Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Amina Yu and Version 2 by Amina Yu.
Taraxerol, an oleanane-type pentacyclic triterpene, is one of the natural compounds that have been investigated extensively for its potential utilization in drug development. It has received major attention for its potential use as a therapeutic agent for the treatment of various diseases. Plants containing taraxerol are Hypericum perforatum, Clitoria ternatea, Mangifera indica, and Strobilanthes crispus.
- taraxerol
- medicinal properties
- biosynthesis
- triterpenoids
1. Distribution of Taraxerol in the Plant Kingdom
Members of the Asteraceae family comprise the greatest number of taraxerol-containing taxa, followed by the Euphorbiaceae and Malvaceae families. It should be noted that within the Euphorbiaceae family, species in the Euphorbia genus have shown considerable accumulation of taraxerol. The most prominent source of taraxerol was found to be chiefly concentrated in the leaves for most taxa [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21], followed by the roots [22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38] and finally the stems [20][24][39][40][41][42][43][44][45]. Some literature has also managed to isolate taraxerol from flowers. However, the distribution of taraxerol is highly diverse in plants, and taraxerol content differ in different parts of plants and across different plant species.
2. Biosynthesis Pathway of Taraxerol
The biosynthesis pathways of taraxerol in plants have yet to be definitively elucidated. Swain et al. (2012) hypothesized that the biosynthesis of taraxerol in plants begins from the mevalonic acid pathway in the plant’s cell cytoplasm [46]. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP, which are the basic building blocks of various terpenoid compounds including taraxerol [47][48]. The DMAPP produced will then undergo condensation with IPP which is catalyzed by geranyl pyrophosphate synthase, producing geranyl pyrophosphate (GPP) that will be further subjected to condensation with IPP to produce farnesyl pyrophosphate (FPP) catalyzed by farnesyl diphosphate synthase (FPS) [13][49]. Squalene synthase catalyzes the condensation of the FPP molecules through reduction by NADPH to produce one molecule of squalene [50][51]. Squalene is then oxidised by NADPH and O2 to produce 2,3-oxidosqualene, which results in the reduction of NADPH into NADP+ and O2 to H2O [52]. 2,3-oxidosqualene is then utilised as a precursor for the biosynthesis of various triterpenoids, starting with a proton-initiated cyclization to produce dammarenyl cation, following which subsequent rearrangement leads to the pentacylic oleanyl cation via baccharenyl and lupenyl cation intermediates [53]. A series of 1,2-hyride shifts and/or methyl groups leads to compound rearrangements. Finally, the rearrangements of compounds via taraxerol synthase eventually lead to the formation of taraxerol in plants, more specifically in the cuticular waxes [52][54][52][55]. A summary of the biosynthesis pathway is illustrated in Figure 1.
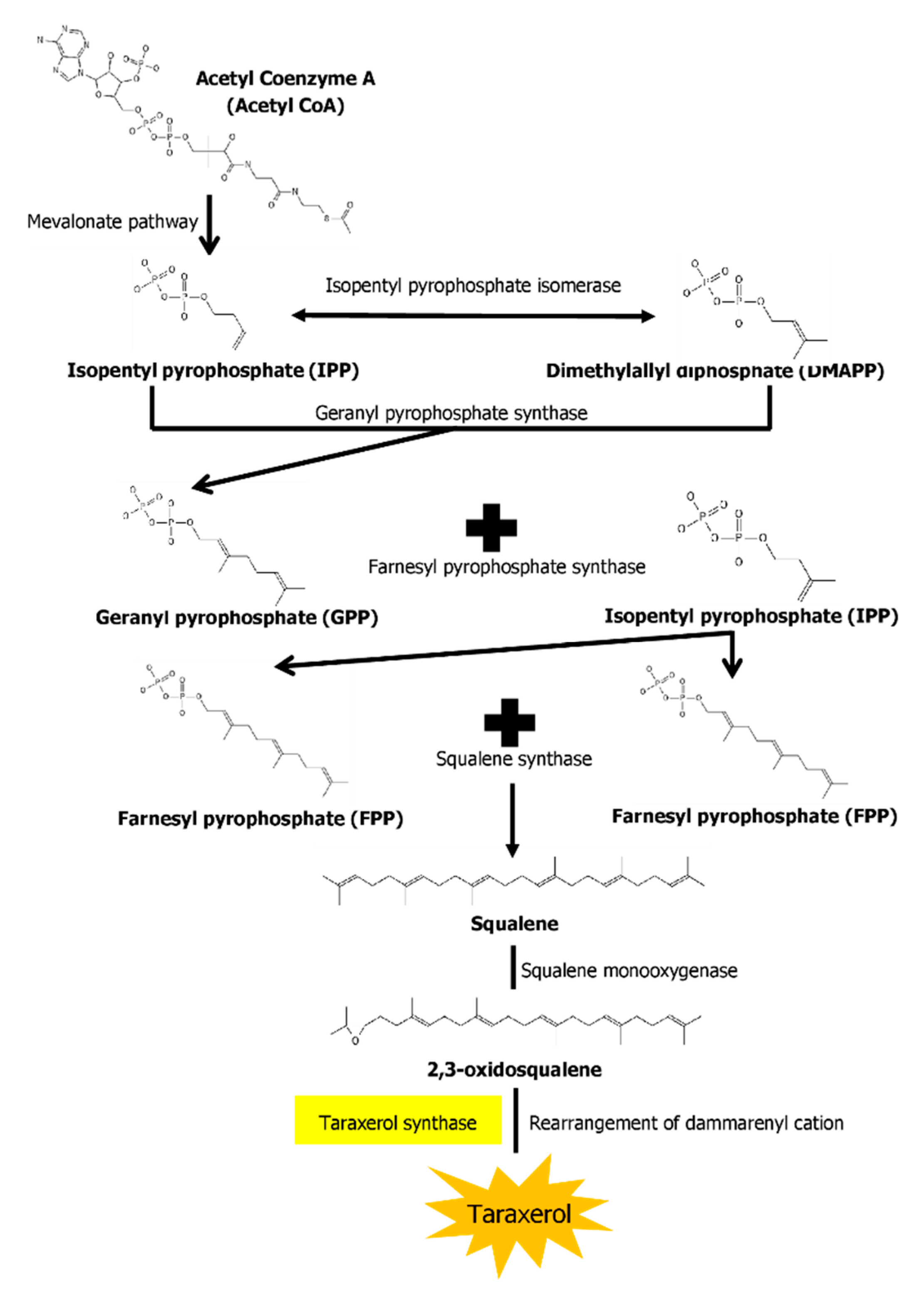
Figure 1. A summary of the biosynthesis pathway of taraxerol. With the aid of taraxerol synthase, dammarenyl cation undergoes rearrangements to produce taraxerol.
3. Medicinal Properties of Taraxerol
3.1. Antioxidative Properties
‘Reactive oxygen species’ (ROS) is a term that encompasses various oxygen free radicals produced during cellular oxidative process. These compounds pose a significant risk factor for various diseases. Hence, antioxidants play an important role as a phytochemical that could inhibit the oxidative process. It was reported that taraxerol isolated from the bark of Styrax japonica exhibited weak radical-scavenging activity in the DPPH assay [56]. Increasing the concentration of taraxerol from 0.05–0.5 mg/mL yielded moderate radical scavenging activity in DPPH assay [57]. Jamila et al. (2015) supported the findings from Min et al. (2004), where taraxerol isolated from Garcinia hombroniana was found to be more potent than trolox and equipotent to gallic acid in DPPH radical scavenging activity, while in ABTS the scavenging activity of taraxerol was higher than trolox but less than gallic acid [58]. The reducing capacity of the extracts is related to the presence of biologically active compounds, particularly the hydrogen donating ability [59]. Owing to the potential chemical structure of taraxerol itself, this might explain the potent antioxidative capabilities of taraxerol. The current body of literature on taraxerol as an antioxidant provides valuable insight on this compound, but the work is not yet completed, and there are aspects that are under-explored.
3.2. Antimicrobial Properties
Singh et al. (2002) observed that 1 mg of taraxerol compound exhibited moderate antimicrobial activity against two Gram-positive (Staphylococcus aureus and Bacillus thuringiensis) and three Gram-negative bacteria (Escherichia coli, Enterobacter cloacae, and Klebsiella pneumonia) [60]. Koay et al. (2013) investigated the minimum inhibitory concentrations (MICs) of taraxerol on several bacteria and found that the compound is active against Gram-positive Bacillus subtilis and Staphylococcus aureus at a concentration of 15.6 µg/mL but is only moderately inhibitive to the Gram-negative Escherichia coli, Klebseilla pneumonia, and Salmonella typhimurium at a concentration of 62.5 µg/mL The taraxerol antimicrobial activity is comparable to that of positive control gentamicin [1]. Meanwhile, Hernandez-Chavez et al. (2012) reported on the anti-gardial activities of taraxerol towards Giardia lambia, a parasitic protozoan [61]. It was found that taraxerol possessed strong anti-gardial activity exhibiting a growth inhibition (IC50) of 50% at a concentration of 16.11 µg/mL and a growth inhibition of 90% at a concentration of 102.4 µg/mL, although the activity is lower compared to the positive control metronidazole. Another was on the cytotoxic activity of taraxerol against parasitic protozoans was conducted by Simelane et al. (2013), targeting malaria-causing Plasmodium falcifarum and Plasmodium berghei [62]. Anti-plasmodial activities were reported for taraxerol at a concentration of more than 100 µg/mL [62], but it was found to have no effect on mycobacteria (Mycobacterium Madagascar and M. indicuspranii), exhibiting a lower activity than the positive control chloroquine (IC50 = 14.1 ng/mL) [57]. Thus, future should focus mainly on the potential of taraxerol as an anti-protozoan drug. Warfield et al. (2014) conducted on the efficacy of taraxerol in combating the parasitic Trypanosoma cruzi [63]. It was characterized the affinity of taraxerol with the sterol 14α-demethylase enzyme from Trypanosoma cruzi and found that the skeletal structure of taraxerol has higa affinity towards the enzyme, therefore providing potent inhibitory activity.
3.3. Anti-Fungal Properties
Earlier, taraxerol at a concentration of 1 mg/disc exhibited weak antifungal activities against four types of fungi namely Aspergillus niger, Aspergillus flavus, Rhizoctonia phaseoli, and Penicillium chrysogenum [60]. On the other hand, Aguilar-Guaddarama et al. (2009) shed some positive light on the anti-fungal potential of taraxerol [64]. It was focused on another type of fungus known as dermatophytes, which are pathogens that cause skin diseases in animals and humans [65]. The compound exhibited strong anti-dermatophytic activities against various dermatophytes, at varying degrees of inhibition. Taraxerol was particularly effective against several species of Trichophyton, for instance T. rubrum and T. mentagrophytes, with an MIC of 12.5 µg/mL, as well as Candida albicans (MIC = 25 µg/mL) and Aspergillus niger at 100 µg/mL [64].
3.4. Cytotoxic Properties
Chaturvedula et al. (2004) found taraxerol at a concentration of 21.8 µg/mL was enough to inhibit 50% (IC50) of the growth of the A2780 ovarian carcinoma cell line, although it performed worse than the positive control doxorubicin (IC50 1–3 ng/mL) [66]. At concentrations lower than 20 µg/mL, it showed little to no effect on the A2780 cell line [14]. Taraxerol also showed cytotoxicity towards the A431 squamous carcinoma cell line at 2.65 µg/mL, even though it was found to be inactive against HeLa, MCF-7, and MRC-5 cancer cell lines. While taraxerol cytotoxicity exhibited low activity compared to positive control doxorubicin, the activity is comparable to that of cisplatin [28]. Taraxerol also showed little to no inhibitory potential against Hypoxia-Induced Factor-1 (HIF-1) protein to reduce hypoxic tumor growth compared to 17-DMAF [17-(dimethylaminoethylamino)-17-demethoxygeldanamycin] [39]. However, taraxerol exhibited strong cytotoxicity towards human AGS gastric epithelial cell line at a concentration of 100 µmol/L by elevating cells arresting from complete mitosis and promoting early cell apoptosis rate from 4.45% to 10.29% [67]. Moreover, the report by Kaennakam et al. (2013) [35] contradicted earlier results from Csupor-Lötfer et al. (2011) [28] whereby the former observed that taraxerol displayed potent cytotoxicity to HeLa cells at a concentration of 14.94 µg/mL and to KB cells at a concentration of 13.58 µg/mL. Based on these results, taraxerol shows potential as a chemotherapeutic agent in cancer therapy.
3.5. Anti-Diabetic Properties
The utility of taraxerol in the treatment of diabetes was reported by Kwon et al. (2008), in which the compound was tested against the protein tyrosine phosphatase 1B (PTP1B)—a negative regulator of the insulin-signalling pathway for the treatment of type 2 diabetes [68]. Taraxerol was shown to exhibit moderate inhibitory properties against PTP1B at concentrations higher than 50 µM. Yet, Sangeetha et al. (2010) discovered that instead of targeting the PTP1B protein, taraxerol holds the potential to treat type 2 diabetes by dual action: as a glucose transport activator and as a glycogen synthesis stimulant [69]. It was also revealed that taraxerol could reverse the effects of dexamethasone-induced insulin resistance back to its normal homoeostasis state. These findings were supported by Gururaja et al. (2015) who claimed that taraxerol is one of the active compounds that shows inhibitory activities against cholesterol esterase enzyme [2]. The antidiabetic properties of taraxerol were mostly attributed to its high affinity towards proteins involved in glucose metabolism [58].
3.6. Anti-Inflammatory Properties
Perhaps the most potent pharmacological properties actively shown by taraxerol is as an anti-inflammatory agent. Singh et al. (2002) investigated the anti-inflammatory activity of taraxerol on carrageenan-induced paw edema on rats and found that applying the triterpenoid extract at a dosage of 20 mg/kg led to edema reduction by 49.66% after 7 h [60]. Naik et al. (2004) further uncovered the anti-inflammatory effects of taraxerol on TPA-induced local inflammation in Swiss Albino mice, in which development of ear edema in rat model was suppressed following its application. A dosage of 1 mg/ear showed the best suppressive effects with a 25.7 mm difference in ear thickness 4 h following the injection [15]. Apart from paw and ear inflammation, taraxerol was also found to be beneficial in inflammatory pulmonary diseases. By directly acting on airway epithelial cells, taraxerol regulates the expression of the Muc5a gene in the cells, thus regulating mucus production in the inflamed airway [31].
Other than in the treatment of edema, taraxerol’s neuroinflammation amelioration effect has also been known. Tsao et al. (2008) examined the effect of taraxerol on the production of nitric oxide (NO) and reactive oxygen species (ROS) by activated microglial cells, which play a number of deleterious roles in central nervous system mediation [8]. The NO and ROS are produced by activated microglial cells through the induction of NADPH oxidase (NOX) and nitric oxide synthase (NOS), which was noted to have been inhibited by 11.6% at 50 µM concentration and 50% at 24.2 µM concentration, respectively. The mechanism through which taraxerol functions as an anti-inflammatory agent was further elucidated by Yao et al. (2013) who showed that taraxerol downregulates the expression of proinflammatory mediators in macrophages through the interference of TAK1 and Akt protein activation, thus preventing NF-κB activation from producing various proinflammatory mediators through a cascade effect [70]. Cellular redox reactions have a critical role in the regulation of immune response, which directly suggested that taraxerol could also mediate inflammatory responses [70].
3.7. Treatment for Neurodegenerative Diseases
Taraxerol has also been extensively known for its potential in treating neurodegenerative diseases. Cholinesterase enzymes were targeted by various target compounds in drug development to find possible treatments for neurodegenerative diseases, particularly Alzheimer’s [71]. Lee et al. (2004) found the potential of taraxerol by inhibiting acetylcholinesterase (AChE) activity in a dose-dependent manner, with an IC50 value of 33.6 µg/mL [72]. This finding was supported by Jamila et al. (2014) in which taraxerol could not exercise its inhibitory effects at concentrations higher than 33.6 µg/mL [58]. Nevertheless, at 50 µg/mL, taraxerol exhibited inhibitory effects on butyrylcholinesterase (BChE) with 98.4% inhibition [58]. The IC50 of taraxerol against BChE was found to be at 17.8 µM.
Furthermore, taraxerol displayed high binding affinity to the monomers and mature fibrils of amyloid peptides, which are critical proteins associated with neurodegenerative disorders [71]. Taraxerol can completely assimilate into the human body and cross the blood-brain barrier, which are the two prerequisites for the development of a potent neurodegenerative drug [73]. In silico analysis of taraxerol affinity towards acetylcholinesterase A and B revealed high affinity towards both enzymes through the formation of hydrogen bonds [58]. This might explain the ability of taraxerol to compete for the active site of acetylcholinesterase, thereby exhibiting potential as a treatment for neurodegenerative diseases.
References
- Koay, Y.C.; Wong, K.C.; Hasnah, O.; Ibrahim, E.M.S.; Mohammad, Z.A. Chemical constituents and biological activities of Strobilanthes crispus L. Rec. Nat. Prod. 2013, 7, 59–64.
- Gururaja, G.; Mundkinajeddu, D.; Dethe, S.; Sangli, G.; Abhilash, K.; Agarwal, A. Cholesterol Esterase Inhibitory Activity of Bioactives from Leaves of Mangifera indica L. Pharmacogn. Res. 2015, 7, 355.
- Du, J.; Gao, L. Chemical constituents of the leaves of Acanthopanax trifoliatus (Linn) Merr. Zhongguo Zhong Yao Za Zhi 1992, 17, 356–357.
- Phan, M.G.; Chinh Truong, T.T.; Phan, T.S.; Matsunami, K.; Otsuka, H. Mangiferonic Acid, 22-Hydroxyhopan-3-One, and Physcion as Specific Chemical Markers for Alnus nepalensis. Biochem. Syst. Ecol. 2010, 38, 1065–1068.
- Correia Da Silva, T.B.; Souza, V.K.T.; Da Silva, A.P.F.; Lyra Lemos, R.P.; Conserva, L.M. Determination of the Phenolic Content and Antioxidant Potential of Crude Extracts and Isolated Compounds from Leaves of Cordia multispicata and Tournefortia bicolor. Pharm. Biol. 2009, 48, 63–69.
- Pinto, N.d.C.C.; Machado, D.C.; da Silva, J.M.; Conegundes, J.L.M.; Gualberto, A.C.M.; Gameiro, J.; Moreira Chedier, L.; Castañon, M.C.M.N.; Scio, E. Pereskia aculeata Miller Leaves Present In Vivo Topical Anti-Inflammatory Activity in Models of Acute and Chronic Dermatitis. J. Ethnopharmacol. 2015, 173, 330–337.
- Mokoka, T.A.; McGaw, L.J.; Mdee, L.K.; Bagla, V.P.; Iwalewa, E.O.; Eloff, J.N. Antimicrobial Activity and Cytotoxicity of Triterpenes Isolated from Leaves of Maytenus undata (Celastraceae). BMC Complement. Altern. Med. 2013, 13, 111.
- Tsao, C.-C.; Shen, Y.-C.; Su, C.-R.; Li, C.-Y.; Liou, M.-J.; Dung, N.-X.; Wu, T.-S. New Diterpenoids and the Bioactivity of Erythrophleum fordii. Bioorg. Med. Chem. 2008, 16, 9867–9870.
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Jiménez-Estrada, M.; Cruz-Ortega, R.; Anaya, A.L. Pentacyclic Triterpenes with Selective Bioactivity from Sebastiania adenophora Leaves, Euphorbiaceae. J. Chem. Ecol. 2006, 33, 147–156.
- Falodun, A.; Qadir, M.I.; Chouldary, M.I. Isolation and characterization of xanthine oxidase inhibitory constituents of Pyrenacantha staudtii. Yao Xue Xue Bao 2009, 44, 390–394.
- Hu, H.; Liu, Q.; Yang, Y.; Yang, L.; Wang, Z. Chemical constituents of Clerodendrum trichotomum Leaves. Zhong Yao Cai 2014, 37, 1590–1593.
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Sahu, R.; Gangopadhyay, M.; De Feo, V.; Zia-Ul-Haq, M. Abroma Augusta L. (Malvaceae) Leaf Extract Attenuates Diabetes Induced Nephropathy and Cardiomyopathy via Inhibition of Oxidative Stress and Inflammatory Response. J. Transl. Med. 2015, 13, 6.
- Wang, J.; Li, Y.; Liu, D. Cloning and Characterization of Farnesyl Diphosphate Synthase Gene Involved in Triterpenoids Biosynthesis from Poria cocos. Int. J. Mol. Sci. 2014, 15, 22188–22202.
- Cao, S.; Brodie, P.; Miller, J.S.; Birkinshaw, C.; Rakotondrafara, A.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Antiproliferative Compounds of Helmiopsis sphaerocarpa from the Madagascar Rainforest. Nat. Prod. Res. 2009, 23, 638–643.
- Naik, D.G.; Mujumdar, A.M.; Waghole, R.J.; Misar, A.V.; Bligh, S.W.; Bashall, A.; Crowder, J. Taraxer-14-En-3β-Ol, an Anti-Inflammatory Compound from Sterculia foetida L. Planta Med. 2004, 70, 68–69.
- Manguro, L.O.A.; Onyango Okwiri, S.; Lemmen, P. Oleanane-Type Triterpenes of Embelia schimperi Leaves. Phytochemistry 2006, 67, 2641–2650.
- Machado, K.E.; Cechinel Filho, V.; Cruz, R.C.B.; Meyre-Silva, C.; Cruz, A.B. Antifungal activity of Eugenia umbelliflora against dermatophytes. Nat. Prod. Commun. 2009, 4, 1181–1184.
- Raja Naika, H.; Krishna, V.; Lingaraju, K.; Chandramohan, V.; Dammalli, M.; Navya, P.N.; Suresh, D. Molecular Docking and Dynamic Studies of Bioactive Compounds from Naravelia zeylanica (L.) DC against Glycogen Synthase Kinase-3β Protein. J. Taibah Univ. Sci. 2015, 9, 41–49.
- Yang, X.; Li, H.; Chen, H.; Li, P.; Ye, B. Chemical constituents in the leave of Rhizophora stylosa L and their biological activities. Yao Xue Xue Bao 2008, 43, 974–978.
- Williams, L.A.D. Rhizophora mangle (Rhizophoraceae) Triterpenoids with Insecticidal Activity. Naturwissenschaften 1999, 86, 450–452.
- Pensec, F.; Szakiel, A.; Pączkowski, C.; Woźniak, A.; Grabarczyk, M.; Bertsch, C.; Fischer, M.J.C.; Chong, J. Characterization of Triterpenoid Profiles and Triterpene Synthase Expression in the Leaves of Eight Vitis vinifera Cultivars Grown in the Upper Rhine Valley. J. Plant Res. 2016, 129, 499–512.
- Kumar, V.; Mukherjee, K.; Kumar, S.; Mal, M.; Mukherjee, P.K. Validation of HPTLC Method for the Analysis of Taraxerol in Clitoria ternatea. Phytochem. Anal. 2007, 19, 244–250.
- Takasaki, M.; Konoshima, T.; Tokuda, H.; Masuda, K.; Arai, Y.; Shiojima, K.; Ageta, H. Anti-Carcinogenic Activity of Taraxacum Plant. I. Biol. Pharm. Bull. 1999, 22, 602–605.
- Okoth, D.A.; Koorbanally, N.A. Cardanols, Long Chain Cyclohexenones and Cyclohexenols from Lannea schimperi (Anacardiaceae). Nat. Prod. Commun. 2015, 10, 1934578X1501000.
- Padmaja, V.; Thankamany, V.; Hisham, A. Antibacterial, Antifungal and Anthelmintic Activities of Root Barks of Uvaria hookeri and Uvaria narum. J. Ethnopharmacol. 1993, 40, 181–186.
- Chen, Y.; Tao, S.; Zeng, F.; Xie, L.; Shen, Z. Antinociceptive and Anti-Inflammatory Activities of Schefflera octophylla Extracts. J. Ethnopharmacol. 2015, 171, 42–50.
- Rashid, M.-U.; Alamzeb, M.; Ali, S.; Ahmad Khan, A.; Igoli, J.O.; Ferro, V.A.; Gray, A.I.; Rafiullah Khan, M. A new ceramide along with eight known compounds from the roots of Artemisia incisa pamp. Rec. Nat. Prod. 2014, 9, 294–304.
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Vasas, A.; Kele, Z.; Hohmann, J. Antiproliferative Constituents of the Roots of Conyza canadensis. Planta Med. 2011, 77, 1183–1188.
- Yang, S.-M.; Liu, X.-K.; Qing, C.; Wu, D.-G.; Zhu, D.-Y. Chemical constituents from the roots of Homonoia riparia. Yao Xue Xue Bao 2007, 42, 292–296.
- Duan, J.; Wang, L.; Qian, S.; Su, S.; Tang, Y. A New Cytotoxic Prenylated Dihydrobenzofuran Derivative and Other Chemical Constituents from the Rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969.
- Yoon, Y.P.; Lee, H.J.; Lee, D.-U.; Lee, S.K.; Hong, J.-H.; Lee, C.J. Effects of Lupenone, Lupeol, and Taraxerol Derived From Adenophora triphyllaon the Gene Expression and Production of Airway MUC5AC Mucin. Tuberc. Respir. Dis. 2015, 78, 210.
- Li, Y.; Yang, X.-W. Studies on chemical constituents of root tuber of cultivated Pseudostellaria heterophylla (Zheshen No. 1). Zhongguo Zhong Yao Za Zhi 2008, 33, 2353–2355.
- Xiang, Y.; Zhang, C.; Zheng, Y. Studies on the chemical constituents of the roots of Rhododendron molle G. Don. J. Huazhong Univ. Sci. Technol. 2004, 24, 202–204.
- Liu, Y.-M.; Tian, D.; Bao, H.; Zhao, G.-L.; Wang, J.-X. Study on chemical constituents of root bark of Discocleidion rufescens. Zhong Yao Cai 2012, 35, 1795–1798.
- Kaennakam, S.; Sichaem, J.; Khumkratok, S.; Siripong, P.; Tip-pyang, S. A New Taraxerol Derivative from the Roots of Microcos tomentosa. Nat. Prod. Commun. 2013, 8, 1934578X1300801.
- Li, S.; Shi, Y.; Shang, X.-Y.; Cui, B.-S.; Yuan, Y.; Chen, X.-G.; Yang, Y.-C.; Shi, J.-G. Triterpenoids from the Roots of Pterospermum heterophyllum Hance. J. Asian Nat. Prod. Res. 2009, 11, 652–657.
- Ango, P.Y.; Kapche, D.W.F.G.; Fotso, G.W.; Fozing, C.D.; Yeboah, E.M.O.; Mapitse, R.; Demirtas, I.; Ngadjui, B.T.; Yeboah, S.O. Thonningiiflavanonol A and Thonningiiflavanonol B, Two Novel Flavonoids, and Other Constituents of Ficus thonningii Blume (Moraceae). Z. Naturforsch. 2016, 71, 65–71.
- Paul, B.D.; Subba Rao, G.; Kapadia, G.J. Isolation of Myricadiol, Myricitrin, Taraxerol, and Taraxerone from Myrica cerifera L. Root Bark. J. Pharm. Sci. 1974, 63, 958–959.
- Jin, W.; Cai, X.F.; Na, M.; Lee, J.J.; Bae, K. Triterpenoids and Diarylheptanoids from Alnus hirsuta Inhibit HIF-1 in Ags Cells. Arch. Pharm. Res. 2007, 30, 412–418.
- Jiang, J.; Li, Y.; Chen, Z.; Min, Z.; Lou, F. Two Novel C29-5β-Sterols from the Stems of Opuntia dillenii. Steroids 2006, 71, 1073–1077.
- Al Muqarrabun, L.M.R.; Ahmat, N.; Aris, S.R.S.; Norizan, N.; Shamsulrijal, N.; Yusof, F.Z.M.; Suratman, M.N.; Yusof, M.I.M.; Salim, F. A New Triterpenoid from Sapium baccatum (Euphorbiaceae). Former. Nat. Prod. Res. 2014, 28, 1003–1009.
- Ragasa, C.Y.; Cornelio, K.B. Triterpenes from Euphorbia hirta and Their Cytotoxicity. Chin. J. Nat. Med. 2013, 11, 528–533.
- Mawa, S.; Jantan, I.; Husain, K. Isolation of Terpenoids from the Stem of Ficus aurantiaca Griff and Their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils. Molecules 2016, 21, 9.
- Somwong, P.; Suttisri, R.; Buakeaw, A. New Sesquiterpenes and Phenolic Compound from Ficus foveolata. Fitoterapia 2013, 85, 1–7.
- Lin, L.-C.; Chou, C.-J.; Kuo, Y.-C. Cytotoxic Principles from Ventilago leiocarpa. J. Nat. Prod. 2001, 64, 674–676.
- Swain, S.S.; Rout, K.K.; Chand, P.K. Production of Triterpenoid Anti-Cancer Compound Taraxerol in Agrobacterium-Transformed Root Cultures of Butterfly Pea (Clitoria ternatea L.). Appl. Biochem. Biotechnol. 2012, 168, 487–503.
- Holstein, S.A.; Hohl, R.J. Isoprenoids: Remarkable Diversity of Form and Function. Lipids 2004, 39, 293–309.
- Miziorko, H.M. Enzymes of the Mevalonate Pathway of Isoprenoid Biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143.
- Delourme, D.; Lacroute, F.; Karst, F. Cloning of an Arabidopsis thaliana CDNA Coding for Farnesyl Diphosphate Synthase by Functional Complementation in Yeast. Plant Mol. Biol. 1994, 26, 1867–1873.
- Dewar, M.J.S.; Ruiz, J.M. Mechanism of the Biosynthesis of Squalene from Farnesyl Pyrophosphate. Tetrahedron 1987, 43, 2661–2674.
- Tansey, T. Structure and Regulation of Mammalian Squalene Synthase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1529, 49–62.
- Abe, I. Enzymatic Synthesis of Cyclic Triterpenes. Nat. Prod. Rep. 2007, 24, 1311.
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic Cyclization of Squalene and Oxidosqualene to Sterols and Triterpenes. Chem. Rev. 1993, 93, 2189–2206.
- Wang, Z.; Yeats, T.; Han, H.; Jetter, R. Cloning and Characterization of Oxidosqualene Cyclases from Kalanchoe daigremontiana. J. Biol. Chem. 2010, 285, 29703–29712.
- Basyuni, M.; Oku, H.; Tsujimoto, E.; Kinjo, K.; Baba, S.; Takara, K. Triterpene Synthases from the Okinawan Mangrove Tribe, Rhizophoraceae. FEBS J. 2007, 274, 5028–5042.
- Min, B.-S.; Na, M.-K.; Oh, S.-R.; Ahn, K.-S.; Jeong, G.-S.; Li, G.; Lee, S.-K.; Joung, H.; Lee, H.-K. New Furofuran and Butyrolactone Lignans with Antioxidant Activity from the Stem Bark of Styrax japonica. J. Nat. Prod. 2004, 67, 1980–1984.
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; de Koning, C.B. A New Cinnamoylglycoflavonoid, Antimycobacterial and Antioxidant Constituents from Heritiera littoralis leaf Extracts. Nat. Prod. Res. 2014, 28, 351–358.
- Jamila, N.; Khairuddean, M.; Yeong, K.K.; Osman, H.; Murugaiyah, V. Cholinesterase Inhibitory Triterpenoids from the Bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2014, 30, 133–139.
- Beaton, J.M.; Spring, F.S.; Stevenson, R.; Stewart, J.L. Triterpenoids. Part XXXVII. The Constitution of Taraxerol. J. Chem. Soc. (Resumed) 1955, 2131–2137.
- Singh, B.; Sahu, P.M.; Sharma, M.K. Anti-Inflammatory and Antimicrobial Activities of Triterpenoids from Strobilanthes Callosus Nees. Phytomedicine 2002, 9, 355–359.
- Hernández-Chávez, I.; Torres-Tapia, L.W.; Simá-Polanco, P.; Cedillo-Rivera, R.; Moo-Puc, R.; Peraza-Sánchez, S.R. Antigiardial Activity of Cupania dentata Bark and Its Constituents. J. Mex. Chem. Soc. 2017, 56, 105–108.
- Simelane, M.; Shonhai, A.; Shode, F.; Smith, P.; Singh, M.; Opoku, A. Anti-Plasmodial Activity of Some Zulu Medicinal Plants and of Some Triterpenes Isolated from Them. Molecules 2013, 18, 12313–12323.
- Warfield, J.; Setzer, W.N.; Ogungbe, I.V. Interactions of Antiparasitic Sterols with Sterol 14α-Demethylase (CYP51) of Human Pathogens. SpringerPlus 2014, 3, 679.
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Rios, M.Y. Active Compounds against Tinea Pedis Dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565.
- Flores, F.C.; Beck, R.C.R.; da Silva, C.d.B. Essential Oils for Treatment for Onychomycosis: A Mini-Review. Mycopathologia 2015, 181, 9–15.
- Chaturvedula, V.S.P.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New Cytotoxic Terpenoids from the Wood of Vepris punctata from the Madagascar Rainforest. J. Nat. Prod. 2004, 67, 895–898.
- Tan, B.; Shi, H.-L.; Ji, G.; Xie, J.Q. Effects of Taraxerol and Taraxeryl Acetate on Cell Cycle and Apoptosis of Human Gastric Epithelial Cell Line AGS. Chin. J. Integr. Med. 2011, 9, 638–642.
- Kwon, J.-H.; Chang, M.-J.; Seo, H.-W.; Lee, J.-H.; Min, B.-S.; Na, M.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Lee, H.K.; et al. Triterpenoids and a Sterol from the Stem-Bark of Styrax japonicaand Their Protein Tyrosine Phosphatase 1B Inhibitory Activities. Phytother. Res. 2008, 22, 1303–1306.
- Sangeetha, K.N.; Sujatha, S.; Muthusamy, V.S.; Anand, S.; Nithya, N.; Velmurugan, D.; Balakrishnan, A.; Lakshmi, B.S. 3β-Taraxerol of Mangifera Indica, a PI3K Dependent Dual Activator of Glucose Transport and Glycogen Synthesis in 3T3-L1 Adipocytes. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 359–366.
- Yao, X.; Li, G.; Bai, Q.; Xu, H.; Lü, C. Taraxerol Inhibits LPS-Induced Inflammatory Responses through Suppression of TAK1 and Akt Activation. Int. Immunopharmacol. 2013, 15, 316–324.
- Godyń, J.; Jończyk, J.; Panek, D.; Malawska, B. Therapeutic Strategies for Alzheimer’s Disease in Clinical Trials. Pharmacol. Rep. 2016, 68, 127–138.
- Lee, J.H.; Lee, K.T.; Yang, J.H.; Baek, N.I.; Kim, D.K. Acetylcholinesterase Inhibitors from the Twigs of Vaccinium Oldhami Miquel. Arch. Pharm. Res. 2004, 27, 53–56.
- Ngo, S.T.; Li, M.S. Top-Leads from Natural Products for Treatment of Alzheimer’s Disease: Docking and Molecular Dynamics Study. Mol. Simul. 2013, 39, 279–291.
More
