Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Aref Zayed.
Steroids are compounds widely available in nature and synthesized for therapeutic and medical purposes. Steroids can be found in various environmental samples, including water, plant, and animal samples as well as in a variety of pharmaceutical forms. Due its sensitivity and selectivity, high performance liquid chromatography with fluorescence detection (HPLC-FLD) is widely used for detection of steroids in pharmaceutical and environmental samples.
- steroids
- Environmental
- Pharmaceutical
- high performance liquid chromatography
- fluorescence
- quantification
1. Pharmaceutical Applications
Due to its simplicity and versatility, HPLC has become a cornerstone tool in pharmaceutical and biomedical analysis. HPLC is routinely used in drug discovery, development, and manufacturing, and routine assessment for the identification and quantification of drugs, both as active pharmaceutical ingredients and within their formulations [106][1]. In addition, it is essential for carrying out product characterizations, including assaying active pharmaceutical ingredients and profiling impurities [107][2], as well as degradation products generated by accelerated aging [108][3]. Moreover, the development of formulations requires studying dissolution properties, stability, and content uniformity of solid dosage forms, as well as conducting assays for the pharmaceutical formulations all of which are carried out using HPLC [106,109][1][4]. Although UV is the common detector for these applications, FLD was employed in several assays particularly in steroid analysis due to its sensitivity and selectivity as discussed below.
One area employing HPLC-FLD for steroid detection is investigating their pharmacokinetic–pharmacodynamic (PK–PD) properties. Vesser et al. [69][5] described HPLC-FLD method for quantification of the neurosteroids alfaxalone and pregnanolone in plasma samples (Figure 16). The method involved sample pretreatment with acetonitrile to precipitate plasma proteins, derivatization with dansyl hydrazine, and LLE with dichloromethane. Analysis was done using isocratic reverse phase HPLC on a 3 µM Microsphere C18 column. The limit of detection using 50 µL plasma sample was 10 ng/mL.
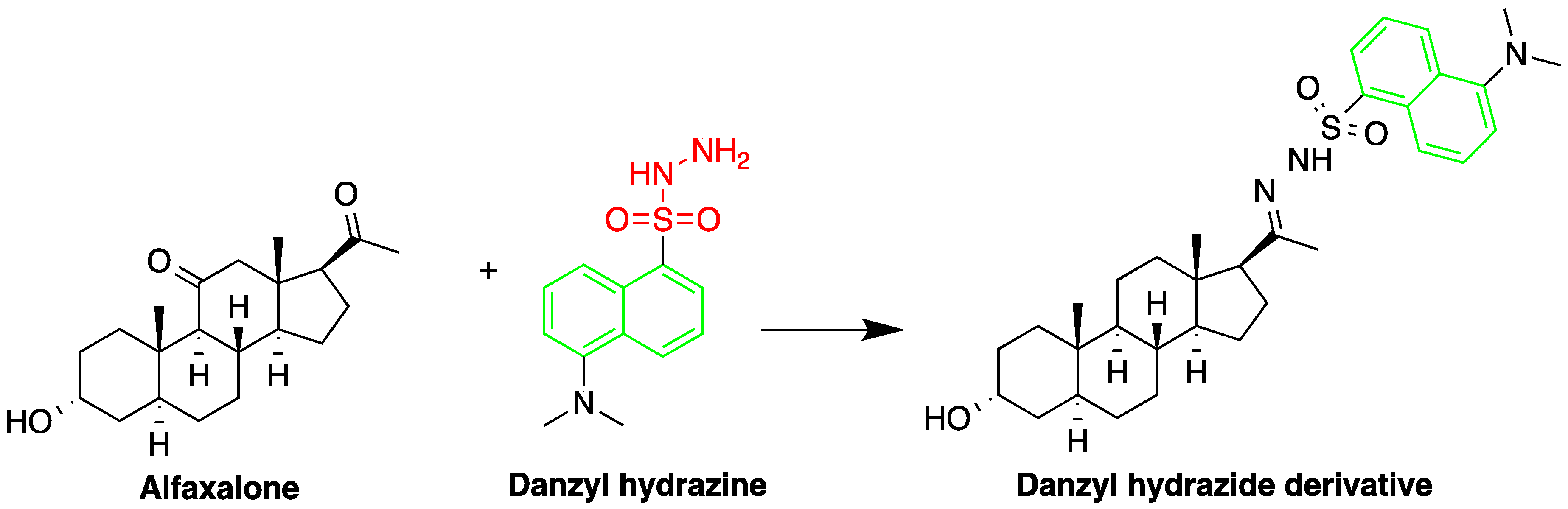
Figure 16.
Derivatization of alfaxalone by dansyl hydrazine.
Butane acid-(5-androsten-17-one-3ß-ol)-diester (A1998), a novel dehydroepiandrosterone (DHEA) derivative antiarrhythmic steroid was successfully quantified by HPLC-FLD. Although LC–MS has the required sensitivity for quantifying low levels of steroids, A1998 is not ionizable and hard to aerosolize and therefore was not suitable for MS detection. HPLC-FLD was therefore ideal to study its PK–PD properties in rat plasma. A mixture of ZnSO4 solution and acetonitrile were used for sample preparation to precipitate sample plasma proteins [70][6] and dansyl hydrazine was used as a derivatizing agent using trifluoroacetic acid (TFA) as a catalyst to enhance the yield. A C18 reverse-phase column (150 mm × 4.6 mm, 5 mm) was used for the analysis and the limit of detection was 25 ng/mL (S/N = 10) using 200 μL of plasma.
Another area employing HPLC-FLD for detection of steroids is for development and characterization of drug formulation. In a recent study HPLC-FLD was used to characterize polypeptide-corticosteroid conjugates as a topical treatment approach to treat psoriasis [110][7]. The study successfully synthesized and characterized a pH-responsive biodegradable poly-L-glutamic acid-fluocinolone acetonide conjugate as a controlled release treatment to reduce skin inflammation. The method employed C-18 LiChrospher analytical column (125 × 4.0 mm), with a flow rate of 1 mL/min, the using a mobile phase of H2O (1% orthophosphoric acid)/ACN (60/40) [110][7].
Due to the low administered dose of ethinyl estradiol, which can be as low as 10 µg/mL, methods employ derivatization or require composite of samples of up to 10 tablets to improve the quantification limits in performing uniformity and dissolution tests [26,111][8][9]. Nonetheless, ethinyl estradiol is a native fluorescent, and it is therefore simpler to measure using direct methods. Strusiak et al. used reversed phase HPLC-FLD with a mobile phase of 0.05 M aqueous KH2PO4-methyl alcohol (2:3) [112][10] for measuring ethinyl estradiol in tablets. The method required minimal sample preparation and can be applied to measure ethinyl estradiol in combination with other compounds, such as methyl testosterone and progesterone.
Glowka et al. measured triamcinolone (TMC) in the presence of endogenous corticosteroids in human plasma, after its administration as tablets to healthy volunteers [88][11]. The samples were subjected to SPE before derivatization with 9-anthroyl nitrile (9-AN) in a basic mixture. An isocratic RP-HPLC was performed using a C18 column and a mixture of acetonitrile and 0.3 mM ortho-phosphoric acid as the mobile phase. The method recorded LOD of 1.0 ng/mL (S/N = 6/1).
The use of HPLC-FLD for the analysis of commercial estrogens using post-column online photochemical derivatization was investigated by Gatti et al. [90][12]. Separation of conjugated and unconjugated estrogens were optimized using a Phenomenex Prodigy column 5 ODS2, and a mobile phase consisting of a TEA phosphate buffer (pH 4.0; 0.05 M) and acetonitrile at different concentrations and flow rates. The addition of photochemical derivatization to the HPLC-FLD method recorded a 1000 times higher sensitivity than UV. The LODs of conjugated estrogens were between 0.03 and 0.19 pmol (S/N = 3). This method provided useful information regarding quality of estrogen in raw materials and conjugated estrogens in dosage forms.
HPLC coupled with UV/FLD was used by Arsova-Sarafinovska et al. for determination of ethinylestradiol and levonorgestrel, present at very low levels in a low-dose oral contraceptive. The method employed Purospher® reversed phase column (150 × 4.0 mm I.D., particle size 5 μm) and a 47%: 53% acetonitrile: water (v/v). Drospirenone, was used as internal standard. FLD showed excellent detection limit for ethinyl estradiol (0.65 ng/mL) which was 83 times lower compared to that produced by UV [12][13].
Similarly, Silva et al. used HPLC coupled with UV/FLD to quantify ethinyl estradiol and the synthetic progestin drospirenone in coated tablets [73][14]. The method conditions were comparable to that developed by Arsova-Sarafinovska et al. but reported higher detection limits using FLD (20 ng/mL) [89][15]. The method was fully validated and was found suitable for routine use in quality control laboratories.
2. Environmental and Food Applications
Steroids can be found in various environmental samples, including water, plant, and animal samples. Water is the transporter medium and reservoir for various synthetic steroids, whereas plants were found to contain many different natural steroids [17[16][17][18][19][20],52,93,94,95], which can be classified according to their biological relevance. For example, plant physiological steroids can be present as hormones (e.g., brassinolide) and pheromones (e.g., antheridiol), whereas plant allelochemical substances are biologically related to animal hormones such as vertebrate hormones (progesterone) and insect hormones (ecdysone). Other plant steroids can act as protective steroids (e.g., digitoxigenin, solanidine) [51,99,113][21][22][23].
Exogenous estrogens can enter the human body through sewage discharge and animal waste disposal. It has been reported that the discharge of industrial and municipal wastewater can introduce up to 60% of the manufactured surfactant alkylphenol ethoxylate (APE) [42][24]. APE can degrade to more toxic products by sewage treatment plants (STP), such as nonylphenol (NP), octylphenol (OP), and their metabolites [28][25], which can cause toxicity at a low concentration range (3.7–6.0 μg/L). Sample preparation procedures and analytical methods used for analysis of steroid hormones in environmental and food samples have been recently reviewed[114][26].
Many studies used HPLC-FLD for the analysis of steroids in environmental samples due to its sensitivity and selectivity. Studies investigated the steroid content in samples collected from drinking water, wastewater, river water, tap surface water, and mineral and underground river water [17,20,37,52,92,93,94,95][16][27][28][17][29][18][19][20]. Steroids were also analyzed in samples taken from effluent, influent, and sediment wastewater in treatment plant [41][30]. Other studies have collected samples from fish, chicken, cows, river buffalos, and measured their steroid content [31,73,95][31][14][20].
Lu et al. have analyzed poultry litters samples to detect estrogens 17β-estradiol (E2) and 17α-ethinylestradiol (EE2). The samples were extracted with a mixture of dichloromethane and methanol followed by a clean-up procedure using normal-phase open column chromatography to remove lipid contents. The detection limits for E2 and EE2 were 4.0 μg/kg and 2.6 μg/kg, respectively [91][32].
In another study, a method was developed to detect four endocrine disruptors: 17-estradiol, estriol, bisphenol A, and 17-ethinylestradiol in environmental water using online monolithic SPME with HPLC-FLD [96][33]. The speed of the analysis was improved using the extraction medium, poly(acrylamide-vinylpyridine-N,N methylene bisacrylamide) monolith, which was synthesized inside a polyether ether ketone (PEEK) tube. The method was applied successfully to the analysis of several types of environmental water samples. Low detection limits in the range of 0.006–0.10 ng/mL (S/N = 3) were achieved for the target compounds.
Another method based on SPME monolithic extraction media coupled with HPLC-FLD was also employed by Fan et al. to determine bisphenol A and 17α-ethinylestradiol in environmental water samples. The method achieved high extraction efficiency and produced a detection limit of 0.064 and 0.12 ng/mL for bisphenol A and 17α-ethinylestradiol, respectively. The method was applied successfully to the analysis of environmental water samples from different sources demonstrating the robustness of the method in real settings [98][34].
Online extraction was also employed by another group using SPE with HPLC-FLD for the quantification of selected EDCs in water. Ying et al. tested three types of SPE cartridges to preconcentrate nonylphenols, octylphenols, POE(1-2) nonyl phenol and bisphenol A as well as hormone steroids estradiol, estriol, ethinylestradiol, and ethinylestradiol 3-methyl ether in deionized water. The highest recoveries were obtained with PLRP-s and PRP-1 polymer cartridges compared to C18 cartridges. The method was applied successfully on a river water sample spiked with the EDCs. The detection limits ranged between 20 and 50 ng/L [97][35].
Another recent study investigating river water samples by HPLC-FLD was carried out by Ali et al. [34][36]. The study employed a derivatization procedure, which targets the ethinyl group rather than the phenolic group to react with an aryl halide in the presence of palladium (Pd) and copper catalysts. The fluorescent aryl halide labeling reagent used in this reaction is 4-(4,5-diphenyl-1H-imidazol-2-yl) iodobenzene (DIB-I); was prepared by the same group. The method achieved a low detection limit (S/N = 3) of 7.4 ng/L [34][36].
Zhang et al. determined eight EDCs in wastewater using precolumn derivatization and SPE-HPLC-FLD. In the study 4-octylphenol, 4-nonylphenol, bisphenol A, diethylstilbestrol, estrone, 17α-ethinylestradiol, 17β-estradiol, and estriol were derivatized with 10-ethyl-acridone-2-sulfonyl chloride (EASC). The method was successfully applied to the determination of the EDC compounds in wastewater samples with a significant improvement in sensitivity compared to traditional HPLC methods. The LODs of the method were between 0.3 and 0.7 ng/L [52][17].
Four trace estrogens were determined in different environmental samples using solid–liquid extraction/auto SPE and HPLC-FLD method developed by Liu et al. The LODs of the method were down to 1.1 × 10−2 (estrone), 4.11 × 10−4 (estradiol), 5.2 × 10−3 (estriol), and 7.18 × 10−3 µg/L (17α-ethynyl estradiol)[21][37]. The study that quantified estrogens in samples collected at livestock farms and a major river in Northeast China, warned that high levels were observed and that estrogenic fate and contamination should be investigated in the region [21][37]. The same HPLC-FLD method was employed in two recent studies investigating steroid-degrading strains of bacteria as an approach for tackling increased environmental pollution of steroids [115,116][38][39].
Municipal wastewater treatment plants were sampled to investigate the presence of the steroids nonylphenol (NP), octylphenol (OP), nonylphenol polyethoxylates (NPE), 17α-estradiol (E2), and ethinylestradiol (EE2) [5][40]. SPE disks were used for extraction, followed by HPLC coupled with FLD or competitive radioimmunoassay (RIA). The recorded LODs for HPLC-FLD were 11, 2, and 52 ng/L of water for NP, OP, and NPE, respectively, but were higher than those obtained with HPLC-RIA for E2 and EE2, recorded as 107 and 53 pg/L, respectively [5][40]. However, safety is a major limitation for routine use of RIA.
Lima et al. developed a low-cost fast method using DLLME with HPLC-FLD for quantification of estrogens in water. The method produced high recoveries and detection limits of 2.0 ng/L and 6.5 ng/L for E2 and EE2, respectively. The method that is suitable for analyzing large number of samples and uses low amount of extraction solvent was applied successfully to the analysis of tap, surface, and waste water samples [37][28].
The adsorption of estrogens on different types of powdered activated carbon (PAC) was investigated by Yoon et al. using HPLC-FLD. The study demonstrated the feasibility of using PAC for removal of >99% of BPA, 17 β-estradiol (E2), and 17α-ethynyl estradiol (EE2) from raw drinking waters at least at initial concentration of 500 ng/L and higher [94][19]. PAC type, dosage, and the presence or absence of natural organic matter determined the percentage of steroid removal. The removal was improved by increasing both PAC dose and contact time. The HPLC-FLD method had detection limits of 0.88, 1.15, and 0.96 nM for BPA, E2, and EE2, respectively.
Kozłowska-Tylingo et al. compared the different methods and extraction procedures for the analysis of five estrogens in drinking water and wastewater samples. Extraction of 17α-ethinylestradiol, estrone, estradiol, estriol, and progesterone produced the best recoveries using extraction disks compared to C18 extraction columns. SPE–LC–MS/MS produced the best sensitivities compared to other methods employing UV and FLD [19][41].
Zhang et al. investigated ionic liquid based pretreatment for quantification of three steroids in water samples by HPLC-FLD. The sample pretreatment has the advantages of being safer and environmentally friendly since no organic solvents is used. The method based on ionic liquid-based homogeneous liquid–liquid microextraction (IF-IHLME) produced detection limits of 0.04, 0.05, and 0.05 ng/mL for 17-α-estradiol, 17-β-estradiol-benzoate, and quinestrol, respectively [101][42].
Estrogens and their metabolites were also quantified in a study by Guedes-Alonso et al. using molecularly imprinted (MIP) solid-phase extraction coupled with UPLC-FLD. The method was used to analyze wastewater from a veterinary hospital as well as influent and effluent samples of a wastewater treatment plant. The limits of detection were between 0.18 and 0.45 ng/mL [41][30].
The occurrence of trenbolone acetate metabolites, which is widely used to promote the growth of beef cattle, was evaluated in samples from a beef cattle feedlot discharge and in river water upstream and downstream from the discharge [95][20]. HPLC-FLD using methanol and water as the mobile phase in gradient flow and C18 column with dimensions of 4.6 mm × 250 mm was employed to quantify 17α- and 17β-trenbolone in the samples. The recorded LOD for was around 4 ng/L. The same metabolites were also quantified in bovine muscle, and liver samples by SPE-HPLC-FLD [33][43]. In the study, Yoshioka et al. examined several extraction solvents, such as methanol, diethyl ether, acetonitrile, and ethyl acetate, with the latter resulting in the cleanest extracts [33][43]. LODs in liver and bovine muscle samples were 0.2 and 1.0 ng/g, respectively.
Determination of natural and synthetic estrogenic compounds in dairy products was carried out using hollow fiber liquid-phase microextraction coupled to HPLC-FLD/PDA. Estriol, 17β-estradiol, 17α-estradiol, estrone, 17α-ethinylestradiol, diethylstilbestrol, dienestrol, and hexestrol) and 2-hydroxyestradiol were quantified in natural yogurt, a probiotic yogurt-type drink and cheese. The method produced LODs in the low lg/kg or lg/L range with good precision and accuracy [36][44].
Ultrasonic assisted dispersive liquid–liquid microextraction (UA-DLLME) method coupled with 9-phenanthreneboronic acid derivatization was used for the quantification of brassinolide a plant hormone by HPLC-FLD. Different extraction and degravitation conditions were investigated and optimized. The method was applied for brassinolide determination in Arabidopsis thaliana, Daucus carota and Brassica campestris L. leaves with higher sensitivity than similar reported methods. LOD of the method was 8.0 ng/L [99][22].
A study by Ito et al. used HPLC-FLD method for the quantification of different phytosterols and cholesterol in land plants and marine algae after derivatization with 1-anthroyl nitrile. The method used a C-30 column and an isocratic mobile phase of acetone/acetonitrile/hexane/water (71:20:4:5, v/v) at 1.0 mL min−1. The LODs for the different sterols were between 0.25 and 0.40 μg/mL [117][45].
Zearalenone an estrogenic mycotoxin that contaminates cereal crops was quantified in edible oil by an automated SPE–HPLC-FLD. Dryzmala et al. employed a SPE for reversible hydrazone formation by zearalenone and a hydrazine moiety covalently attached to a solid phase. The results of the HPLC-FLD method were found in agreement with an isotopic dilution LC–MS/MS method. The LOD of the method was 10 µg/kg [100][46].
Alkylphenol polyethoxylates are nonionic surfactants that act as EDCs and can exert estrogenic effects on fish [118,119][47][48]. To assess their contamination in fish and shellfish samples, Tsuda et al. developed a simple and highly sensitive HPLC method [32][49]. The method was used to detect 4-nonylphenol (NP), 4-nonylphenol mono-(NP1EO), diethoxylates (NP2EO), bisphenol A (BPA), 4-tert-butylphenol (BP), and 4-tert-octylphenol (OP). Acetonitrile was used for extraction, followed by clean-up with a magnesia silica gel adsorbent, Florisil PR, before analysis with HPLC-FLD. The mobile phase was water and methanol with gradient flow. The detection limit was 2 ng/g for NP, NP1EO, and NP2EO, and 1 ng/g for BPA, BP, and OP.
Similarly, Datta et al. examined fish tissue for presence of alkylphenolic compounds using an HPLC-FLD method [28][25]. Extraction of ground fish tissue was conducted using pressurized fluid extraction, followed by purification on amino propyl silica cartridges. Hexane and ethanol were used as the mobile phase. The recorded LOD was 4–15 ng/mL.
