Carbon nanodots are fluorescent, quasi-spherical nanoparticles that have been investigated and modified for numerous applications. In recent years, the utility of these structures for antimicrobial photodynamic therapy (APDT) has been of increasing interest. For this technology, light is used to trigger the generation of reactive oxygen species which subsequently inactivate or kill pathogenic microorganisms. Carbon nanodots are of interest for this application due to their simple, "green" synthesis methods and their tunable organic structures and luminescent properties. Herein we share some recent developments in the field of antimicrobial carbon nanodots, highlighting their increasing relevance and potential in this area.
- carbon dots
- carbon nanodots
- carbon quantum dots
- photosensitization
- antimicrobial photodynamic therapy
- antibacterial
- photocatalytic disinfection
- antimicrobial materials
- carbon nanomaterials
- reactive oxygen species
1. Introduction
Although the threat of antibiotic resistance development in pathogenic bacteria is not a new challenge, it remains to date a pressing concern in regard to global health. Antibiotics have been a primary tool for combating infectious diseases, yet the timescale of development for next-generation antibiotics remains lengthy due to the complexities and cost of not only drug discovery, but also clinical testing and ultimately approval. Additionally, bacteria have a multitude of mechanisms by which they can rapidly acquire resistance,[1][2] which is of particular concern for high-risk populations such as those in healthcare settings.[3][4] For those with concurrent conditions, such as cancer, bacterial infections can severely worsen patient health;[5][6] infections may also delay wound recovery even when treated, with some negative health impacts associated with current disinfection techniques.[7] These cumulative effects place a premium on developing techniques to mitigate the threat of infection from deadly pathogens, namely by improved sterilization technologies. A selection of current disinfection strategies include spray disinfectants containing harsh chemicals, ultra-violet radiation that is deleterious to human health, and antimicrobial nanotechnology—for example silver nanoparticles—which have an inherent antimicrobial character that is not controllable except by dose/exposure.
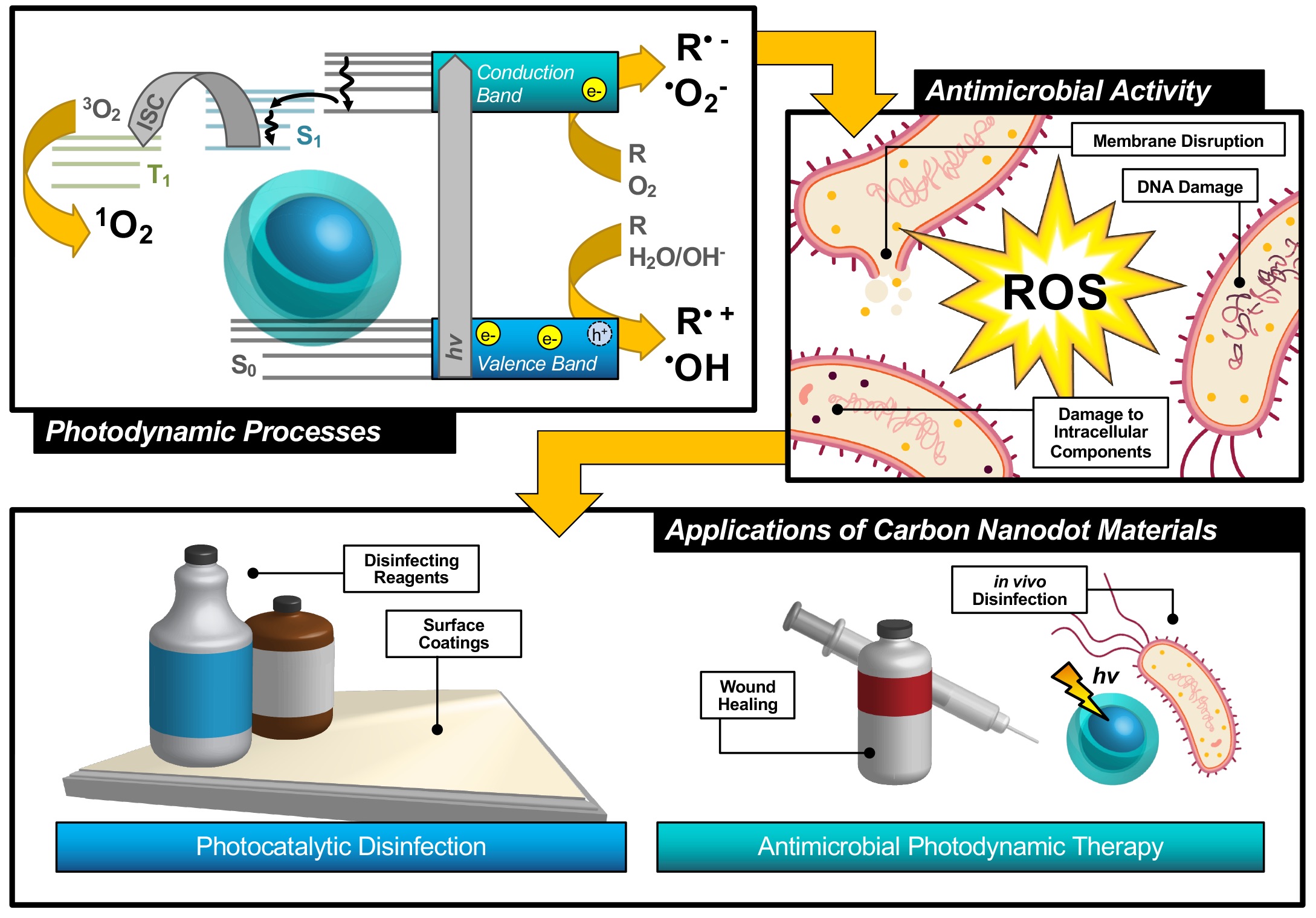
An alternative technique known as antimicrobial photodynamic therapy (APDT)[8][9][10]—or for variations in application: antimicrobial photodynamic inactivation (APDI)[11] of bacteria or photocatalytic disinfection[12]—presents an intriguing option for a different class of disinfection materials. This process is based in the photodynamic response of a photosensitizing agent, as shown in the first step illustrated in Figure 1. As shown by the simplified Jablonski diagram, incident light induces an excitation event in the photosensitizer (S0 → S1); from this stage, the excited electron may undergo a spin flip in a process known as intersystem crossing (ISC, S1 → T1) to form a triplet excited state. Occupation of this state permits triplet-triplet interactions with ground-state molecular oxygen; energy transfer, in this case, produces highly reactive singlet oxygen species (ROS), for example, singlet oxygen (1O2), as a result of a ‘Type II’ photosensitization mechanism.[13][14]
Figure 1.
Schematic description of antimicrobial photodynamic therapy and photocatalytic disinfection using carbon nanodots. Reproduced from reference .
Photosensitizers such as carbon nanodots (CNDs), the subject of the review, are known to exhibit fluorescence due to the generation of electron/hole pairs (e−/h+), also shown in the first step of Figure 1. Either of these (e− or h+) can undergo electron transfer, known as Type I photosensitization, with different proximal species such as water, molecular oxygen, or organic agents in solution to produce radical species, including hydroxyl radical (•OH) and superoxide anion radical (O2•−) to name a few.[14] Both Type I and II mechanisms result in highly reactive ROS and are known to inflict severe oxidative damage on microbial cells as illustrated in Figure 1.[15] Destruction may occur at the membrane causing morphological changes or cytoplasmic leakage; additionally, diffusing ROS can cause DNA impairment or damage to other intracellular components, such as ribosomes. Oxidative damage from ROS has been of increasing interest in the context of antibiotic-resistant bacteria, as it proves more complex for bacteria to develop resistance to this method.[16][17] Additionally, there is no known enzyme to detoxify •OH and 1O2.[18] Furthermore, studies have shown that APDT may be tuned to permit high spatial control, thereby reducing deleterious impacts on nearby mammalian cells during treatment.[17][19] Alternatives such as photothermal therapies have promise but do not share the spatial resolution possible with APDT.[20] Accordingly, an emphasis has been placed on optimizing the properties of photosensitizing molecules for antimicrobial applications, particularly in tuning the photophysical properties of those agents[21] for maximized ROS quantum yields.[22][23] By optimizing these characteristics, it is possible to improve the efficiency of light utilization by the photosensitizer, and even to select for activation wavelengths that are themselves not inherently harmful, unlike short-wavelength ultraviolet light (UV-C).[24] In contrast, TiO2 materials, which are commonly used for photocatalytic disinfection applications, suffer from low utilization of solar energy[22][25] and require UV activation.[26][27] Beyond quantum efficiency, it is also desirable to tune the proximity or adherence of the photosensitizer to bacterial cells.[21] This is of particular importance since ROS are by nature highly reactive; to prevent off-target interactions, the species must be generated locally to the cell of interest. Beyond these properties, an ideal APDT photosensitizer would also have a dynamic scaffold structure that would permit facile adaptation for multi-modal purposes. This could include modification for varied delivery platforms, changing surface structures to achieve solubility in different disinfectant reagents, or even covalent attachment for antimicrobial surface coating materials, as in photocatalytic disinfection (Figure 1). Dynamic structures could also improve theranostic applications, permitting simultaneous imaging/detection in the context of wound healing or in vivo disinfection therapies (Figure 1). Beyond medical therapies, applications could be found in food safety, water disinfection, and solar-driven disinfection.[3][25][28] Ultimately, an inexpensive and sustainable photosensitizer would be optimal for a broad-use, up-scaled commercial APDT photosensitizer.
Although numerous small molecule photosensitizers have been developed for APDT, these are limited in commercial scalability due to complex syntheses, high cost, and purification requirements. Recently, carbon nanodots have received increasing attention for use as a nanoparticulate photosensitizer for APDT and photocatalytic disinfection. These fluorescent nanoparticles are known to be a combustion byproduct and/or the product of incomplete carbonization from biomass, resulting in quasi-spherical, fluorescent particles less than 10 nm in size with broad-spectrum absorption profiles. Due to their synthetic sources, these particles present a potentially inexpensive and ‘green’ option for a scalable photocatalytic disinfection material. Other recent reviews have focused on carbon nanodots in general as antimicrobial agents;[29][30][31][32] herein, we present and discuss recent developments surrounding the application of carbon nanodots in antimicrobial photodynamic therapy.
2. Carbon Nanodots as Photodynamic Antimicrobial Agents
“Other carbon-based structures, for example fullerenes or graphene quantum dots, have been known for some time to generate reactive oxygen species and have found applications in anti-cancer photodynamic therapies.[33][34] Carbon nanodots, also referred to as ‘carbon dots’ or ‘carbon quantum dots,’ differ from these in both their chemical structure and fluorescent properties. Regarding the latter, carbon dots in general are composed of a core consisting of many-layered sheets of oxidized graphene.[24][35] Each sheet varies in the extent of its ring system and pi conjugation, resulting in a quasi-spherical morphology after these sheets have assembled.[36] An additional passivation layer of organic nature is frequently functionalized to the graphitic core to achieve desirable properties; these may include reduced particle aggregation, optimized solubility, or an improved quantum yield to name a few. In total, this structure is on the order of less than 10 nm in size. Typical absorption spectra inherent to carbon dot structures demonstrate strong UV absorption with a visible absorption tail; studies have been conducted to further extend the absorption of carbon dots into the visible or even NIR region.[37][38][39] Carbon dots are also known to be capable of up-conversion photoluminescence [27,40].[27][40] Beyond synthetic optimization of excitation/emission properties, carbon dot samples also uniquely have an excitation-dependent emission profile. This allows, to a degree, luminescence tuning simply by adjusting the excitation source used in an experiment; however, varying carbon dot quantum efficiencies at each wavelength must be carefully considered. Studies have indicated exciton or electron/hole (e−/h+) recombination, surface trap states, and quantum confinement effects to all contribute to carbon dot fluorescent properties.[41][42][43] Studies have also demonstrated that excitation of the carbon dot core, rather than surface states, results in different observed photodynamic properties.[44]”
With these luminescent properties, and the basis of other carbon nanostructures as effective APDT agents, researchers have begun focusing attention on the ROS-generating capabilities of carbon nanodots specifically. Recent studies have reported general ROS from excited carbon dots,[3][16][19][25][28][45] with more specific detection reporting both Type I[12][25][45][46] and Type II[17][25][36][45][46] photosensitization products. Studies by us, Zhao et al. and Zhang et al., for example, have detected both superoxide anion radical[25][45] and hydroxyl radical.[25][46] Detection of Type I products, conducted by Sidhu et al., has even occurred in hypoxic environments;[19] it is likely that interaction between water and h+ generated upon excitation is able to generate •OH. Type II products, namely singlet oxygen, have also been detected from excited carbon nanodot structures. Stankovic et al., for example, report a singlet oxygen quantum yield of 0.31; further, the authors discuss how carbon nanodots should not effectively quench singlet oxygen based on reported lifetimes.[36] Detection of both types of photosensitization products has been conducted via a number of experimental methods, including fluorescent/colorimetric probes (conducted either in situ[3][16][25][47][48] or for cell-less, solution-based systems[46][19][5][25][17][45][49]), direct detection from 1O2 luminescence,[36][45] electron paramagnetic resonance (EPR),[36][12][25][45] and scavenging reagents employed with a number of these, and other, methods.[12][25][45]
By extension, many studies reporting the generation of ROS from their carbon nanodot structures have additionally investigated the antibacterial effects from these activated structures as well. In fact, one report by Zhang et al. demonstrated comparable and even superior antibacterial efficiencies of their carbon dot structures relative to even small-molecule photosensitizers.[45] Other studies have reported antibacterial efficiency from photo-activated carbon nanodots, using strains such as Gram-negative Escherichia coli,[46][28][25][60][19][17] Salmonella,[45] and P. aeruginosa,[36] and Gram-positive Staphylococcus aureus,[46][22][50][19][3] Bacillus subtilis,[24][26] and Listeria monocytogenes.[46][50] Resistant strains have also been studied, including multi-drug resistant E. coli[19] and methicillin-resistant S. aureus (MRSA).[19] Although many reports of carbon nanodot photosensitizers detail no significant dark toxicity effects,[3][16][17][19][22][45][46][50] this is not always the case and in fact some studies do note toxicity with no incident excitation.[3][25][26][28][36][46] Dark toxicity effects have been discussed in a review by Anand et al.,[31] with consequences such as cell wall disruption[51][52][53][54] and DNA/RNA damage[51][55][56] being reported from carbon dots. This disparity in results remains to be carefully characterized by researchers in the field, classifying features of specific carbon dot structures influencing both dark and light-activated toxicity mechanisms. It is interesting to note also that disparities arise across studies even for similar strains of bacteria. Positive results have been reported for carbon dot APDT for both E. coli and Salmonella, yet different authors have also noted no significant APDT effects for these same strains.[45][50] The factors influencing these differences are currently under investigation in the field, such that specificity and selectivity may be obtained with future developments.[50][57][58]
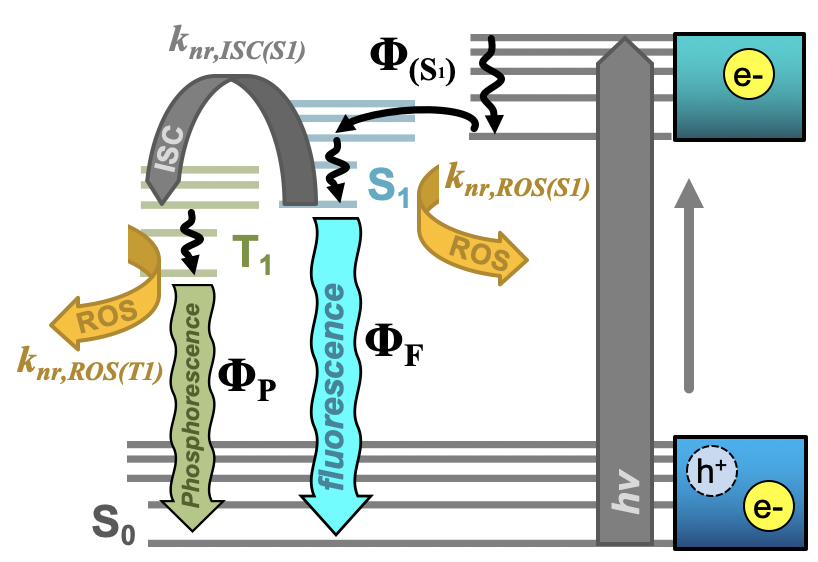
Further, researchers have begun to evaluate the tunable properties of carbon nanodots to achieve even greater photodynamic antimicrobial responses. Key in this approach is the adjustment of quantum yields to achieve higher overall ROS yields. Both fluorescence and phosphorescence quantum yields are important in this, as excitation in general permits Type I mechanisms and formation of triplet excited states further supports the generation of singlet oxygen (Type II). Both of these pathways are described in the modified Jablonski diagram shown in Figure 2. It is important to note here, however, that direct correlations between luminescence versus ROS quantum yields are difficult for carbon dots, as these are complex photophysical structures whereby excitation may initially form non-emissive states. Relaxation from these to emissive states (for example, S1 in Figure 2), results in fluorescence emission after decay to S0 (S1 → S0); therefore, both the efficiency of S1 formation (ΦS1) and efficiency of radiative decay from S1 both contribute to the overall fluorescence quantum yield. It would be intuitive to state that decreased fluorescence quantum yield should correlate to a heightened ROS quantum yield, as the excited state forms ROS rather than radiatively decaying. A study by Al Awak et al. reports just the opposite, where higher fluorescence quantum yield carbon dots instead also yield more ROS.[24] The authors suggest that both observations could be a consequence of improved efficiency in forming the emissive state following excitation, rather than an improvement in radiative efficiency from that state itself. Another study by Jijie et al. similarly saw a direct correlation between fluorescence quantum yield and ROS formation.[17] Tuning of phosphorescence quantum yields similarly has provided researchers a method through which to tune APDT from carbon dots. In this case, however, phosphorescence is largely observed through the minimization of its interaction with oxygen—that is, APDT agents are either fixed in a matrix[45] or suspended in a viscous solution.[59] Deactivation by ROS then is reduced, and one can expect higher ROS generation in ‘diffusion-allowed’ media (i.e. no matrix, etc.) to correspond to higher phosphorescence quantum yields in the ‘diffusion-limited’ system. This is in fact reported both by Zhang et al[45] and us[46] using different methods to achieve phosphorescent carbon dot structures.
Figure 2.
Simplified Jablonski diagram for carbon nanodots depicting initial e-/h+ formation upon excitation, conversion to an emissive S
1
state (Φ
(S1)
) and the various relaxation pathways available from this point. From the S
1
state, radiative relaxation as fluorescence (Φ
F
), non-radiative (k
nr
) decay by intersystem crossing (ISC) to the triplet emissive state (T
1
), or non-radiative decay by reactive oxygen species (ROS) generation are detailed. From the T
1
state, radiative relaxation as phosphorescence (Φ
P
) or non-radiative decay by ROS formation are detailed. “Φ” in all cases refers to quantum yield. Adapted from reference .
Interestingly, the fluorescence quantum yield effect is not the only factor influencing photodynamic efficiency of antimicrobial effects from carbon dots. A study by Abu Rabe et al demonstrated that the quantum yield effect could be dominated instead by surface charge effects. The positively charged, low quantum yield particles in fact exhibited higher antibacterial efficiency than the high yield, neutrally charged analogs.[21] In the same report, the authors noted that for structures displaying different charges but comparable sizes/quantum yields, positively charged structures were more APDT active; the efficacy of the positively-charged particles was further increased when shorter surface passivating agents were used, possibly due to decreased ROS diffusional distances.[21] The effects of surface charge, however, require further investigation to clarify. Reports by others have also demonstrated APDT effects from carbon dots carrying negative surface charges,[17][50][46][60] and many studies do not characterize charge at all. Yet for all of these mentioned factors influencing APDT efficiency, the characteristics may be tuned by careful selection of carbon dot synthesis precursors or reagents. “In the previously noted Zhang et al. study, the authors generated their phosphorescent structures by altering the amount of ethylenediamine precursor in synthesis to tune final nitrogen concentration;[45] in our work, a bromine component was added as a precursor.[46][59] In fact, precursor tuning has been a mainstay strategy in carbon dot literature to achieve new and varied properties for a multitude of applications, and APDT is no exception. In the vein of phosphorescence discussion, Mandal et al. selected an anthraquinone derivative known as Anthrarufin for their carbon dot precursor specifically due to its known triplet character.[3] Beyond photophysical tuning, precursor selection has prioritized retaining antimicrobial properties[50][61][62] or even cell targeting capabilities[19] by using antibacterial small molecule drugs as precursors. In a general sense, precursors may also be selected simply to improve the stability of and reduce defects in the resulting carbon dots after carbonization, for example in the study by Kavitha et al. which used date palm fronds (featuring a high lignin content) as a biomass precursor.[60] Although many studies cite the rationale for precursor selection, comprehensive and methodical examinations of precursor variations and their downstream influence on carbon dot properties are limited. Nonetheless, this technique will likely receive expanded attention in regard to the development of new photodynamic antimicrobial carbon dots.”
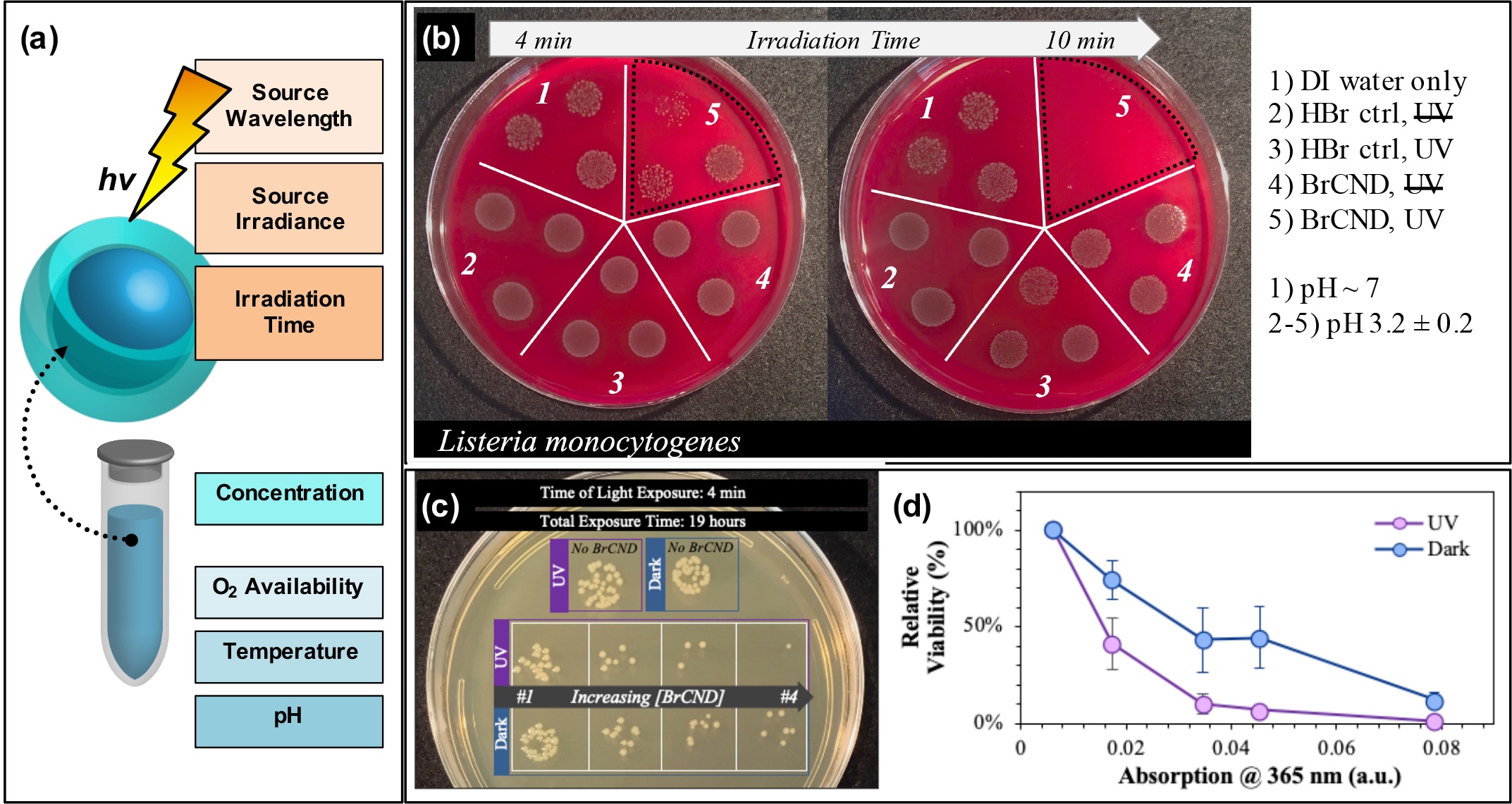
In addition, researchers can tune the APDT efficiency from their structures via conventional PDT techniques (Figure 3a), including but not limited to excitation wavelength,[24][46] irradiance,[17] irradiation time (example in Figure 3b)[17][19][24][25][26][28][45][46], photosensitizer concentration (example in Figure 3c/d),[17][19][24][25][26][28][46] oxygen availability,[46] reaction temperature,[45] and even solution pH.[59]
Figure 3.
Parameters for tuning carbon nanodot photodynamic antimicrobial effects. (a) Schematic displaying some of the experimental parameters that must be adjusted to achieve maximal photodynamic antimicrobial effects from carbon nanodots. (b) Sample data set demonstrating the impact of irradiation time on the colony growth for Listeria monocytogenes exposed at 365 nm with brominated carbon nanodot (BrCND) photosensitizers. (c,d) Viability of Escherichia coli (c) grown on plates and (d) plotted as a function of photosensitizer concentration (plotted here by the BrCND sample absorption at 365 nm). Panels (b–d) adapted from Reference
[46]. For additional details regarding the reported studies, the reader is referred to Reference . Reproduced from reference .
. For additional details regarding the reported studies, the reader is referred to Reference . Reproduced from reference .
Even as researchers strive to understand the chemical, molecular, and photophysical factors contributing to carbon dot efficiency as APDT photosensitizers, still others have begun to implement these in hybrid systems and/or materials. Carbon dot photosensitizers have, for example, been co-administered with small-molecule photosensitizers,[16] oxidizing agents,[63] and antibiotics[3][17][19] for synergistic antimicrobial activity. Recent research by us has also shown that ROS yields may be improved by combination of carbon dot structures in tandem with plasmonic metal nanoparticle systems (i.e. nano-silver coated films).[49] “This amplification platform has its mechanistic basis in metal-enhanced fluorescence (MEF), whereby a plasmonic metal substrate can act as a “nanoantennae” for excitation light; the resulting field generated by the substrate may then excite larger populations of fluorophores in the near-field than would be possible under classical conditions. This effectively expands the absorption cross-section well beyond what would be possible for the fluorophore alone and is known as the enhanced absorption mechanism. MEF may also occur as a result of enhanced emission pathways, where fluorophore quanta couple to the nanoparticle plasmons such that the whole entity radiates as a unit.[64] MEF, and even metal-enhanced phosphorescence (MEP), from carbon dots on silvered substrates have been previously reported by our lab.[65][66] However, the enhancement of radiative emission could actually run counter to the metal enhancement of ROS (ME-ROS), as radiative decay versus ROS generation are competitive decay pathways for excited carbon dots. Nonetheless, ME-ROS has been detected for small molecule photosensitizers, potentially via enhanced absorption, yielding ME1O2[67][68] and MEO2•−,[67][69]” similar to what is reported for carbon dots.[49]” Other materials capitalizing on photophysical interactions have been developed, where carbon dots are used as Forster Resonance Energy Transfer (FRET) donors to improve material short-wavelength absorption as in a study by Kumari et al.,[48] or as agents for up-conversion luminescence whereby longer wavelengths may be used to activate a previously UV-excited material as was demonstrated for zinc oxide-coated brackets by Zhang et al.[40] Carbon dots were also used to improve light absorption by Zhang et al.[12] and Hazarika et al.[27] for different hybrid materials.
3. Conclusion
Herein we have provided a discussion of the current literature for carbon nanodots as photosensitizers for antimicrobial photodynamic therapies and photocatalytic disinfection. Although still an evolving field, the publications reviewed herein set the foundation for future growth and optimization of carbon nanodots with intrinsic photodynamic antibacterial capabilities. By tuning properties such as fluorescence or phosphorescence quantum yield, precursors and passivation agents, surface charge and more, still more effective antibacterial and antibiofouling materials may yet be achieved using carbon nanodots as a scaffold. Furthermore, hybrid materials incorporating carbon dots both with or without intrinsic antibacterial activity show immense promise in the development and optimization of new, inexpensive, and potentially even ‘green’ materials. Still, we propose deeper characterization and methodical examination of trends for structural/chemical tuning, selectivity and activity against strains of differing Gram stains, carbon dot photosensitization-induced cytotoxicity, characterization of carbon dots themselves,” which are thoroughly discussed in the full review . “In all, the research reviewed herein provides a solid foundation for the future pursuit, characterization, and development of these carbon nanodot materials for APDT and photocatalytic disinfection applications.
References
- Silvia Buroni; Simona Pollini; Gian Maria Rossolini; Elena Perrin; Editorial: Evolution of Genetic Mechanisms of Antibiotic Resistance. Frontiers in Genetics 2019, 10, 3, 10.3389/fgene.2019.00983.
- José M Munita S; Cesar A Arias; Mechanisms of Antibiotic Resistance. Microbiology Spectrum 2016, 4, 481-511, 10.1128/microbiolspec.vmbf-0016-2015.
- Saptarshi Mandal; Surendra Rajit Prasad; Debabrata Mandal; Prolay Das; Bovine Serum Albumin Amplified Reactive Oxygen Species Generation from Anthrarufin-Derived Carbon Dot and Concomitant Nanoassembly for Combination Antibiotic–Photodynamic Therapy Application. ACS Applied Materials & Interfaces 2019, 11, 33273-33284, 10.1021/acsami.9b12455.
- E. Sib; A.M. Voigt; G. Wilbring; C. Schreiber; H.A. Faerber; D. Skutlarek; M. Parcina; R. Mahn; D. Wolf; P. Brossart; et al.F. GeiserS. EngelhartM. ExnerG. BierbaumRicarda Maria Schmithausen Antibiotic resistant bacteria and resistance genes in biofilms in clinical wastewater networks. International Journal of Hygiene and Environmental Health 2019, 222, 655-662, 10.1016/j.ijheh.2019.03.006.
- Yadan Liu; Xiaolin Liu; Yue Xiao; Fangman Chen; Fangnan Xiao; A multifunctional nanoplatform based on mesoporous silica nanoparticles for imaging-guided chemo/photodynamic synergetic therapy. RSC Advances 2016, 7, 31133-31141, 10.1039/C7RA04549B.
- S.P. Russell; C. Neary; S. Abd Elwahab; J. Powell; N. O'connell; L. Power; S. Tormey; B.A. Merrigan; A.J. Lowery; Breast infections – Microbiology and treatment in an era of antibiotic resistance. The Surgeon 2020, 18, 1-7, 10.1016/j.surge.2019.03.008.
- N. Sattarahmady; M. Rezaie-Yazdi; G.H. Tondro; N. Akbari; Bactericidal laser ablation of carbon dots: An in vitro study on wild-type and antibiotic-resistant Staphylococcus aureus. Journal of Photochemistry and Photobiology B: Biology 2016, 166, 323-332, 10.1016/j.jphotobiol.2016.12.006.
- Deema Al-Shammery; Dimitrios Michelogiannakis; Zain Uddin Ahmed; Hameeda Bashir Ahmed; P. Emile Rossouw; Georgios E. Romanos; Fawad Javed; Scope of antimicrobial photodynamic therapy in Orthodontics and related research: A review. Photodiagnosis and Photodynamic Therapy 2019, 25, 456-459, 10.1016/j.pdpdt.2019.02.011.
- Maximiliano L. Agazzi; M. Belén Ballatore; Andrés M. Durantini; Edgardo N. Durantini; Augusto C. Tomé; BODIPYs in antitumoral and antimicrobial photodynamic therapy: An integrating review. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2019, 40, 21-48, 10.1016/j.jphotochemrev.2019.04.001.
- Rebeca Boltes Cecatto; Laís Siqueira De Magalhães; Maria Fernanda Setúbal Destro Rodrigues; Christiane Pavani; Adriana Lino-Dos-Santos-Franco; Mariana Teixeira Gomes; Daniela Fátima Teixeira Silva; Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagnosis and Photodynamic Therapy 2020, 31, 101828, 10.1016/j.pdpdt.2020.101828.
- Tim Maisch; Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. Journal of Photochemistry and Photobiology B: Biology 2015, 150, 2-10, 10.1016/j.jphotobiol.2015.05.010.
- Jingtao Zhang; Xing Liu; Xueying Wang; Lilong Mu; Mingming Yuan; Bingkun Liu; Hengzhen Shi; Carbon dots-decorated Na2W4O13 composite with WO3 for highly efficient photocatalytic antibacterial activity. Journal of Hazardous Materials 2018, 359, 1-8, 10.1016/j.jhazmat.2018.06.072.
- Kanofsky, J.R.. Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 93-103.
- Maurício S. Baptista; Jean Cadet; Paolo Di Mascio; Ashwini A. Ghogare; Alexander Greer; Michael R. Hamblin; Carolina Lorente; Silvia Cristina Nunez; Martha S. Ribeiro; Andrés H. Thomas; et al.Mariana VignoniTania Mateus Yoshimura Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochemistry and Photobiology 2017, 93, 912-919, 10.1111/php.12716.
- Magaraggia, M.; Jori, G.. Advances in Photodynamic Therapy: Basic, Translational, and Clinical; Mróz, P., Hamblin, M.R., Eds.; Artech House: Boston, MA, USA, 2008; pp. 337-357.
- Xiuli Dong; Ambrose E Bond; Nengyu Pan; Montrez Coleman; Yongan Tang; Ya-Ping Sun; Liju Yang; Synergistic photoactivated antimicrobial effects of carbon dots combined with dye photosensitizers. International Journal of Nanomedicine 2018, 13, 8025-8035, 10.2147/IJN.S183086.
- Roxana Jijie; Alexandre Barras; Julie Bouckaert; Nicoleta Dumitrascu; Sabine Szunerits; Rabah Boukherroub; Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloids and Surfaces B: Biointerfaces 2018, 170, 347-354, 10.1016/j.colsurfb.2018.06.040.
- Mans Broekgaarden; Ruud Weijer; Thomas M. Van Gulik; Michael R. Hamblin; Michal Heger; Tumor cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategies.. Cancer and Metastasis Reviews 2015, 34, 643-90, 10.1007/s10555-015-9588-7.
- Jagpreet Singh Sidhu; Mayank; T. Pandiyan; Navneet Kaur; Harpreet Singh; The Photochemical Degradation of Bacterial Cell Wall Using Penicillin-Based Carbon Dots: Weapons Against Multi-Drug Resistant (MDR) Strains. ChemistrySelect 2017, 2, 9277-9283, 10.1002/slct.201701810.
- Ifat Nissan; Vijay Bhooshan Kumar; Ze’Ev Porat; Darko Makovec; Orit Shefi; Aharon Gedanken; Sonochemically-fabricated Ga@C-dots@Ga nanoparticle-aided neural growth.. Journal of Materials Chemistry B 2017, 5, 1371-1379, 10.1039/c6tb02508k.
- Dina I. Abu Rabe; Mohamad M. Al Awak; Fan Yang; Peter A. Okonjo; Xiuli Dong; Lindsay R. Teisl; Ping Wang; Yongan Tang; Nengyu Pan; Ya-Ping Sun; et al.Liju Yang The dominant role of surface functionalization in carbon dots' photo-activated antibacterial activity.. International Journal of Nanomedicine 2019, 14, 2655-2665, 10.2147/IJN.S200493.
- Anupma Thakur; Pooja Devi; Shefali Saini; Rishabh Jain; Ravindra Kumar Sinha; Praveen Kumar; Citrus limetta Organic Waste Recycled Carbon Nanolights: Photoelectro Catalytic, Sensing, and Biomedical Applications. ACS Sustainable Chemistry & Engineering 2018, 7, 502-512, 10.1021/acssuschemeng.8b04025.
- Ruth Prieto-Montero; Alejandro Prieto-Castañeda; Rebeca Sola-Llano; Antonia R. Agarrabeitia; David García-Fresnadillo; Íñigo López‐Arbeloa; Angeles Villanueva; Maria J. Ortiz; Santiago De La Moya; Virginia Martinez-Martinez; et al. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochemistry and Photobiology 2020, 96, 458-477, 10.1111/php.13232.
- Mohamad M. Al Awak; Ping Wang; Shengyuan Wang; Yongan Tang; Ya-Ping Sun; Liju Yang; Correlation of Carbon Dots' Light-Activated Antimicrobial Activities and Fluorescence Quantum Yield. RSC Advances 2017, 7, 30177-30184, 10.1039/C7RA05397E.
- Fangdong Zhao; Wei Gu; Jian Zhou; Qiang Liu; Yu Chong; Solar-excited graphene quantum dots for bacterial inactivation via generation of reactive oxygen species. Journal of Environmental Science and Health, Part C 2019, 37, 67-80, 10.1080/10590501.2019.1591701.
- Mohammed Jaouad Meziani; Xiuli Dong; Lu Zhu; Les P. Jones; Gregory Ethan LeCroy; Fan Yang; Shengyuan Wang; Ping Wang; Yiping Zhao; Liju Yang; et al.Ralph A. TrippYa-Ping Sun Visible-Light-Activated Bactericidal Functions of Carbon “Quantum” Dots. ACS Applied Materials & Interfaces 2016, 8, 10761-10766, 10.1021/acsami.6b01765.
- Deepshikha Hazarika; Devabrata Saikia; Kuldeep Gupta; Manabendra Mandal; Niranjan Karak; Photoluminescence, Self cleaning and Photocatalytic Behavior of Waterborne Hyperbranched Polyester/Carbon dot@TiO2 Nanocomposite. ChemistrySelect 2018, 3, 6126-6135, 10.1002/slct.201801160.
- Zhe Gao; Chun-Xi Zhao; Yan-Yan Li; Ya-Ling Yang; Beer yeast-derived fluorescent carbon dots for photoinduced bactericidal functions and multicolor imaging of bacteria. Applied Microbiology and Biotechnology 2019, 103, 4585-4593, 10.1007/s00253-019-09782-3.
- Fengming Lin; Yan-Wen Bao; Fu‐Gen Wu; Carbon Dots for Sensing and Killing Microorganisms. C—Journal of Carbon Research 2019, 5, 33, 10.3390/c5020033.
- Xiuli Dong; Weixiong Liang; Mohammed J. Meziani; Ya-Ping Sun; Liju Yang; Carbon Dots as Potent Antimicrobial Agents. Theranostics 2019, 10, 671-686, 10.7150/thno.39863.
- Anisha Anand; Binesh Unnikrishnan; Shih-Chun Wei; C. Perry Chou; Li-Zhi Zhang; Chih-Ching Huang; Graphene oxide and carbon dots as broad-spectrum antimicrobial agents – a minireview. Nanoscale Horizons 2018, 4, 117-137, 10.1039/c8nh00174j.
- Ahmed Al-Jumaili; Surjith Alancherry; Kateryna Bazaka; Mohan V. Jacob; Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066, 10.3390/ma10091066.
- Hamblin, M.R.; Sperandio, F.F.; Gupta, A.. Photodynamic Therapy Mediated by Fullerenes and Their Derivatives; Al-Jumaily, A.; Ahmed, W.; Jenkins, C.H.M., Eds.; Momentum Press: New York, NY, USA, 2013; pp. 12-24.
- Jovanovi´c, S.; Markovi´c, Z.; Todorovi´c Markovi´c, B.. Carbon Based Nanomaterials as Agents for Photodynamic Therapy In Photodynamic Therapy (PDT): Principles, Mechanisms and Applications; Fitzgerald, F., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 45-108.
- Qi- Long Yan; Michael Gozin; Feng-Qi Zhao; Adva Cohen; Si-Ping Pang; Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 2015, 8, 4799-4851, 10.1039/c5nr07855e.
- Nenad K. Stanković; Michal Bodik; Peter Siffalovic; Mario Kotlár; Matej Mičušík; Zdenko Špitálský; Martin Danko; Dušan D. Milivojević; Angela Kleinová; Pavel Kubát; et al.Zdenka CapákováPetr HumpolíčekMarián LehockýBiljana Todorović MarkovićZoran Marković Antibacterial and Antibiofouling Properties of Light Triggered Fluorescent Hydrophobic Carbon Quantum Dots Langmuir–Blodgett Thin Films. ACS Sustainable Chemistry & Engineering 2018, 6, 4154-4163, 10.1021/acssuschemeng.7b04566.
- Lei Jiang; Haizhen Ding; Mingsheng Xu; Xiaolong Hu; Shengli Li; Mingzhu Zhang; Qiong Zhang; Qiyang Wang; Siyu Lu; Yupeng Tian; et al.Hong Bi UV–Vis–NIR Full‐Range Responsive Carbon Dots with Large Multiphoton Absorption Cross Sections and Deep‐Red Fluorescence at Nucleoli and In Vivo. Small 2020, 16, 9, 10.1002/smll.202000680.
- Xiao-Lu Guo; Zhao-Yang Ding; Si-Min Deng; Chang-Chun Wen; Xing-Can Shen; Bang-Ping Jiang; Hong Liang; A novel strategy of transition-metal doping to engineer absorption of carbon dots for near-infrared photothermal/photodynamic therapies. Carbon 2018, 134, 519-530, 10.1016/j.carbon.2018.04.001.
- M. Carmen Ortega-Liebana; M. Mar Encabo-Berzosa; María J. Ruedas-Rama; Jose Luis Hueso; Nitrogen-Induced Transformation of Vitamin C into Multifunctional Up-converting Carbon Nanodots in the Visible-NIR Range. Chemistry - A European Journal 2016, 23, 3067-3073, 10.1002/chem.201604216.
- Jingxiang Zhang; Xiaoli An; Xiaoan Li; Xiaozhu Liao; Yingying Nie; Zengjie Fan; Enhanced antibacterial properties of the bracket under natural light via decoration with ZnO/carbon quantum dots composite coating. Chemical Physics Letters 2018, 706, 702-707, 10.1016/j.cplett.2018.06.029.
- Shoujun Zhu; Yubin Song; Xiaohuan Zhao; Jieren Shao; Junhu Zhang; Bai Yang; The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Research 2015, 8, 355-381, 10.1007/s12274-014-0644-3.
- Hui Ding; Xue-Hua Li; Xiao-Bo Chen; Ji-Shi Wei; Xiao-Bing Li; Huan-Ming Xiong; Surface states of carbon dots and their influences on luminescence. Journal of Applied Physics 2020, 127, 231101, 10.1063/1.5143819.
- Lian Xiao; Yue Wang; Yi Huang; Teck Neng Wong; Handong Sun; Self-trapped exciton emission from carbon dots investigated by polarization anisotropy of photoluminescence and photoexcitation. Nanoscale 2016, 9, 12637-12646, 10.1039/c7nr03913a.
- Schmitz, R. Synthesis, Characterization, and Plasmonic Enhancement of Fluorescent Carbon Nanodots.Ph.D. Thesis, University of Maryland Baltimore County, Baltimore, MD, USA, 2016.
- Jinyi Zhang; Xiaomei Lu; Dandan Tang; Shihong Wu; Xiandeng Hou; Juewen Liu; Peng Wu; Phosphorescent Carbon Dots for Highly Efficient Oxygen Photosensitization and as Photo-oxidative Nanozymes. ACS Applied Materials & Interfaces 2018, 10, 40808-40814, 10.1021/acsami.8b15318.
- Knoblauch, R.; Harvey, A.; Ra, E.; Greenberg, K.M.; Lau, J.; Hawkins, E.; Geddes, C.D. AntimicrobialCarbon Nanodots: Photodynamic Inactivation and Dark Toxicity E ects on Bacteria by Brominated CarbonNanodots. ACS Appl. Mater. Interfaces 2020. submitted.
- M. Shahshahanipour; Behzad Rezaeia; Ali A. Ensafi; Zahra Etemadifar; An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug.. Materials Science and Engineering: C 2019, 98, 826-833, 10.1016/j.msec.2019.01.041.
- Sonam Kumari; Surendra Rajit Prasad; Debabrata Mandal; Prolay Das; Carbon dot-DNA-protoporphyrin hybrid hydrogel for sustained photoinduced antimicrobial activity. Journal of Colloid and Interface Science 2019, 553, 228-238, 10.1016/j.jcis.2019.06.034.
- Knoblauch, R.; Harvey, A.; Geddes, C.D. Metal-enhanced photosensitization of singlet oxygen (ME1O2) frombrominated carbon nanodots on silver nanoparticle substrates. Plasmonics 2020. submitted.
- Zhe Gao; Dezhi Yang; Yang Wan; Yaling Yang; One-step synthesis of carbon dots for selective bacterial inactivation and bacterial differentiation.. Analytical and Bioanalytical Chemistry 2020, 412, 871-880, 10.1007/s00216-019-02293-0.
- Hao Li; Jian Huang; Yuxiang Song; Mengling Zhang; Huibo Wang; Fang Lu; Hui Huang; Yang Liu; Xing Dai; Zonglin Gu; et al.Zaixing YangRuhong ZhouZhenhui Kang Degradable Carbon Dots with Broad-Spectrum Antibacterial Activity. ACS Applied Materials & Interfaces 2018, 10, 26936-26946, 10.1021/acsami.8b08832.
- Bo Ju; Hui Nie; Xiao-Guang Zhang; Qiaonan Chen; Xiaowei Guo; Zhen Xing; Minjie Li; Sean Xiao‐An Zhang; Inorganic Salt Incorporated Solvothermal Synthesis of Multicolor Carbon Dots, Emission Mechanism, and Antibacterial Study. ACS Applied Nano Materials 2018, 1, 6131-6138, 10.1021/acsanm.8b01355.
- Huan-Huan Ran; Xiaotong Cheng; Yan-Wen Bao; Xian-Wu Hua; Ge Gao; Xiaodong Zhang; Yao-Wen Jiang; Ya-Xuan Zhu; Fu‐Gen Wu; Multifunctional quaternized carbon dots with enhanced biofilm penetration and eradication efficiencies. Journal of Materials Chemistry B 2018, 7, 5104-5114, 10.1039/c9tb00681h.
- Cheng Zhu; Hao Li; Huibo Wang; Bowen Yao; Hui Huang; Yang Liu; Zhenhui Kang; Negatively Charged Carbon Nanodots with Bacteria Resistance Ability for High-Performance Antibiofilm Formation and Anticorrosion Coating Design.. Small 2019, 15, e1900007, 10.1002/smll.201900007.
- Mariadoss Asha Jhonsi; Devanesan Arul Ananth; Gayathri Nambirajan; Thilagar Sivasudha; Rekha Yamini; Soumen Bera; ArunKumar Kathiravan; Antimicrobial activity, cytotoxicity and DNA binding studies of carbon dots. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 196, 295-302, 10.1016/j.saa.2018.02.030.
- Theodoros G. Chatzimitakos; Athanasia I. Kasouni; Anastassios N. Troganis; Constantine Stalikas; Exploring the antibacterial potential and unraveling the mechanism of action of non-doped and heteroatom-doped carbon nanodots. Journal of Nanoparticle Research 2020, 22, 36, 10.1007/s11051-019-4736-6.
- Jingjing Yang; Xiaodong Zhang; Yong-Hao Ma; Ge Gao; Xiaokai Chen; Hao-Ran Jia; Yan-Hong Li; Zhan Chen; Fu‐Gen Wu; Carbon Dot-Based Platform for Simultaneous Bacterial Distinguishment and Antibacterial Applications. ACS Applied Materials & Interfaces 2016, 8, 32170-32181, 10.1021/acsami.6b10398.
- Jingjing Yang; Ge Gao; Xiaodong Zhang; Yong-Hao Ma; Xiaokai Chen; Fu‐Gen Wu; One-step synthesis of carbon dots with bacterial contact-enhanced fluorescence emission: Fast Gram-type identification and selective Gram-positive bacterial inactivation. Carbon 2019, 146, 827-839, 10.1016/j.carbon.2019.02.040.
- Rachael Knoblauch; Brian Bui; Ammar Raza; Chris D. Geddes; Heavy carbon nanodots: a new phosphorescent carbon nanostructure. Physical Chemistry Chemical Physics 2017, 20, 15518-15527, 10.1039/c8cp02675k.
- T. Kavitha; S. Kumar; Turning date palm fronds into biocompatible mesoporous fluorescent carbon dots. Scientific Reports 2018, 8, 16269, 10.1038/s41598-018-34349-z.
- Junjun Liu; Siyu Lu; Qiuling Tang; Kai Zhang; Weixian Yu; Hongchen Sun; Bai Yang; One-step hydrothermal synthesis of photoluminescent carbon nanodots with selective antibacterial activity against Porphyromonas gingivalis. Nanoscale 2016, 9, 7135-7142, 10.1039/C7NR02128C.
- Peng Hou; Tong Yang; Hui Liu; Yuanfang Li; Chengzhi Huang; An active structure preservation method for developing functional graphitic carbon dots as an effective antibacterial agent and a sensitive pH and Al( iii ) nanosensor. Nanoscale 2016, 9, 17334-17341, 10.1039/C7NR05539K.
- Xiuli Dong; Mohamad Al Awak; Nicholas Tomlinson; Yongan Tang; Ya-Ping Sun; Liju Yang; Antibacterial effects of carbon dots in combination with other antimicrobial reagents. PLOS ONE 2017, 12, e0185324, 10.1371/journal.pone.0185324.
- Knoblauch, Rachael; Geddes, C.D.. Reviews in Plasmonics 2017; Geddes, C.D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 253-283.
- Rachel D. Schmitz; Jan Karolin; Chris D. Geddes; Plasmonic enhancement of intrinsic carbon nanodot emission. Chemical Physics Letters 2015, 622, 124-127, 10.1016/j.cplett.2015.01.035.
- Rachael Knoblauch; Estelle Ra; Chris D. Geddes; Heavy Carbon Nanodots 2: Plasmon Amplification in Quanta Plate™ Wells and the Correlation with the Synchronous Scattering Spectrum. Physical Chemistry Chemical Physics 2018, 21, 1254-1259, 10.1039/c8cp06299d.
- Jan Karolin; Chris D. Geddes; Metal-enhanced fluorescence based excitation volumetric effect of plasmon-enhanced singlet oxygen and super oxide generation. Physical Chemistry Chemical Physics 2012, 15, 15740, 10.1039/c3cp50950h.
- Yongxia Zhang; Kadir Aslan; Michael J. R. Previte; Chris D. Geddes; Plasmonic engineering of singlet oxygen generation. Proceedings of the National Academy of Sciences 2008, 105, 1798-1802, 10.1073/pnas.0709501105.
- Yongxia Zhang; Kadir Aslan; Michael J. R. Previte; Chris D. Geddes; Metal-enhanced superoxide generation: A consequence of plasmon-enhanced triplet yields. Applied Physics Letters 2007, 91, 023114, 10.1063/1.2753718.