The race towards the development of user-friendly, portable, fast-detection, and low-cost devices for healthcare systems has become the focus of effective screening efforts since the pandemic attack in December 2019, which is known as the coronavirus disease 2019 (COVID-19) pandemic. Currently existing techniques such as RT-PCR, antigen–antibody-based detection, and CT scans are prompt solutions for diagnosing infected patients. However, the limitations of currently available indicators have enticed researchers to search for adjunct or additional solutions for COVID-19 diagnosis. Meanwhile, identifying biomarkers or indicators is necessary for understanding the severity of the disease and aids in developing efficient drugs and vaccines. Therefore, clinical studies on infected patients revealed that infection-mediated clinical biomarkers, especially pro-inflammatory cytokines and acute phase proteins, are highly associated with COVID-19. These biomarkers are undermined or overlooked in the context of diagnosis and prognosis evaluation of infected patients.
1. Introduction
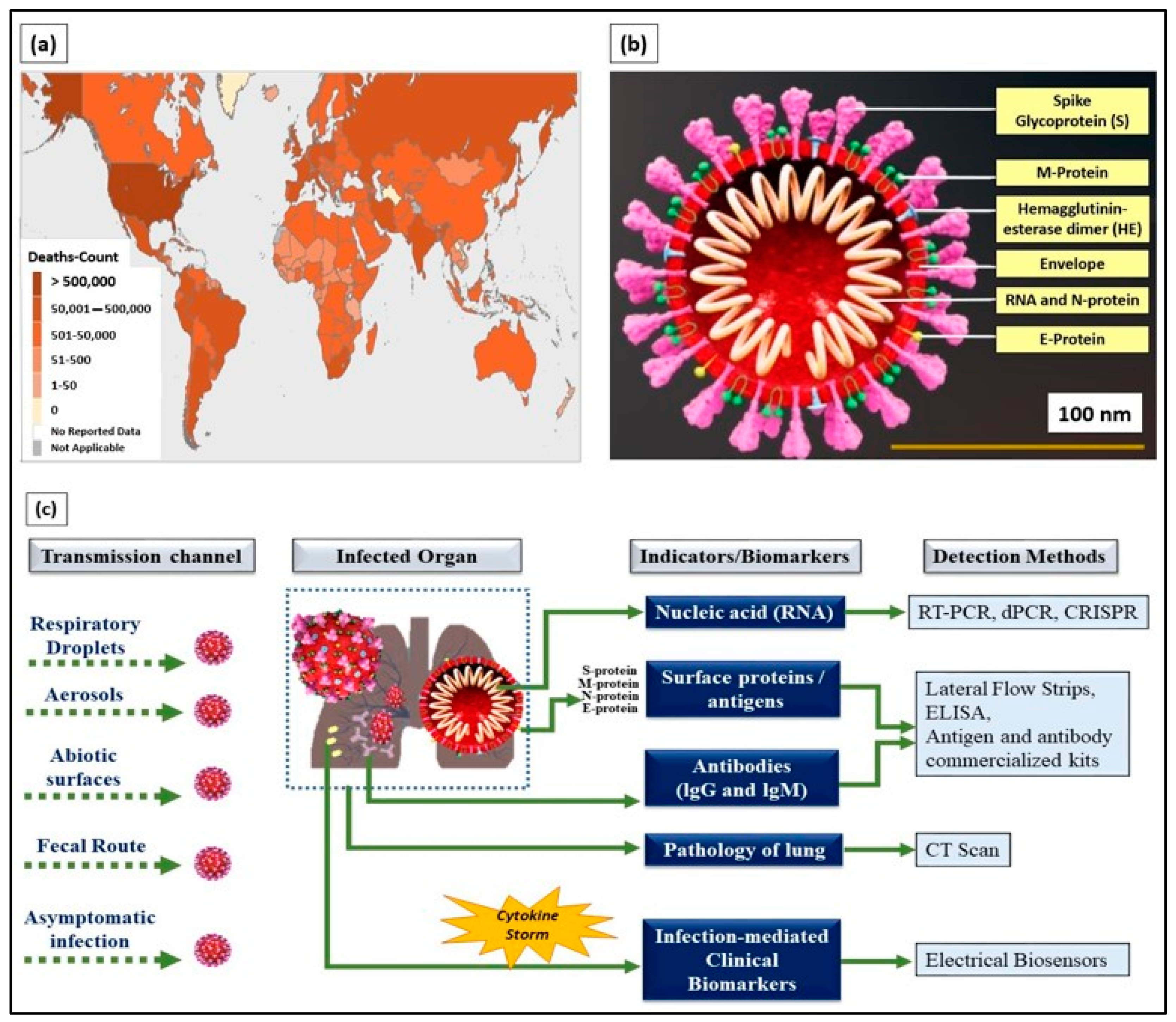
The coronavirus disease 2019 (COVID-19), an infectious disease that caused a global pandemic, originated from the causative virus “SARS-CoV-2”, also known as the coronavirus. With the initial outbreak in Wuhan, China, in November 2019, the World Health Organization (WHO) declared 155,665,214 confirmed cases and 3,250,648 deaths globally as of the 6th of May 2021 (4.43 pm CEST). The detailed death counts are shown in the choropleth map of
Figure 1a. This single-stranded and positive ribonucleic acid (RNA) viral strain originated from the subfamily Orthocoronavirinae
[1]. Its genome has a size range of 26–32 kilobases, which has ~80%, ~50%, and ~96% similarity to the genomes of SARS-CoV, the Middle East respiratory syndrome virus (MERS-CoV), and bat coronavirus RaTG13, respectively
[2].
Figure 1. (a) World Health Organization (WHO) coronavirus disease 2019 (COVID-19) dashboard (choropleth map) indicating death count globally as of the 6th of May 2021 (4.43 pm CEST). (b) Cross-section of SARS-CoV-2 virus structure (picture credits: Scientific Animations/Wikimedia Commons). (c) Current existing indicators and biomarkers with transmission channels and detection methods are illustrated.
Typically, SARS-CoV-2 viral genomic RNA is enveloped by membrane proteins and has embedded spike (S), envelope (E), nucleocapsid (N), and hemagglutinin esterase (HE)
[3] proteins (
Figure 1b). Upon entering the human body through respiratory droplets, aerosols, abiotic surfaces, fecal routes, and asymptomatic transmission
[4], the SARS-CoV-2 virus binds to the alveolar epithelium in the lungs with the help of the angiotensin-converting enzyme-2 (ACE2) host receptor
[5]. This, in turn, activates the immune system
[6] and induces a “cytokine storm” as more immune cells attack the virus.
Infected patients exhibit a wide range of symptoms, including cough, sneeze, and fever, which are commonly observed in most patients. Moreover, a definite number of asymptomatic and pre-symptomatic infected patients have contributed to the bloom of daily cases around the globe. The continuous alteration of the genetic material on SARS-CoV-2 through mutation exacerbates the current condition. The D614G spike-mutated SARS-CoV-2 discovered in July 2020 is 10 times as infectious as its predecessor (D614)
[7]. The emergence of another mutated COVID strain (B.1.1.7) from the United Kingdom (UK) revealed the capability of SARS-CoV-2 to continuously modify its genetic information
[8].
Owing to its deleterious nature, it has enticed researchers, scientists, physicians, virologists, and multidisciplinary experts around the world to collaborate and play their roles in handling the current situation. Currently, the distribution of COVID-19 vaccines from Pfizer, Moderna, SINOVAC, Sputnik V, and the University of Oxford has restored hope worldwide. Amid this release, researchers are working continuously on providing better solutions or finding an endpoint to restore the world into its pre-pandemic conventional state.
Currently, detection methods based on biological indicators, such as ribonucleic acid (RNA), viral proteins (e.g., S, M, N, and E), the response of the body’s immune system (antibody- and antigen-based responses), and clinical imaging (e.g., chest X-rays and CT scans), have been used in diagnosing COVID-19
[9] (
Figure 1c). These methods are the gold standard and provide prompt solutions for diagnosing infected patients. However, laborious RNA extraction protocols, lengthy assays, prolonged time consumption
[10], the need for centralized laboratories and trained laboratory technicians
[11], and alterations, especially mutations in viral RNA sequences, are the key factors limiting the use of nucleic acids as indicators for COVID-19 diagnosis.
Meanwhile, antigen-based detection is only applicable for diagnosis in symptomatic patients with high viral loads compared with patients at the initial stage of infection. Often, it leads to an increasing rate of false negatives in low-viral-load patients, causes super-spreading, and contaminates the environment that had been previously been kept virus free
[12]. Access to CT scan imaging facilities is limited to large centralized hospitals and is limited in terms of specificity toward SARS-CoV-2 infection, as COVID-19 cannot be distinguished from other infections through CT scan
[4]. Therefore, adjunct indicators for detecting pre-symptomatic and symptomatic patients with false negatives in epidemic surroundings should be explored.
Moreover, suitable prognostic indicators are necessary for evaluating the severity of an infection and are vital to the management of an outbreak. In particular, personnel from hospitals and clinical research centers need a solid indicator to retrieve data on the prognosis of COVID-19. Prognostic indicators are essential to the understanding of the nature of SARS-CoV-2 infection, the formulation of strategies for prompt treatment, and the development of effective drugs and vaccines.
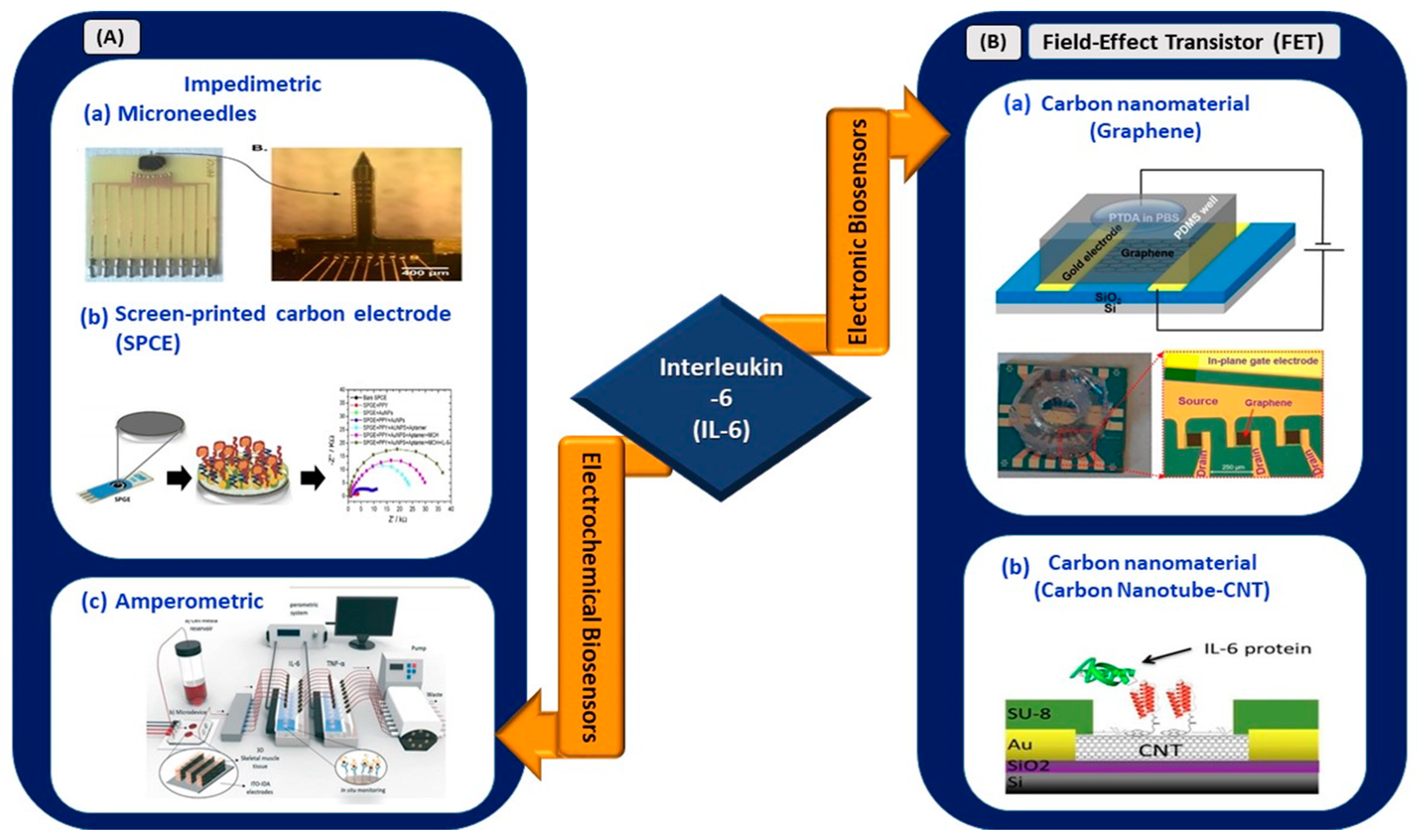
Therefore, infection-mediated clinical biomarkers released by the body’s immune system can be used as prognostic indicators of COVID-19. The elevated concentrations of these biomarkers in infected individuals can be monitored and used in evaluating the severity of the illness. Elevated concentrations of infection-mediated clinical biomarkers provide a better understanding of the nature of the infection and aid in developing efficient vaccines that can support clinical research on handling the current pandemic. In addition, certain biomarkers, especially interleukin-6 (IL-6), serum ferritin, and serum amyloid A (SAA) released during an immune response or “cytokine storm”, can be used as adjunct indicators for COVID-19 diagnosis, particularly for symptomatic patients, but show false negative results in conventional and “gold-standard” detection methods. Pre-symptomatic and symptomatic patients from epidemic surroundings can be diagnosed by monitoring the secretion range of these biomarkers in their bodies.
Therefore, the most promising tools for detecting these infection-mediated clinical biomarkers are biosensors. Biosensors are analytical devices composed of a biorecognition element and a physical transducer that converts a recognition phenomenon into a measurable signal
[13]. Typically, biosensing platforms offer ultra-sensitivity, high selectivity, cost effectiveness, easy fabrication, and a quick turn-around time for the detection of pathogens and biomolecules
[14,15][14][15]. In particular, electrical-based biosensors comprising electrochemical and electronic biosensors allow for direct translation of the conjugation of targets with probes into electrical signals. Hence, a fast response time and easy operation are achieved, paving the way for the application of the biosensors in point-of-care (POC) tests.
The first biosensor for the accurate detection of SARS-CoV-2 was reported by Qiu et al.
[16] in April 2020. The researchers implemented dual-functional plasmonic biosensors by combining the plasmonic photothermal effect and localized surface plasmon resonance for precise SARS-CoV-2 detection, with a limit of detection (LOD) value of 0.22 pM. Seo et al.
[17] reported a graphene-based field-effect transistor (GFET) biosensor for the rapid detection of SARS-CoV-2. The GFET exhibited an LOD value of as low as 1.6 × 101 pfu/mL and 2.42 × 102 copies/mL in the culture medium and clinical samples, respectively.
The biosensors mentioned above mainly detect nucleic acids and surface proteins as biomarkers for COVID-19 diagnosis. However, repurposing the roles of infection-mediated clinical biomarkers released due to “cytokine storm” demonstrated the potential of the biomarkers as prognostic and adjunct diagnostic indicators for COVID-19 infection. Therefore, detecting and monitoring these infection-mediated clinical biomarkers facilitates severity analysis of the illness and provides additional options for diagnosing infected patients.
2. Pathophysiology of SARS-CoV-2 Infection and Clinical Types of Patients
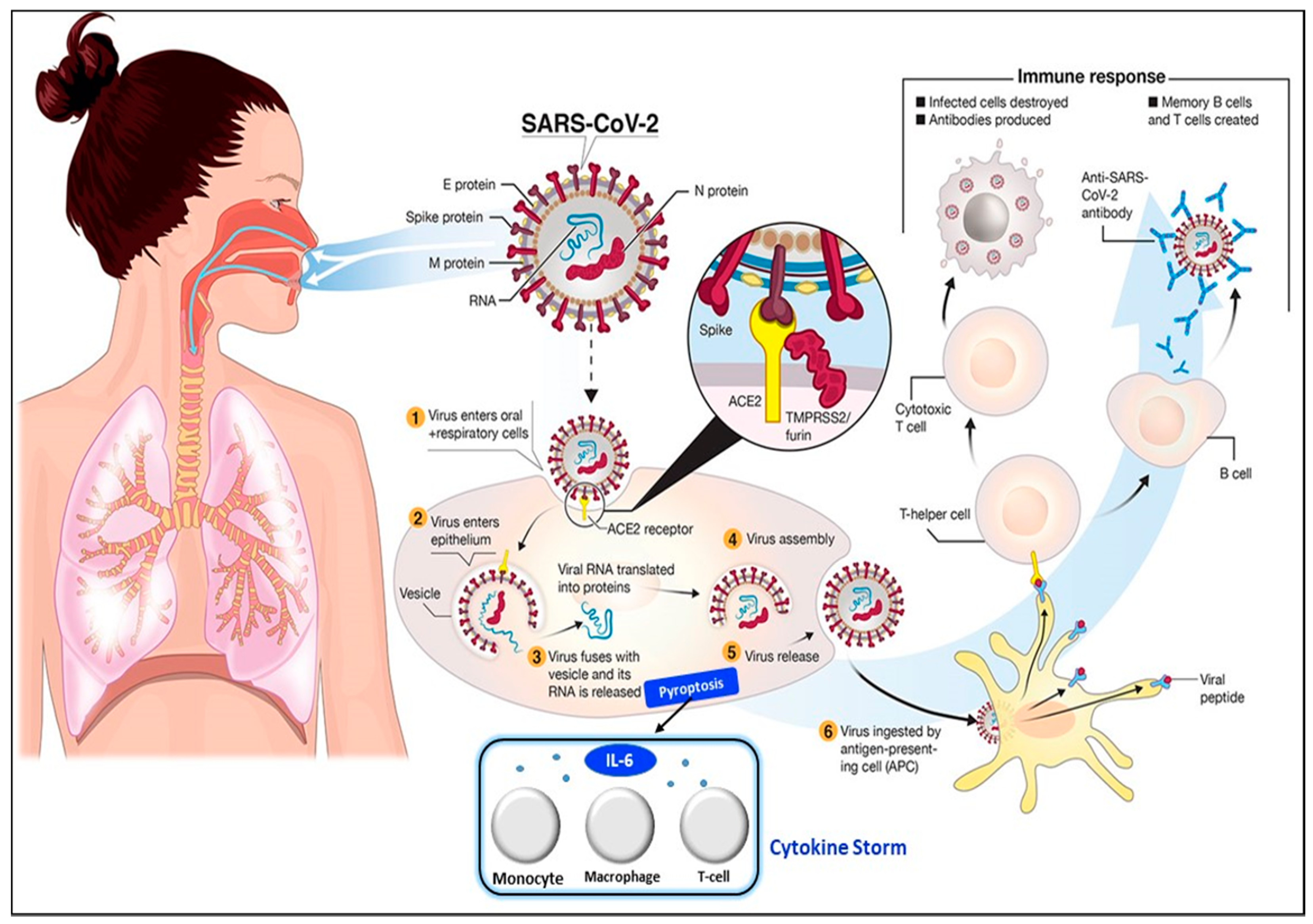
The transmission of SARS-CoV-2 into the human body is facilitated by nasal secretions; abiotic surfaces, such as air, water, and atmosphere; feces, and asymptomatic carriers
[4]. Upon invading the body, the causative virus binds to angiotensin-converting enzyme-related carboxypeptidase (ACE2) receptors, which are widely present in cardiopulmonary tissues, and then enters the cells
[18]. The presence of a pathogen activates the immune system (e.g., innate and adaptive immunity)
[19], inducing the release of a large quantity of pro-inflammatory cytokines in a process known as the “cytokine storm”
[20]. The cytokine storm starts with the release of IL-6 upon infection with SARS-CoV-2. Then, other cytokines and acute phase proteins, such as vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), interleukin-2R, procalcitonin, and serum ferritin, are released in the blood vessels
[21], while SAA and C-reactive protein (CRP) are released in the liver
[2,18][2][18]. The prolonged inflammatory response of the body leads to acute respiratory distress syndrome (ARD), which results in severe lung injury, decreased level of oxygen saturation, multiple organ failure, and even death
[22[22][23],
23], which are evident in the current pandemic. Hence, the excessive production of pro-inflammatory cytokines in SARS-CoV-2 infection is one of the major factors contributing to the increasing rate of fatality in COVID-19 patients
[24,25,26][24][25][26]. The detailed steps of the SARS-CoV-2 virus invasion in the human body are illustrated in
Figure 2.
Figure 2. Pathophysiology of the SARS-CoV-2 virus. SARS-CoV-2 virus invasion in the human body undergoes the following processes: (
1) virus adhesion with ACE2 receptor; (
2) virus entry into cells; (
3) release of viral RNA and replication; (
4) assembly of new viral content, which causes the pyroptosis of the host cell and induces the secretion of pro-inflammatory cytokines and chemokines. These processes attract monocytes, macrophages, and T cells and result in an inflammatory response. (
5) Release of virus; (
6) antigen-presenting cells engulf viral particles and activate T helper cells. This activates an adaptive immune response that stimulates B cells to produce antibodies against the virus and T cytotoxic cells to recognize and destroy infected cells. Hence, the accumulation of immune cells at infection sites after an excessive pro-inflammatory stimulus primarily begins with the release of IL-6, which induces the cytokine storm. Reprinted from
[27] and modified with the insertion of the cytokine storm part.
The described pathophysiology is the conventional response of the body’s immune system for pathogenic infection; however, the secretion range of each infection-mediated clinical biomarker in the blood and serum varies between COVID-19 and other infections. This finding is supported by data from clinical studies in which the secretion range of these infection-mediated clinical biomarkers exhibited a specific range for each clinical category of patients. Four distinct clinical categories of patients with SARS-CoV-2 are known—namely mild, moderate, severe, and critical. The severity of the illness and changes in body chemistry can be distinguished in these four categories.
According to the COVID-19 Diagnosis and Treatment Plan issued by the National Health Committee of China
[28], patients with mild clinical symptoms and without pneumonia according to radiological imaging are classified as mild patients, whereas patients with fever, respiratory symptoms, and pneumonia according to radiological imaging are categorized as moderate patients. Severe patients are identified with the following criteria: (1) respiratory distress, respiratory rate of ≥30 bpm; (2) peripheral capillary oxygen saturation at rest ≤ 93%; and (3) arterial oxygen partial pressure/fraction of inspiration O
2 of ≤300 mmHg (1 mmHg = 0.133 kPa). Finally, patients with any one of the following criteria are grouped into the critical category: (1) respiratory failure and mechanical ventilation required, (2) shock, and (3) combination with other organ failures and ICU monitoring and treatment needed
[29]. Therefore, the detection and monitoring of biomarkers within a specific concentration range facilitates the diagnosis and increases the understanding of disease severity in an infected individual.
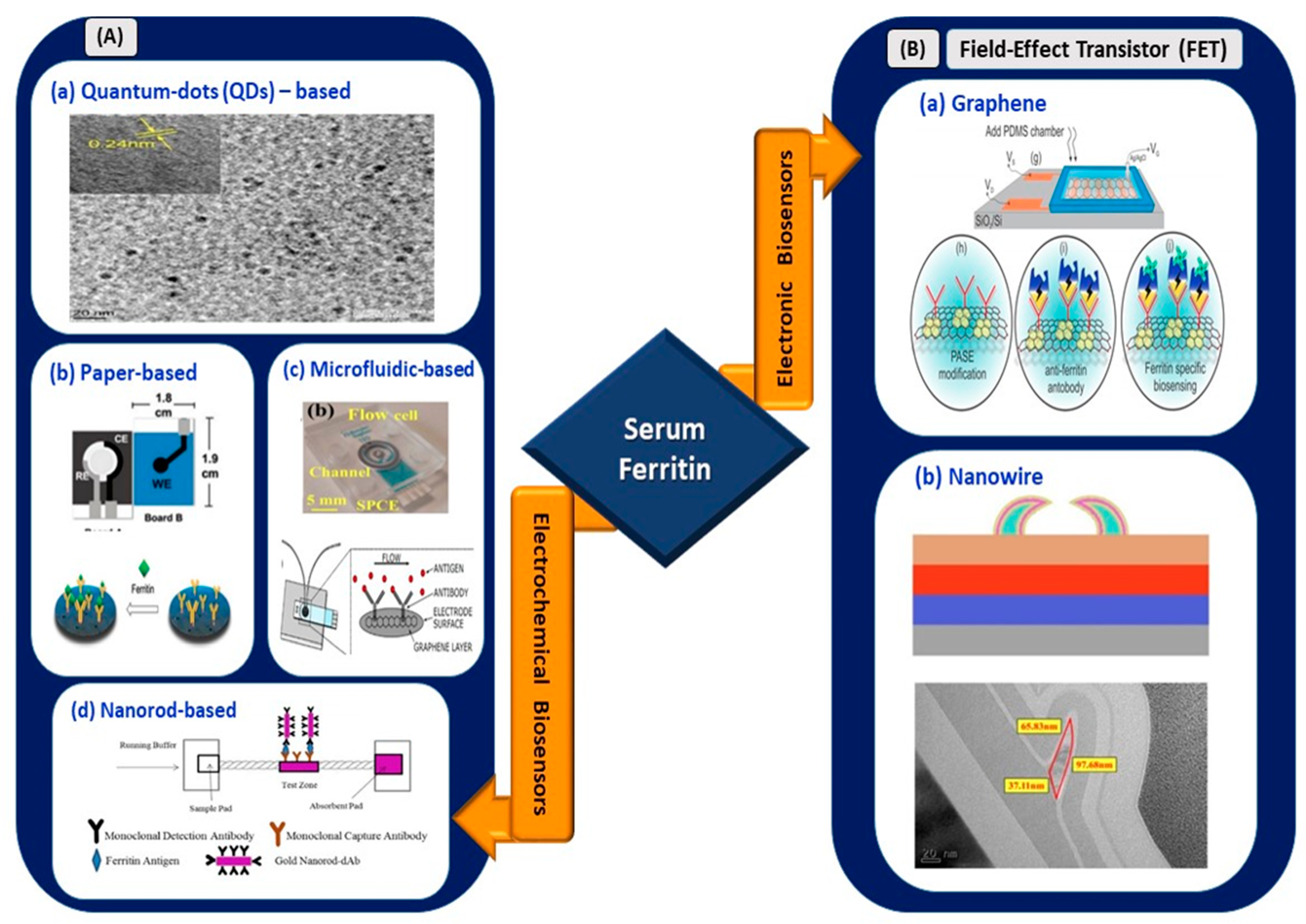
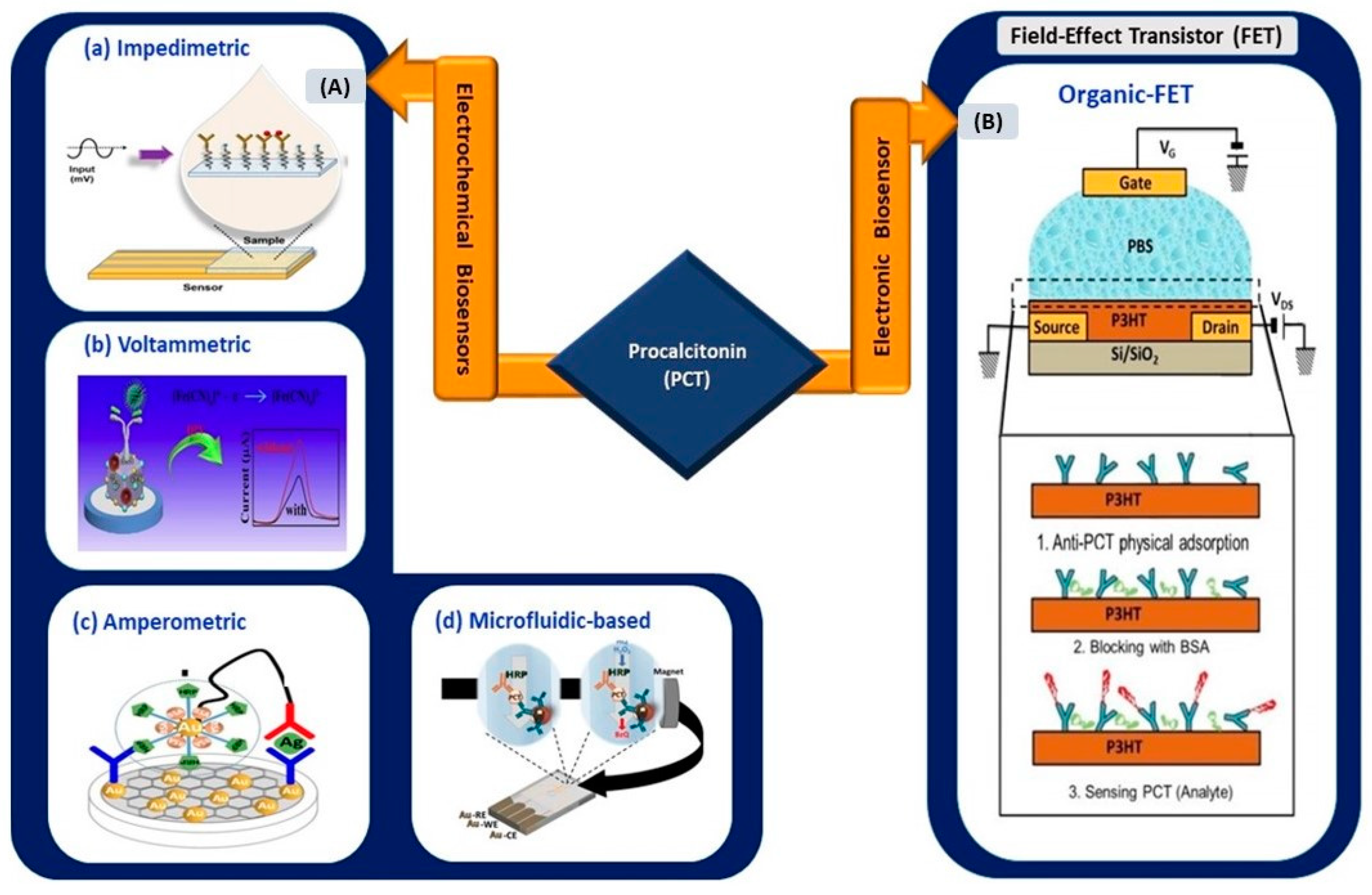
3. Infection-Mediated Clinical Biomarkers and Their Electrical Biosensors
This section reviews and highlights potentially effective infection-mediated clinical biomarkers as prognostic and adjunct diagnostic indicators in SARS-CoV-2 infection. The secretion range of each biomarker for four different types of clinical patients (mild, moderate, severe, and critical) was reviewed based on data supported by clinical studies (
Table 1)
[29,30][29][30]. Advancements in electrical biosensors comprising electrochemical and electronics biosensors for these biomarkers are presented in the subsections below. At the end of this section, the applicability of these sensors for COVID-based biosensing applications is assessed and listed as (1) clinically viable, (2) less viable, or (3) not viable in
Table 2.
Table 1. Concentrations of infection-mediated clinical biomarkers in normal and four clinical types (mild, moderate, severe, and critical) of COVID-19 patients.
Infection-Mediated Clinical
Biomarkers |
Interleukin-6 (IL-6) a
(pg/mL) |
Vascular Endothelial Growth Factor
(VEGF) b (mg/mL) |
Serum
Amyloid-A
(SAA) c
(mg/mL) |
Serum Ferritin d
(ng/mL) |
C-Reactive
Protein (CRP) e
(mg/mL) |
Procalcitonin
(PCT) f
(ng/mL) |
Baseline
(normal range in a healthy individual) |
0.00–7.00 |
27.00–30.00 |
0.00–10.00 |
15.00–150.00 |
0.00–1.00 |
0.00–0.10 |
| Clinical Categories (mean + standard deviation) |
| Mild |
5.36 ± 1.84 |
N/A |
N/A |
148.22 ± 196.53 |
7.79 ± 10.88 |
N/A |
| Moderate |
13.76 ± 9.07 |
N/A |
123.57 ± 75.81 |
396.02 ± 260.29 |
12.79 ± 7.70 |
0.08 ± 0.279 |
| Severe |
15.94 ± 14.88 |
25.90 (12.30, 40.60) |
171.91 ± 56.89 |
469.53 ± 188.18 |
17.84 ± 15.09 |
0.14 ± 0.353 |
| Critical |
33.21 ± 28.58 |
62.90 (45.80, 79.60) |
181.00 ± 40.66 |
1,005.16 ± 577.32 |
46.75 ± 37.66 |
0.44 ± 0.512 |