Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 3 by Conner Chen.
Breast cancer is a cancer that develops from breast tissue. Symptoms of breast cancer may include a breast lump, a change in the shape of the breast, sunken skin, fluid leaking from the nipple, new sunken nipple, or red or scaly patches on the skin. Distant spread may present with bone pain, swollen lymph nodes, shortness of breath, or yellowing of the skin. Breast cancer is very complex and includes several subtypes with distinct pathological features. Most of the immunotherapy efforts have focused on the most immunogenic subtypes: triple-negative breast cancer and HER2-positive breast cancer.
- triple negative breast cancer
- breast cancer
- immunotherapy
- cancer vaccine
- adoptive cell transfer therapies
- (CAR) T-cell therapy
- immunopheresis
- immune checkpoint inhibition (ICI)
- anti-HER2 antibody
- antibody-drug conjugates (ADC)
1. Introduction
Breast cancer (BC) is the most diagnosed cancer in the world and the leading cause of cancer death in women [1]. This disease is very complex and comprises a heterogeneous group of cancer types with distinct pathological features and therapeutic implications. The clinical classification of BC is based on the expression of three clinically validated biomarkers associated with prognosis and treatment options: estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) [2]. Clinical BC types include hormone receptor (HR)-positive (HR+/HER2-, also ER+/PR+/HER2-), triple positive (HR+/HER2+, also ER+/PR+/HER2+), HER2-positive (ER-/PR-/HER2+, also HER2+), and triple-negative breast cancer (TNBC) (HR-/HER2-, also ER-/PR-/HER2-) [2]. Additionally, based on genomic DNA analysis (including copy number, DNA mutations, DNA methylation), gene expression profiling, and protein profiling, phenotypically diverse breast cancers were molecularly characterized and classified into four molecular (intrinsic) groups: luminal A (ER+/PR+/HER2-, low proliferation factor Ki67+ (<14%), low grade), luminal B (ER+/PR±/HER2±, high proliferation factor Ki67+ (≥14%), high grade), HER2-enriched (ER-/PR-/HER2+, any Ki67 level, high proliferation), and basal-like (ER-/PR-/HER2-, any Ki67 level, high grade and proliferation, necrosis) [2][3]. Intrinsic BC subtypes partially overlap with clinical subtypes and show significant intragroup molecular heterogeneity [3]. Basal-like tumors are enriched in TNBCs, although 21.4% of TNBCs are not basal-like [4].
2. Immune Mechanisms in Breast Cancer and Importance of Immune Responses in TNBC Prognosis and Treatment
While the current clinical decision-making strongly relies on the assessment of receptor expression by tumor cells, it is clear that immune cells and immune signaling are also important in cancer prognosis as well as response to therapy. The tumor microenvironment (TME) is characterized by the presence of innate and adaptive immune cells [5][6]. Studies have identified that tumor-infiltrating lymphocytes (TILs) and tumor-associated macrophages (TAMs) are prominent players in the BC tumor microenvironment. TME also comprises non-cellular components (e.g., inflammation mediating cytokines and growth factors, which correlate with cancer prognosis) and non-immune cells (such as cancer-associated fibroblasts and cancer-associated adipocytes) (Figure 1) [7].
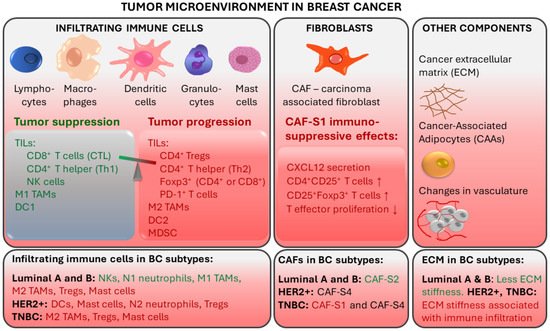

Figure 1. Tumor-associated immune responses in breast cancer. Major players in breast TME include tumor-infiltrating lymphocytes (TILs) and tumor-associated macrophages (TAMs). Non-immune cells such as cancer-associated fibroblasts (CAFs) and cancer-associated adipocytes (CAAs) contribute to an immunosuppressive environment. CTL: cytotoxic CD8+ T lymphocytes; Th: CD4+ T helper cells; NK: natural killer cells; DC: Dendritic cells; Tregs: regulatory T cells; MDSCs: myeloid-derived suppressor cells. Red text color indicates tumor progression and immunosuppressive effects. Green text color indicates tumor suppression.
2.1. Tumor-Infiltrating Lymphocytes
TILs are immune cells that have left the bloodstream and infiltrated the tumor tissue. They are thought to represent pre-existing antitumor immunity and are clinically meaningful [5]. Elevated tumor TIL presence has been shown to predict a response to neoadjuvant chemotherapy (NAT) in all BC molecular subtypes [8]. TIL levels in the intratumoral stroma have been shown to strongly correlate with good prognosis in TNBC [7][9]. TIL presence at diagnosis of TNBC was positively associated with pathologic response (pCR) to neoadjuvant therapy and disease-free and overall survival after adjuvant chemotherapy, suggesting that anti-tumor immune responses are important in chemotherapeutic sensitivity of BC [10][11]. For every 10% increase in stromal TILs, a risk reduction regarding TNBC recurrence or death was observed [10]. On the other hand, increased TILs was an unfavorable prognostic factor for survival in luminal-HER2-negative patients, suggesting a different biology of the immunological infiltrate in this BC subtype [8]. The prognostic value of TILs in TNBC has also been shown in the absence of adjuvant chemotherapy, identifying a subgroup of early TNBC patients for whom adjuvant systemic therapy might be safely withheld [12][13].
The degree of TIL infiltration assessed by simple hematoxylin and eosin staining of tumor sections is predictive and prognostic in TNBC and HER2-positive BC even without the detailed information on the immune subpopulations of the infiltrate [14]. Nonetheless, deeper immune profiling revealed that the type of TILs, their location and density correlated with BC prognosis and specific outcomes, including pCR [14][15][16]. Better outcomes were associated with infiltrates enriched in CD8+ cytotoxic T lymphocytes (CTLs), which are the major effector cell type in BC, and with CD4+ T helper cells, natural killer (NK) cells, and dendritic cells (DCs) [16][17][18]. On the other hand, worse prognosis was observed when infiltrates were enriched in regulatory T cells (Tregs), which can suppress immune responses and help to create an immune environment that promotes tumor cell survival and carcinogenesis. Tumor infiltration with Foxp3+ Tregs and PD-1+ T cells was linked with immune escape [16][18][19]. Furthermore, immature DCs and eosinophils in immune infiltrates were associated with a worse overall survival in TNBC [20]. In TNBC patients who received NAT, pCR was correlated with a higher ratio of CD3+:CD68+ cells and closer spatial proximity of T cells to tumor cells [15].
In breast TME, immune infiltration is correlated with the presence of hormone receptors and differs between ER+, HER2+, and TNBC tumors. ER-positive breast tumors show an enrichment in NKs and neutrophils, while immune infiltrates in ER-negative breast tumors are enriched in Tregs, activated mast cells (associated with poor prognosis) and M2-macrophages [6].
Tumors can evade recognition by the host immune mechanisms at the level of immune checkpoints. This has led to the identification of immune checkpoint inhibition (ICI) as a promising approach to enhancing antitumor immunity. Immune checkpoint receptor programmed death 1 (PD-1), which is found on T-cells in the TIL infiltrate, functions as a negative regulator of the immune system [21]. Programmed cell death ligand 1 (PD-L1) binds to PD-1, sends a suppressive signal to T cells, and mediates local immune evasion in various types of cancer. Expression of PD-L1 is enriched in basal-like breast tumors compared to other BC subtypes [21][22]. Clinical trials studying PD-1/PD-L1 immune checkpoint inhibition therapy in TNBC have shown positive results and have led to new therapy options for TNBC (Figure 2) [23][24][25][26][27].
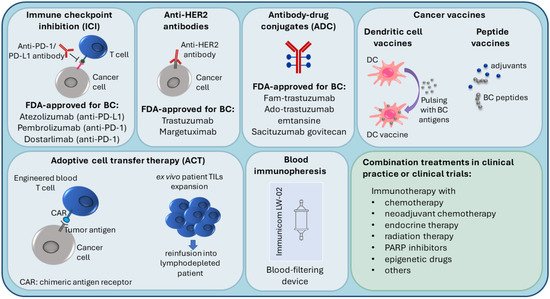

Figure 2.
Current immunotherapy strategies in breast cancer treatment.
2.2. Tumor-Associated Macrophages
TAMs derived from peripheral blood monocytes are recruited into the cancer microenvironment and undergo M1/M2 polarization in response to multiple stimuli [28][29]. They are involved in the interaction between the immune system and cancer cells. M1 (CD11c+) macrophages have a proinflammatory function, while M2 (CD163+) TAMs show immunosuppressive action [30]. The tumor stroma of TNBC and basal-like BC is enriched with CD163+ M2-macrophages, which are associated with higher tumor grade and proliferation [31]. In TNBC, TAMs promote tumor growth and progression in various ways: by secreting inhibitory cytokines, by reducing the effector functions of TILs, by promoting Tregs, and by modulating PD-1/PD-L1 expression in the tumor environment [28]. M2 macrophages are prognostic for lower, while M1 macrophages are prognostic for higher overall and disease-free survival [30]. Luminal A tumors contain fewer CD11c+ and CD163+ cells compared to TNBC [30].
2.3. Other TME Components
Fibroblasts are stromal cells involved in supporting tissues by secreting proteins and remodeling the extracellular matrix (ECM). Breast TME contains carcinoma-associated fibroblasts (CAFs), which contribute to tumor progression by secreting tumor-promoting factors (e.g., chemokines and matrix metalloproteinases) [32][33][34]. Specific CAF subsets were detected in different BC subtypes. CAF-S2 is characteristic for luminal-like tumors [35]. The significant presence of CAF-S2 is also seen in normal breast tissue, suggesting that CAFs in luminal BC may be derived from normal resident fibroblasts [34][35]. In aggressive BC subtypes, CAF-S1 and CAF-S4 are characteristic: HER2+ tumors are enriched in CAF-S4, while TNBC tumors have a high presence of CAF-S4 and CAF-S1 [35]. CAF-S1 fibroblasts were found to promote an immunosuppressive environment by increasing the capacity of Tregs to inhibit T effector proliferation, by attracting (via CXCL12 secretion) and retaining CD4+CD25+ TILs, and by promoting their differentiation into CD25+FOXP3+ TILs [35].
Breast cancer is associated with extensive remodeling of ECM. Particularly in TNBC and HER2+, stiffening related to collagen deposition and linearization, which is associated with enhanced immune cell infiltration, is observed [36]. In contrast, luminal-like breast cancers undergo less ECM remodeling. Additionally, breast TME contains cancer-associated adipocytes (CAAs), which express proteases that degrade the ECM. CAAs are characterized by reduced lipid content, expression of specific adipokines and proteases, and increased pro-inflammatory cytokine production [34]. Fragmentation of ECM by metalloproteinases and remodeling enzymes can promote the penetration of CAFs [37].
3. Immunotherapy in Breast Cancer
Due to higher TIL and TAM levels, increased PD-L1 expression, greater mutational burden, and genomic instability, TNBC and HER2+ breast cancers are considered more immunogenic compared to luminal BC, with the immune responses of these patients affecting the disease outcome [16][24][35][38][39]. Because of this, TNBC and HER2+ patients are most suitable for immunotherapy, which is an important emerging treatment approach in BC (Figure 2), and most of the BC immunotherapy efforts have focused on these subtypes [16][39]. Several targets in immune pathways have been explored [38].
3.1. Immune Checkpoint Inhibition (ICI), Anti-HER2 Antibodies and Antibody-Drug Conjugates (ADC)
Active BC immunotherapies have been designed to target the immune checkpoints (such as PD-1/PD-L1) on cancer cells and in the TME. Checkpoint inhibitors used in clinical practice for BC target either the PD-1 receptor or the PD-L1 ligand. The disruption of PD-1/PD-L1 interaction enhances the ability of T cells to attack the tumor [40]. The U.S. Food and Drug Administration (FDA) (https://www.fda.gov/, accessed on 14 November 2021) recently (in 2019) approved atezolizumab (anti-PD-L1 antibody) with paclitaxel as a combination therapy for PD-L1-positive unresectable locally advanced or metastatic TNBC [41]. In 2021, the FDA-approved pembrolizumab (anti-PD-1 antibody) in combination with chemotherapy for high-risk early-stage TNBC and for locally recurrent unresectable or metastatic PD-L1-positive TNBC tumors [42]. Moreover, in TNBC clinical trials, PD-1/PD-L1 inhibitors are being investigated in combination with a number of other treatments, such as radiation therapy (NCT04683679), PARP inhibitors (i.e., DNA damage response inhibitors) [43], anti-CTLA4 (cytotoxic T lymphocyte–associated antigen 4) ICI immunotherapy (NCT03982173), and others [38].
Several immune therapies and immunotherapy combination treatments have received FDA approval for HER2-positive BC. They include trastuzumab (anti-HER2 antibody), margetuximab (anti-HER2 antibody), fam-trastuzumab (antibody-drug conjugate (ADC); combination of trastuzumab and the chemotherapeutic DXd), ado-trastuzumab emtansine (ADC; combination of trastuzumab and the chemotherapeutic emtansine) [44][45][46][47]. Trastuzumab can be used alone or combined with chemotherapy and/or pertuzumab (a HER2 dimerization inhibitor) [45]. ADCs are also being investigated in TNBC [48]. In 2021, the FDA approved sacituzumab govitecan (combination of anti-Trop2 antibody and the chemoterapeutic SN-38) to treat unresectable locally advanced or metastatic TNBC [49].
Luminal BC tumors, which are less immunogenic, are the least likely to respond to immunotherapy. Consequently, fewer immunotherapy studies have been carried out in this subtype [38]. In ER-positive tumors, PD-1/PD-L1 inhibitors are being investigated in combination therapies such as endocrine therapy (NCT02997995) and neoadjuvant chemotherapy (NCT03356860).
3.2. Personalized Immunotherapy Approaches
Several novel immunotherapy approaches are currently being tested, including vaccines, cell-based immunotherapies, and immunopheresis.
In peptide-based cancer vaccines, BC-specific antigens are used to stimulate the host immune system. Endogenous antigens (prepared from the patient’s tumor) or exogenous antigens (known to be tumor-specific, for example, HER2 peptide) can be introduced with adjuvants into the patient to stimulate an effector and memory T cell response [50][51][52].
Dendritic cell (DC)-based cancer immunotherapy (i.e., DC vaccination) involves ex vivo pulsing of patient DCs with tumor cell lysates or tumor peptides prior to reinfusion into the patient. Patient DCs are prepared from peripheral blood precursors that are in vitro differentiated into DCs [53][54].
Adoptive cell transfer (ACT) therapies come in different variations. For example, lymphocytes can be isolated from the patient, expanded ex vivo and then reinfused into the patient, who undergoes lymphodepletion to reduce cells that contribute to immunosuppression [51][55]. Another adoptive cell transfer strategy is the use of genetically modified cells; autologous immune cells are removed from peripheral blood, genetically engineered to recognize a specific tumor antigen, and then reinfused into the patient. For instance, in chimeric antigen receptor (CAR) T-cell therapies, T cells are engineered to express a protein that can recognize tumor antigens on the tumor cell and a signaling domain that can turn the T cell on [51][56].
Recent advances in immunopheresis have made it possible to selectively remove specific immune-suppressive molecules from the blood of TNBC patients in order to re-energize the immune system to aggressively fight cancer (NCT04142931) (https://immunicom.com/ (accessed on 14 November 2021)) [57].
Overall, the emergence of immunotherapy has transformed the treatment standard of BC. After the completion of numerous clinical trials, immunotherapy is expected to become even more widespread. Transcriptome studies may prove useful in identifying new therapeutic targets for immunotherapy and novel BC markers that could be useful in disease/treatment monitoring. Additionally, transcriptome analyses may enable further characterization of patient immune responses, which would optimize patient stratification and selection of patients who are likely to benefit from immunotherapy.
