Clinically significant atherosclerosis of the coronary arteries, known as coronary artery disease (CAD), is an endemic condition that is associated with significant morbidity and mortality. The introduction of artificial intelligence (AI) and machine learning over the last two decades has unlocked new dimensions in the field of cardiovascular medicine. From automatic interpretations of heart rhythm disorders via smartwatches, to assisting in complex decision-making, AI has quickly expanded its realms in medicine and has demonstrated itself as a promising tool in helping clinicians guide treatment decisions. Understanding complex genetic interactions and developing clinical risk prediction models, advanced cardiac imaging, and improving mortality outcomes are just a few areas where AI has been applied in the domain of coronary artery disease.
- artificial intelligence
- coronary artery disease
- major adverse cardiovascular events
1. Integration of Genetics and AI in Cardiovascular Diseases
Over the last two decades, the emergence of technologies able to measure biological processes at a large scale have resulted in an enormous influx of data. For instance, the completion of the Human Genome Project has paved the way to design single-nucleotide polymorphism (SNP) and mRNA microarrays, which can broadly test for millions of genetic variants in a simple point-of-care test. This has paved the way for the emergence of modern data-driven sciences such as genomics and other “omics” [1][12]. Genome-wide association studies (GWASs) operate by simultaneous comparison of millions of SNPs between diseased individuals and disease-free controls to detect a statistically significant association between an SNP locus and a particular condition [1][12]. Machine learning (ML) and particularly deep learning (DL)DL algorithms are inherently designed to extract patterns and associations from large-scale data, including clinical and genomic data. Given the complexity and multifaceted nature of cardiovascular diseases in general, and CAD in particular, an approach that integrates all these factors into a risk-stratification model would be expected to better predict incident events than existent models [2][17].
Multiple studies have emphasized the role of ML in identifying genetic variants and expression patterns associated with CAD from mRNA arrays using differential expression analysis and protein–protein interaction networks [3][4][18,19]. For example, Zhang et al. used ML to perform differential expression analysis on mRNA profiles from CAD patients and healthy controls to identify a set of differentially expressed genes between the two groups, then built a network representation of functional protein–protein interaction. The top 20 genes in the network were identified using a maximal clique centrality (MCC) algorithm. Finally, to test the performance, a logistic regression model was built using the top five predictor genes to classify individuals into the presence or absence of CAD. The model achieved an AUC of 0.9295 and 0.8674 in the training and internal validation sets respectively [5][20].
Dogan et al. built an ensemble model of eight random-forest (RF) classifiers to predict the risk of symptomatic CAD using genetic and epigenetic variables along with clinical risk factors. The model was trained on a cohort derived from the Framingham heart study (n = 1545) and utilized variables derived from genome-wide array chips to extract epigenetic (DNA methylation loci) and genetic (SNP) profiles. The initial number of available variables were 876,014 SNP and DNA methylation (CpG) loci, which required multiple reduction steps, ending up with 4 CpG and 2 SNP predictors fed into the model along with age and gender. The model predicted symptomatic CAD with an accuracy, sensitivity, and specificity of 0.78, 0.75, and 0.80, respectively, in the internal validation cohort (n = 142).
Finally, the coronary artery calcium (CAC) score, calculated using the Agatston method on noncontrast ECG-gated cardiac computed tomography, is an established strong predictor of major adverse cardiovascular events in asymptomatic individuals. Genomic studies have previously focused on identifying genetic loci linked to CAC [6][7][24,25]. Oguz et al. suggested the use of ML algorithms to predict advanced CAC from SNP arrays and clinical variables. They identified a set of SNPs that ranked the highest in predictive importance and correlated with advanced CAC scores, defined as the 89th–99th percentile CAC scores in the derivation and replication cohorts, and trained different RF models to predict advanced CAC scores using clinical and genetic variables.
2. Risk Prediction Models and Imaging Modalities for Estimating Pretest Probability of CAD
When available, a CAC score has been shown to add to the PTP of CAD, with a CAC score of zero identifying low-risk patients who might not need additional testing [17][18][7,36]. ML models, combining clinical and imaging parameters, have been shown to have higher predictive power than traditional risk scores when predicting the PTP of obstructive CAD [19][20][37,38].
Various ML algorithms based on stress imaging, particularly single-photon emission computed tomography (SPECT), have been devised to facilitate the prediction of CAD. These models combined the clinical and demographic characteristics with the quantitative variables, as evaluated via SPECT to better predict CAD compared with the visual interpretation or quantitative variables alone [21][22][23][24][25][26][39,40,41,42,43,44].
34. Artificial Intelligence in Management of CAD in the Emergency Department
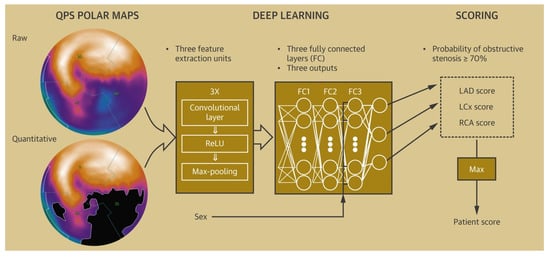
Chest pain is a common emergency department presentation, and distinguishing cardiac from noncardiac pain causes is crucial for optimal management. Modalities such as electrocardiography (ECG) serve as a quick way to recognize patterns associated with unstable CAD, and in particular acute coronary syndromes (ACSs). Deep neural networks have shown a consistent performance in image recognition, and models have hence been devised to identify patterns related to CAD and myocardial infarction (MI) [30][31][32][52,53,54]. By reducing interobserver variability and providing accurate results efficiently, this approach holds the promise of improving workflow across healthcare systems, while helping patients in areas of limited medical infrastructure and specialized care. The 2021 American College of Cardiology/American Heart Association (ACC/AHA) chest pain guidelines advocate for the use of coronary CT angiography (CCTA) in intermediate-risk patients presenting with acute chest pain who either have no known history or a history of nonobstructive CAD (defined as coronary artery disease with less than 50% diameter stenosis) [18][36]. Given the ability of CCTA to accurately define coronary anatomy and extent/distribution of atherosclerotic plaque, it has been consistently shown to be a useful noninvasive imaging modality for patient selection, particularly for those who might require further invasive evaluation. However, interpretation of CCTA scans requires expertise and is time-intensive. Therefore, automatic interpretation of CCTA, which can lead to a significant reduction in the processing times, is highly desirable. ML algorithms have recently been developed, achieving a 70–75% reduction in reading time compared to that required for human interpretation (2.3 min for AI vs. 7.6–9.6 min for human readers). Though the model described performed slightly lower than highly experienced readers in interpreting CCTA (AUC 0.93 vs. 0.90 for human vs. AI, p < 0.05), when combined with low-experience human readers, it augmented the reader’s ability to correctly reclassify obstructive CAD (per-vessel net reclassification index (NRI) 0.07, p < 0.001) [33][65]. In addition, ML has been applied for various segmentation and classification tasks on cardiac CT imaging, from automatic segmentation of calcified and noncalcified plaque to automated calculation of the Agatston CAC score, and finally quantification of cardiac structures on CT imaging (Figure 12) [34][35][36][37][38][39][40][41][66,67,68,69,70,71,72,73]. Therefore, the application of ML could provide reliable results in real time, while bridging the dearth of experts in low-resource settings.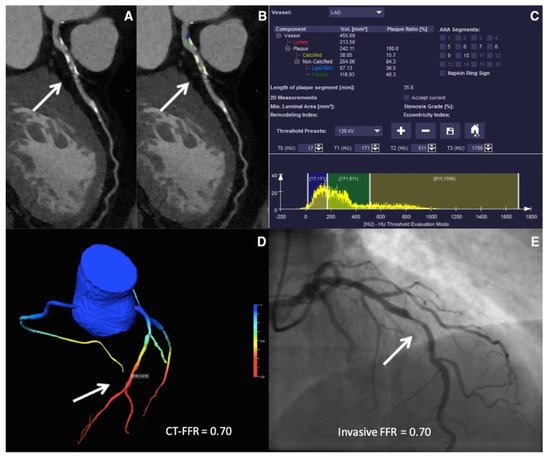
45. Artificial Intelligence to Predict Functionally Obstructive CAD and Lesion-Specific Ischemia—As a Gatekeeper to the Catheterization Laboratory
4.1. ML-Based CT-FFR Estimation and Diagnostic Accuracy
5.1. ML-Based CT-FFR Estimation and Diagnostic Accuracy
4.2. Impact of Calcification Burden on the Performance of CT-FFR
5.2. Impact of Calcification Burden on the Performance of CT-FFR
ML
The impact of coronary calcification on the diagnostic performance of CCTA has been well-established, with more extensive calcification limiting the ability of CCTA to evaluate for the presence of obstructive CAD [67][68][69][108,109,110]. Multiple indices have been devised to compute a CAC score, with the Agatston score, calcium volume, calcification remodeling index (CRI), and segmental arc calcification method being common examples [70][111]. The Agatston Score (AS) is the most widely validated approach, which summates the calcium score (function of peak density and area of the lesion) of the individual lesions across all coronary artery segments [71][112]. CRI provides a lesion-specific calcium estimate and is calculated as a ratio of the cross-sectional luminal area of the most severely calcified site to the proximal luminal area [72][113]. The segmental arc calcification method estimates lesion-specific calcium burden by measuring the greatest circumferential extent of coronary calcium, grading as nil (noncalcified), mild (0–90°), moderate (90–180°), and severe (>180°) calcification [69][73][110,114].4.3. CT-FFR
5.3. CT-FFR
ML
in Predicting Revascularization Events
56. Artificial Intelligence in the Field of Intracoronary Imaging
5.1. Artificial Intelligence to Optimize Peri-Intervention Workflow
6.1. Artificial Intelligence to Optimize Peri-Intervention Workflow
To predict OCT-derived TCFA on IVUS images, Bae et al. created a ML model, enrolling 517 patients who underwent ICA [81][137]. A total of 40,908 IVUS-OCT co-registered sections in 517 coronary arteries were divided into training and testing sets in a ratio of 4:1. An artificial-neural-network-based model using 17 features achieved the highest performance with a sensitivity and specificity of 85 ± 4% and 79 ± 6%, respectively, and good discriminatory power (AUC of 0.80 ± 0.08). Larger plaque burden, minimal diameter, decreased lumen area, and increased lumen eccentricity were seen to be strongly associated with OCT-derived TCFAs. Min et al. utilized a deep learning algorithm (densely connected convolutional neural network) on 35,678 OCT frames to automatically detect TCFAs from OCT images [82][138]. After the frames were interpreted for the presence/absence of TCFA, data was fed into the algorithm to devise a deep-learning model. By achieving high sensitivity and specificity of 88.7 ± 3.4% and 91.8 ± 2.0% on the test data, such deep-learning models can significantly reduce processing times and allow for easy interpretation when it comes to identifying a vulnerable high-risk plaque.5.2. Applications of Artificial Intelligence in Intra and Post-Intervention Workflow
6.2. Applications of Artificial Intelligence in Intra and Post-Intervention Workflow
67. Artificial Intelligence-Based Post-Procedure Risk Prediction Models
In addition to early detection and the institution of guideline-directed therapy in the appropriate risk strata, accurate prediction of unheralded adverse events forms the cornerstone for managing CAD. Identifying the high-risk target population can potentially provide a window for aggressive risk factor modulation, thereby reducing mortality and contributing towards better health at a population level. Multiple risk-prediction models have been developed to predict in-hospital mortality and the long-term risk of MACE in high-risk cohorts [90][91][92][93][94][95][96][97][151,152,153,154,155,156,157,158]. PCI is a relatively safe procedure, with a reported overall in-hospital mortality rate of 1–2% [98][159]. The risk of complications increases with increasing patient morbidity, with an incidence of technical difficulties and periprocedural complications 2.2 times higher than in the average population [99][160]. The Mayo clinic risk score (MCRS) and New York State risk score (NYSRS) were developed to predict in-hospital and 30-day mortality in patients undergoing PCI. Both scores performed equivalently well, showing an excellent discriminative ability to identify patients at a higher risk for in-hospital and 30-day mortality [100][161]. They employed regression-based models, assuming a linear interplay between patient variables and mortality outcomes. ML models have been recently developed to potentially uncover complex and nonlinear relationships between multiple factors, hence improving diagnostic accuracy over current models.78. Artificial Intelligence-Based Long-Term Mortality and MACE Prediction Models
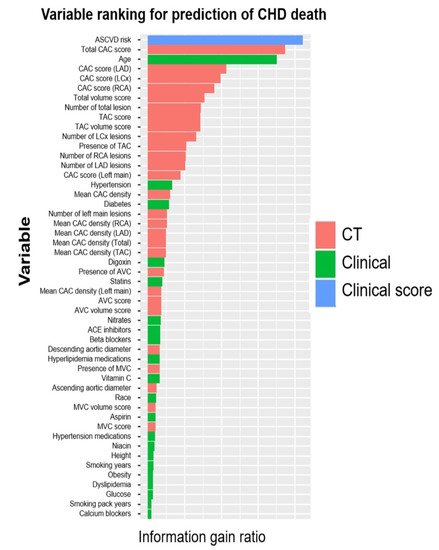
Prognostic modeling via ML has been validated with the use of electronic health records (EHRs) integrated with clinical scores and imaging modalities to predict MACE [101][102][103][173,174,175]. Utilizing the array of data available in EMR and identifying patterns based on clinical course, ML models have been used to create a personalized treatment algorithm (ML4CAD) for every patient, based on risk factors, past medical history, time present in the EMR system, and medications. The illustrated model makes clinical decisions for patients based on these factors and suggests a decision with an aim to increase prescription effectiveness, evaluated in the terms of time from initial diagnosis to the first potential adverse event (time to adverse event, TAE). The model had superior performance when compared to standard of care, increasing the time to adverse event (TAE) from 4.56 to 5.66 years (24.3% increase), hence furthering the idea of precision medicine [102][104][174,176]. Multiple ML techniques have been proposed to automatically evaluate CAC score from dedicated cardiac and non-EKG gated chest CT scans [34][35][105][106][107][66,67,183,184,185]. ML techniques incorporating CAC score and other imaging parameters have been shown to be a better predictor than the traditional risk scores employed for cardiovascular disease risk stratification [108][109][110][111][112][181,186,187,188,189]. An ensemble-boosting model developed by Nakanishi et al. incorporating a total of 77 clinical and imaging variables had a superior discriminatory power for predicting coronary heart disease deaths than imaging and clinical data alone (AUC for ML model: 0.845 compared to 0.821 and 0.781 for clinical data and CAC respectively, p < 0.001) (Figure 35) [113][190].