosulfate sulfotransferase or cyanide localization; this provided the rationale for investigating the erythrocyte carrier as an alternative approach to cyanide antagonism. In vivo studies in mice demonstrated that erythrocytes containing sodium thiosulfate and rhodanase could rapidly metabolize cyanide to the less toxic thiocyanate and antagonize the effects of a lethal dose of potassium cyanide [11][12][13]. Moreover, by replacing sodium thiosulfate with butanethiosulfonate, a more reactive sulfur donor substrate, an enhanced protective effect against cyanide was found [14].
The application of the erythrocyte carrier as an antagonist of the lethal effects of parathion, a once widely used agricultural organophosphorous insecticide was also investigated as an alternative antidote approach to paraoxon intoxication. The toxicity of parathion is attributed to its in vivo metabolism to paraoxon which inhibits acetylcholinesterase, leading to an accumulation of acetylcholine and ultimately altering cholinergic synaptic transmission at neuroeffector junctions, at skeletal myoneural junctions and autonomic ganglia in the central nervous system [15]. Two antidotes for parathion poisoning are atropine, a competitive antagonist of acetylcholine at the muscarinic receptor and pralidoxime, which regenerates acetylcholinesterase [16]. However, neither of these antidotes are able to degrade parathion. In vivo studies in mice investigated the efficacy of erythrocyte encapsulated phosphotriesterase (EC 3.1.8.1) in antagonizing the lethal effects of paraoxon through its hydrolysis to the less toxic 4-nitrophenol and diethylphosphate [17]. The results indicated that although the phosphotriesterase-loaded erythrocytes were more effective than the classic antidotal combination of atropine and pralidoxime, a combination of the loaded erythrocytes with the classic antidot, provided a 1000-fold protection against paraoxon. The same group also investigated the application of erythrocyte encapsulated recombinant paraoxonase as an approach to directly hydrolyze paraoxon; treated mice showed no signs of intoxication at paraoxon dose levels that were lethal when using the classical antidotal combination of atropine and pralidoxime. Moreover, erythrocyte encapsulated paraoxonase, in combination with the classic antidotal combination, provided the highest antidotal efficacy ever reported against any chemical toxicant [18].
Another category of detoxifying enzymes that have been investigated are those associated with the metabolism of ethanol and methanol. Ethanol detoxification requires two enzymic reactions: the oxidation of ethanol to acetaldehyde by alcohol dehydrogenase (EC. 1.1.1.1), followed by the oxidation acetaldehyde to acetate by aldehyde dehydrogenase (EC 1.2.1.5). Chronic alcohol consumption decreases acetaldehyde oxidation, either due to decreased aldehyde dehydrogenase activity or impaired mitochondrial function. The application of the erythrocyte carrier as an alcohol detoxifier was first proposed by Magnani et al. They demonstrated that mice administered acetaldehyde dehydrogenase-loaded erythrocytes intraperitoneally had 35% less blood acetaldehyde compared to controls, one hour after receiving an acute dose of ethanol [19]. Lizano et al. investigated the co-encapsulation of alcohol dehydrogenase and aldehyde dehydrogenase in human erythrocytes, demonstrating their superior ability to metabolize ethanol in vitro, compared to erythrocytes loaded with alcohol dehydrogenase alone [20]. In vivo studies in mice receiving acute doses of ethanol and treated with co-encapsulated enzymes showed blood ethanol to be eliminated at rates of 1.7 mmol/L to 4 mmol/L loaded erythrocytes/hour [21][22]. However, these rates of plasma ethanol clearance were lower by an order of magnitude than those expected from the activities of encapsulated enzymes. By employing a mathematical modeling approach and then conducting a subsequent in vitro study, Alexandrovich et al. were able to theorize and then demonstrate the rate limiting step of external ethanol oxidation. They found this was due to the rate of nicotinamide–adenine dinucleotide (NAD+) generation in erythrocyte glycolysis, rather than the activities of the loaded enzymes. By supplementing the erythrocytes with NAD+ and pyruvate they were able to demonstrate an elimination of 17 mmol ethanol/L loaded erythrocytes/hour [23].
In mammalian species, methanol is metabolized to formaldehyde via alcohol dehydrogenase, followed by the conversion of formaldehyde into formic acid via aldehyde dehydrogenase. Formic acid metabolism is mediated through a tetrahydrofolate-dependent pathway by folate-dependent enzymes. Humans have 60% less liver folate concentrations compared to mice and rats, and for this reason humans are more sensitive to methanol poisoning [24]. Specifically, formic acid inhibits mitochondrial cytochrome c oxidase, leading to cellular hypoxia and metabolic acidosis. Magnani et al. investigated the application of erythrocyte-encapsulated methylotrophic yeast alcohol oxidase (EC 1.1.3.13) as an approach to the detoxification of methanol. In vivo studies showed that two hours following an acute dose of methanol (0.7 g/kg), mice that had received enzyme-loaded erythrocytes had 50% less blood methanol compared to controls, with antagonism persisting for at least one week [25]. On the basis that formate dehydrogenase (EC 1.2.1.2) converts formate into CO2 in the presence of NAD, Muthuvel et al. administered formate dehydrogenase-loaded erythrocytes to methanol-intoxicated folate-deficient rats, which were pre-treated with carbicarb to correct the metabolic acidosis, and demonstrated a marked elimination of formate [26].
A detoxifying enzyme that has been investigated in relation to lead poisoning is δ-aminolevulinic acid dehydratase (E.C. 4.2.1.24). Lead is a potent inhibitor of δ-aminolevulinic acid dehydratase through its displacement of zinc from the enzyme’s active site. The resulting inactivation leads to an accumulation of its substrate, aminolevulinic acid, and this has been shown to have a neuropathogenic effect [27]. The in vitro and in vivo mouse studies of Bustos et al. showed that it was possible to correct the defective δ-aminolevulinic acid dehydratase activity in erythrocytes by encapsulating exogenous human enzyme, thus providing a rationale for this approach for treating lead intoxication [28][29]. In the first clinical application, 100 mL autologous erythrocytes loaded with 25,600 units of human δ-aminolevulinic acid dehydratase were administered to a single patient with a history of chronic lead intoxication, producing a reduction in blood lead concentration and decrease in urinary porphyrin excretion, which was sustained for a period of four years [30].
Biochemical decompression has been proposed as a method for reducing the amount of time required for deep-sea divers to return to the surface. Divers breathing H2/O2 mixtures would be administered hydrogenase (EC 1.12.1.4), thus accelerating decompression through the removal of excess hydrogen from the tissues. The application of erythrocyte encapsulated hydrogenase as an approach to converting dissolved hydrogen gas into non-gaseous forms was investigated by Axley et al. Hydrogenase activities of 7-µmol H2/minute/mL packed human erythrocytes were successfully encapsulated, however the cells were not able to consume hydrogen due to the enzyme’s dependence on NAD for activity. The co-encapsulation of FAD with hydrogenase was shown to overcome this [31].
3.2. Thrombolytic Therapy
The application of erythrocytes as a strategy for thrombolytic therapy has been explored by several groups. Thrombolytics (or fibrinolytics), also known as plasminogen activators, facilitate the removal of pre-existing thrombotic clots through degrading the fibrin meshwork into plasmin. However, their application is limited by their rapid clearance and inactivation by plasminogen activator inhibitor-1, the risk of bleeding caused by dissolution of hemostatic clots and filtration into extravascular tissues, such as the central nervous system, causing toxicity [32]. The rationale for the erythrocyte encapsulation of fibrinolytics was based on addressing these issues with the study of Delahousse et al. who successfully demonstrated the encapsulation of the fibrinolytic enzymes urokinase (EC 3.4.21.31), streptokinase (EC 3.4.99.0) and recombinant tissue plasminogen activator (tPA; EC 3.4.21.68) in human erythrocytes. However, in vitro stability studies using human erythrocytes and in vivo studies in the mouse were not able to support the objective of a sustained half-life and demonstrated a rapid destruction of the encapsulated enzymes [33]. Flynn and coworkers reported that Aspergillus oryzae brinase (EC. 3.4.99) encapsulated into rabbit erythrocytes was able to lyse clotted blood in vitro [34]. The coupling of fibrinolytics to the surface of the erythrocyte carrier was proposed as a preferable alternative to encapsulation [35]. Murciano et al. investigated the conjugation of tPA to erythrocytes as a strategy for thromboprophylaxis, by employing mouse and rat models of venous and arterial thrombosis. Following intravenous injection, the fibrinolytic activity of tPA conjugated to erythrocytes was shown to persist in the circulation at least tenfold longer than that of free tPA. Free tPA was able to lyse pulmonary clots that had lodged before administration, but not those that lodged after injection. Erythrocyte conjugated-tPA, however was more selective in lysing nascent over preexisting pulmonary emboli and arterial clots, an effect that is most likely a result of restricting the diffusion of tPA into fibrin [36]. The utility of this approach in preventing cerebrovascular thrombosis was also examined using mouse and rat models of cerebrovascular thromboembolism and ischemia, with the results indicating that erythrocyte-conjugated tPA was an effective thromboprophylaxis, whereas free tPA was ineffective up to a 10-fold higher dose [37].
3.3. Enzyme Replacement for Metabolic Diseases
The application of the erythrocyte as a carrier of enzymes for the treatment of inherited metabolic diseases and other metabolic disturbances has generated a large amount of interest from those working in a field where there are enormous unmet needs. With the exception of phenylketonuria, these therapeutic applications have focused on the use of encapsulated enzyme.
3.4 Antitumour Therapy
The implementation of the erythrocyte carrier in antitumor therapy has focused on the use of encapsulated enzymes and represents one of the most clinically advanced applications is for the treatment of acute lymphoblastic leukemia (ALL). This strategy is based on tumour cells not being able to synthesize adequate amounts of amino acids and therefore depend on extracellular sources. The administration of specific enzymes enables the depletion of plasma amino acids, thus starving the tumour cells of amino acids necessary for DNA, RNA and protein synthesis and leading ultimately to cell death.
The implementation of the erythrocyte carrier in antitumor therapy has focused on the use of encapsulated enzymes and represents one of the most clinically advanced applications is for the treatment of acute lymphoblastic leukemia (ALL). This strategy is based on tumour cells not being able to synthesize adequate amounts of amino acids and therefore depend on extracellular sources. The administration of specific enzymes enables the depletion of plasma amino acids, thus starving the tumour cells of amino acids necessary for DNA, RNA and protein synthesis and leading ultimately to cell death.