Constipation is a very common disorder, mostly functional in nature, that may persist for years in up to 35–52% of children. Food allergy prevalence, severity and persistence are increasing over time, and cows’ milk protein is the commonest food allergen recognised to affect gastrointestinal motility in children. There is mounting evidence of the role of cows’ milk (CM) allergy (CMA) in children with constipation.
- constipation
- allergic disease
- food allergy
- cows’ milk allergy
1. Diagnostic Work-Up
Despite the ongoing debate within the paediatric gastroenterology community, constipation is listed as a ‘common’ gastrointestinal symptom of CMA in some recent national guidelines [1][2]. All major European and international allergy and paediatric gastrointestinal guidelines (BSACI, DRACMA, EACI, ESPGHAN) mention constipation as a possible allergic manifestation [1][2][3][4][5][6][7][8]. However, the pleomorphic nature of CMA and the heterogeneity of the studied populations accentuate the lack of a specific phenotype of patients with FA-constipation.
Constipation is a considered to be non-IgE mediated manifestation of food allergy. Therefore, testing for antigen specific IgE using skin prick, RAST or immune-CAP is not informative or recommended {Irastorza, 2010 #23169;Dehghani, 2012 #15935} Allergy tests are not indicated. {Tabbers, 2014 #93929}. A variety of invasive and non-invasive tests have been evaluated; however, none have proven accurate to detect children with FA-C.
In the absence of inflammatory bowel disease, ileal and colon lymphoid nodular hyperplasia has been associated with FA in children [9][10]. However, LNH is absent in a significant proportion of cases with FA-C [9][10]. Similarly, mucosal eosinophilia has been found in FA-C [9][11][12][9], but it is not present universally. Both findings improve on elimination diet in children with GI symptoms due to FA [12]. Nevertheless, invasive tests such as sigmoidoscopy or colonoscopy are unsuitable as diagnostic tests for food allergy in constipated children [13].
Faecal calprotectin is a non-invasive marker of gastrointestinal inflammation as it correlates with the level of mucosal inflammation [14][15][16]. It is widely used in inflammatory bowel disease. However, it has not proved to be useful in FA. A recent review concluded that despite elevation of faecal calprotectin levels in some infants [17][18] and older children [19] with CMA, there was no significant change in faecal calprotectin levels after challenge, and the specific cut-off values for allergic disease and FA-C remained indefinable [20].
Similarly, faecal biomarkers derived from eosinophils, such as eosinophil derived neurotoxin (EDN) and eosinophilic cationic protein (ECP), have been evaluated as possible biomarkers of non-IgE mediated food allergy but very limited data support their role in populations with gastrointestinal disorders [21][22][23]. Increased baseline mRNA levels of some cytokines (IL-13 and IL-10) in combination with faecal calprotectin and EDN have been reported as predictive of a positive food challenge outcome in a small study of children with both IgE and non-IgE mediated reactions. These markers have also been proposed as prognostic markers for symptomatic, IgE-mediated food allergy, but they need further validation in a larger patient cohort [23].
The atopy patch test (APT) has been evaluated for the diagnosis of non-IgE mediated food allergy and late phase reactions mediated by T lymphocytes [24]. Syrigou et al. applied APT in children with constipation, showing a positive correlation with a clinical response to dietary eliminations. Interestingly, wheat was found to be the main allergen diagnosed using APT in chronic constipation, followed by egg and milk [25]. However, these results have not been reproduced, and APT has fallen out of favour in some studies due to poor sensitivity. A recent meta-analysis found a pooled sensitivity of 44.2% and 86.9% specificity of APT for detection of CMA in children [26]. Studies focusing on gastrointestinal disorders, including constipation, have reported even lower sensitivity values [27]. As such, the role of APT in clinical practice remains controversial [25][27][28][29][27][30][31], and they are not currently recommended [4][32].
The allergen-specific lymphocyte stimulation test (ALST) is a promising tool in paediatric populations with non-IgE-mediated food allergies [33]. However, prolonged incubation times (up to 5 days) and large blood sample volumes limit its utility in children [22][34]. Although recently developed variation on traditional ALST allows shorter incubation times and lower blood sample volumes, this test still requires validation for the diagnosis of non-IgE mediated FA in children [35].
In the absence of reliable laboratory tests, an allergy-focused history and elimination with open food challenge remain the recommended diagnostic steps for dairy allergic constipation [36]. The UK NICE guidelines recommend this approach in all children with chronic GI symptoms, including constipation [37] and have used this strategy as a health care quality indicator since 2016 [38].
The Cows’ Milk-Related Symptom Score (CoMiSS) has been recently developed as an awareness tool in infants. It is based on quantification of crying, regurgitation, respiratory and skin symptoms, and stool consistency according to the Bristol scale, creating a combined score from 0 to 33. The authors’ ambition was to create an easy and an accurate tool that would help in the early recognition of CMA and, possibly, the evolution of symptoms during a diet intervention [39][40]. The original proposed cut-off of 12 was found to be highly predictive of the response to CMP elimination diet and the reaction at challenge in a number of studies, and it clearly discriminated symptomatic infants from controls [41][42]. Recently, Calvani et al. reviewed the current literature on CoMiSS and concluded that although it can already be considered a useful screening tool, it is not a substitute for elimination diet and food challenge for the diagnosis of CMA. This score requires further validation in different target populations, and optimal cut-offs need to be identified [22].
Families of older children with CMA constipation often describe a history of multiple symptoms in infancy and early childhood [43][44][45], including irritability, formula intolerance, colic and regurgitation, similar to those listed in the CoMiSS for infants [46][45][47]. As such, questions about the child’s health in infancy are an important component of the allergy-focused history in a child with constipation. A clinician’s guide to allergy-focused history has been proposed by the Royal College of Paediatrics and Child Health in the UK [48]. It contains different modules for allergy screening of children as well as questions for children with suspected food allergy, focusing particularly on clinical manifestations rather than established diagnoses (e.g., itchy rash in skin creases rather than eczema).
The ESPGHAN diagnostic and therapeutic guidelines do not recommend routine testing for CMA in children with functional constipation [49][4], but the constipation guidelines suggest consideration of a CMP elimination trial for 2–4 weeks after failure of first line therapy (diary, education, toilet training, oral medication) and after excluding other underlying organic causes of constipation [49]. No guidance is given on how to select children for CMP elimination diet and there is no compulsion to implement it. A missed opportunity to diagnose CMA at this point poses a significant risk of inappropriate tests and treatments. These may involve biopsies, ionizing radiation, bowel preparation, colonoscopy, manometry and stoma surgery or resection [49]. Traditionally, CMP elimination diet has been offered to children based on high-risk features in the history, such as a personal history of prior food allergy, atopy or a family history of allergy [50][51][52][53]. However, some children with CMA constipation lack any such risk factors. Therefore, given the high pre-test probability of CMA in children with chronic constipation, some have advocated for the consideration of elimination diet trial in all children with chronic [54] or medication-resistant constipation [55][56][9][57][45][13]. Most trials have used a four-week elimination diet [9][12][55][58][56][9][59][60][57][61]. Improvement of constipation has been reported within days of CM elimination, regardless the presence of specific IgE towards cows’ milk proteins. Similarly, relapse usually occurs after 1–2 weeks of reintroduction of dairy product. Thus, avoidance of cows’ milk protein for a minimum of two to four weeks must be considered in children with chronic intractable constipation [49][62][63].
Taking all of the evidence into account, a 4-week CMP elimination diet could be considered not only in constipated children at high-risk of CMA (based on a personal history of atopy, prior food allergy or a family history of allergy), but also in all children with chronic constipation and/or unexplained outlet obstruction resistant to conventional treatment, absent any alarm sign/symptoms, before performing other diagnostic investigations (such as transit studies).
As with the other non-IgE mediated FA, a formal diagnosis of CMA constipation is made by CM elimination and challenge. If the symptoms disappear during CMP elimination diet and reappear with reintroduction, a causal link can then be established [3][64]. Dairy elimination can therefore be done for diagnostic or therapeutic purposes. Improvement and relapse of symptoms is usually within days of elimination and challenge [58][56][65]. However, symptom onset can be delayed by two to four weeks [51][44][51]. Due to the delayed onset of constipation symptoms, an in clinic/hospital challenge is not required in most cases. However, a challenge may be initiated in hospital if there is high risk of severe acute reaction. After confirming CMA as the cause of constipation with the 4-week CMP elimination diet and challenge, the CM can be eliminated for therapeutic purposes and then gradually reintroduced as tolerated [1][66].
2. Food Allergy-Associated Constipation Management
Current guidelines for constipation recommend education, behaviour modifications, adequate physical activity, a normal-for-age intake of fibre and fluids, progressing if needed to pharmacological treatments [49]. Elimination diet is commonly used as an add on treatment to conventional laxative and behavioural therapies [20,27,29,30,31,32], although CM avoidance diet solely may lead to symptom relief, especially in younger infants.
Overall, the quality of evidence for the efficacy of laxative therapy in children is low, due to sparse data, a high risk of bias, the clinical heterogeneity of subjects and treatment and a short follow up in the available studies [67][68]. Nevertheless, the use of laxatives in clinical paediatric practice is well established [69], both for initial faecal disimpaction, if required, and subsequently for maintenance therapy [49]. Unfortunately, paediatric constipation often requires prolonged laxative treatment [70][71], including regular use of high-dose osmotic laxatives and intermittent use as rescue therapy of stimulant laxatives as part of the conventional therapy for functional constipation.
Of children seen in tertiary clinics for functional constipation, approximately 70% experience symptom control on laxatives, leaving 30% medication-resistant [72][73]. Using figures from the DBPCFC studies showing response to CMP elimination diet in medication-resistant cases, a further 47% of children achieved treatment response while dairy free [11][12][59][51][74]. An expanded elimination diet, removing soy and egg in addition to dairy detected 12% with non-dairy allergies [51]. These outcomes are illustrated in Figure 1.
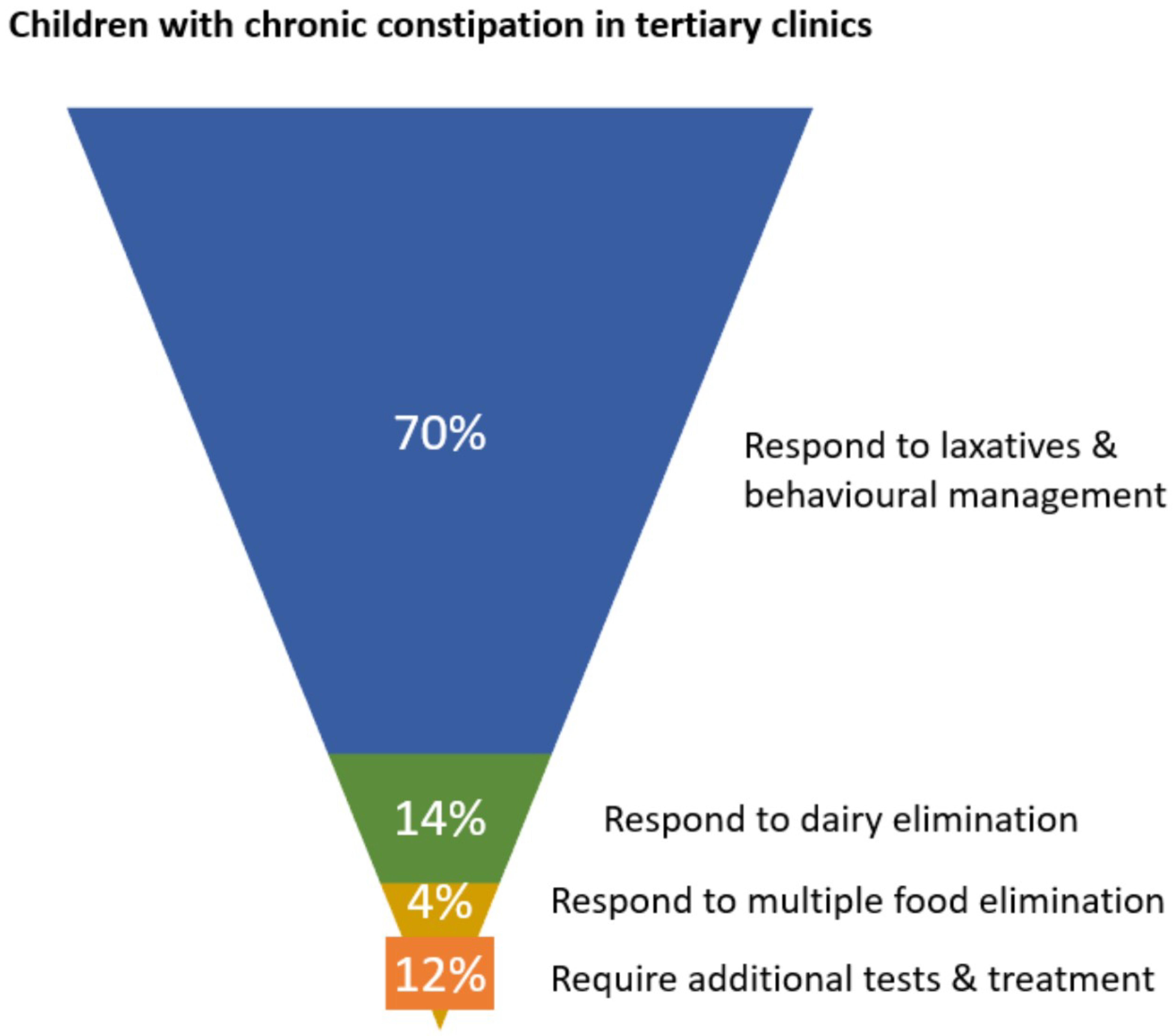
Figure 1. Schematic showing proportions of children in tertiary clinics responding to initial phases of management for chronic constipation.
For infants requiring CM elimination, all guidelines on the management of CMA recommend continued breast feeding, with the mother avoiding all dairy products, as the ideal nutrition [13][21][34][42][6][7][8][36][75][76][77][78]. A maternal CMP elimination diet during breast feeding requires close supervision and support to protect the nutritional safety of both mother and baby, in accordance with published guidelines [5]. When human milk is not available or it is insufficient, extensively hydrolysed CM formula, rice hydrolysate or amino acid formula are recommended for the management of CMA [36]. Hypoallergenic formulas may vary for protein source and content, method and degree of hydrolysis and additional components, which affect tolerance and efficacy [36][79]. In formula-fed infants with food-allergic motility disorders, the use of extensively hydrolysed formulas has been shown to be effective [79]. Amino acid-based formula can be used if symptoms persist on extensively hydrolysed cow’s milk protein-based formula or if GI symptoms are associated with malabsorption and faltering growth [80]. This may also be required in infants whose symptoms were present while breastfed, if feeding with hydrolysed formula failed [81]. In the countries where soy formula is available, it may be considered for infants with CMA older than 6 months [13][31][4][6][7][82][83][84]. However, soy may exacerbate GI manifestations, as concomitant allergy with CMA has been reported in 10–15% of cases [85]. Rice hydrolysate formula is also a suitable alternative to CM-based hydrolysed formulas. Other plant-based milk alternatives are not nutritionally complete, and they are contraindicated as cows’ milk substitutes in infants [36][79].
The direct effect of hydrolysed proteins on gastrointestinal motility has been considered. A number of studies indicated that source protein (casein vs. whey) and degree of hydrolysis (intact protein vs. partial or extensively hydrolysed protein) may influence gastric emptying time [79][86]. Conversely, very limited data have been published on the effect of different proteins on intestinal motility. The modulatory effects on colonic motility of bovine whey and casein milk protein hydrolysates were tested in an ex vivo rat model [87]. Intestinal propagation frequency was decreased by casein protein hydrolysate, increased by a combination of 60% whey to 40% casein hydrolysate and unaffected by intact whey proteins [87].
No difference in stool consistency and frequency was found when a partially hydrolysed CM formula (hydrolysed whey/intact casein = 63/37) was used in healthy term infants [88] compared to an intact CMP formula (whey/casein = 61/39). Both groups of formula-fed infants had significantly fewer evacuations per day than breastfed infants. The colour and the volume of the stools of infants fed the hydrolysed formula resembled those of breastfed infants [88].
Most children with FA-C respond to elimination of CMP alone. Step up to oligoantigenic diet only benefits a minority. Step up to multiple food elimination may be considered in non-responders to CMP elimination, if justified by the severity or persistence of symptoms, particularly in young children with atopic comorbidities. A multiple food elimination diet requires close medical and dietetic support to optimize adherence and nutrition [89][90].
In addition to relieving current symptoms, the identification and the treatment of CMA may be related to long-term outcomes. A number of studies suggest a possible role of CMA in the development of functional gastrointestinal disorders (FGID) later in life [91][92][93][94][95][96]. The nature of this association remains to be evaluated.
Interestingly, Nocerino et al. found that dietary intervention with an extensively hydrolysed casein-based (EHCF) formula enriched with LGG in infants with CMA could influence the subsequent development of FGID at preschool age. A significant difference between the EHCF and the EHCF+LGG groups was observed for the rate of functional dyspepsia, abdominal pain and constipation (incidence rate ratio 0.44, 95% CI, 0.17–1.06; p = 0.07). The authors suggested that specific strain of probiotics may induce a beneficial epigenetic regulation of immune and non-immune gene expression of gut microbiota structure and function with increased production of the short-chain fatty acid butyrate [97]. In addition to accelerating the rate of resolution of CMA [98][99][100], this molecule may regulate gastrointestinal motility and pain perception, which are pivotal factors for the development of FGIDs [97].
Families should be advised that CMA tends to remit with time in most cases [60][1][4][60][25]. In children with multiple foods contributing to constipation, each should be challenged separately [25]. In most cases, FA-C improves after 6–24 months [60][25]. However, occasionally it persists into adult life [101].
Ideally all diets should be monitored and implemented by trained dieticians. For diagnostic purposes simple, time-limited, single food elimination diets in well-nourished children can be administered effectively from the paediatric gastroenterology or allergology clinic, using appropriate verbal instruction and written patient information. However, children who prove to have CMA and who require ongoing allergen elimination need supervision by an appropriately qualified dietician to ensure adequate intake of micro and macronutrients, including calcium and protein [13][31][4][102][103][104][105]. A recent study suggested that compared to healthy controls, patients with FA who lack confidence in FA issues and those following an uncontrolled, restrictive elimination diet are more prone to food aversion and eating disorders [106]. Conversely, another recent study reported an increased risk of eating disorders in children who had a previous history of chronic diarrhoea or constipation [107]. However, the possible relationship of the observed gastrointestinal symptoms to food allergy was not explored. Eating disorders themselves are commonly associated with gastrointestinal problems, including constipation [108]. Therefore, elimination diets require careful implementation with nutritional counselling and regular monitoring of growth and of compliance to ensure safety [13][31][4][106].
References
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672.
- El-Hodhod, M.A.; El-Shabrawi, M.H.F.; AlBadi, A.; Hussein, A.; Almehaidib, A.; Nasrallah, B.; AlBassam, E.M.; El Feghali, H.; Isa, H.M.; Al Saraf, K.; et al. Consensus statement on the epidemiology, diagnosis, prevention, and management of cow’s milk protein allergy in the Middle East: A modified Delphi-based study. World J. Pediatr. 2021, 17, 576–589.
- Fiocchi, A.; Brozek, J.; Schuenemann, H.; Bahna, S.L.; Von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr. Allergy Immunol. 2010, 21 (Suppl. 21), 1–125.
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: Espghan gi committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229.
- Meyer, R.; Lozinsky, A.C.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy 2019, 75, 14–32.
- Allen, K.J.; Davidson, G.P.; Day, A.S.; Hill, D.J.; Kemp, A.S.; E Peake, J.; Prescott, S.L.; Shugg, A.; Sinn, J.K.; Heine, R.G. Management of cow’s milk protein allergy in infants and young children: An expert panel perspective. J. Paediatr. Child Health 2009, 45, 481–486.
- Matthai, J.; Sathiasekharan, M.; Poddar, U.; Sibal, A.; Srivastava, A.; Waikar, Y.; Malik, R.; Ray, G.; Geetha, S.; Yachha, S.K.; et al. Guidelines on Diagnosis and Management of Cow’s Milk Protein Allergy. Indian Pediatr. 2020, 57, 723–729.
- Guler, N.; Cokugras, F.; Sapan, N.; Selimoglu, A.; Turktas, I.; Cokugras, H.C.; Aydogan, M.; Beser, O. Diagnosis and management of cow’s milk protein allergy in Turkey: Region-specific recommendations by an expert-panel. Allergol. Immunopathol. 2020, 48, 202–210.
- Turunen, S.; Karttunen, T.J.; Kokkonen, J. Lymphoid nodular hyperplasia and cow’s milk hypersensitivity in children with chronic constipation. J. Pediatr. 2004, 145, 606–611.
- Iacono, G.; Ravelli, A.; Di Prima, L.; Scalici, C.; Bolognini, S.; Chiappa, S.; Pirrone, G.; Licastri, G.; Carroccio, A. Colonic Lymphoid Nodular Hyperplasia in Children: Relationship to Food Hypersensitivity. Clin. Gastroenterol. Hepatol. 2007, 5, 361–366.
- Iacono, G.; Cavataio, F.; Montalto, G.; Florena, A.; Tumminello, M.; Soresi, M.; Notarbartolo, A.; Carroccio, A. Intolerance of Cow’s Milk and Chronic Constipation in Children. N. Engl. J. Med. 1998, 339, 1100–1104.
- Carroccio, A.; Scalici, C.; Maresi, E.; Di Prima, L.; Cavataio, F.; Noto, D.; Porcasi, R.; Averna, M.R.; Iacono, G. Chronic constipation and food intolerance: A model of proctitis causing constipation. Scand. J. Gastroenterol. 2005, 40, 33–42.
- Putnam, P.E. The milk of human constipation. J. Pediatr. 2004, 145, 578–580.
- Canani, R.B.; Rapacciuolo, L.; Romano, M.; de Horatio, L.T.; Terrin, G.; Manguso, F.; Cirillo, P.; Paparo, F.; Troncone, R. Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig. Liver Dis. 2004, 36, 467–470.
- Chang, M.-H.; Chou, J.-W.; Chen, S.-M.; Tsai, M.-C.; Sun, Y.-S.; Lin, C.-C.; Lin, C.-P. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 522–526.
- Degraeuwe, P.L.; Beld, M.P.; Ashorn, M.; Canani, R.B.; Day, A.S.; Diamanti, A.; Fagerberg, U.L.; Henderson, P.; Kolho, K.-L.; Van de Vijver, E.; et al. Faecal Calprotectin in Suspected Paediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 339–346.
- Beşer, Ö.F.; Sancak, S.; Erkan, T.; Kutlu, T.; Cokugras, H.C.; Çokuğraş, F.Ç. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow’s Milk Protein Allergy? Allergy Asthma Immunol. Res. 2014, 6, 33–38.
- Belizón, C.T.; Páez, E.O.; Claros, A.F.M.; Sánchez, I.R.; González, A.R.; Medialdea, R.V.; Salguero, J.M.R. Calprotectina fecal como apoyo al diagnóstico en la alergia a las proteínas de leche de vaca no IgE mediada. An. Pediatría 2016, 84, 318–323.
- Lendvai-Emmert, D.; Emmert, V.; Fusz, K.; Prémusz, V.; Boncz, I.; Tóth, G. Quantitative Assay of Fecal Calprotectin in Children with Cow’s Milk Protein Allergy. Value Health 2018, 21, S128.
- Xiong, L.-J.; Xie, X.-L.; Li, Y.; Deng, X.-Z. Current status of fecal calprotectin as a diagnostic or monitoring biomarker for cow’s milk protein allergy in children: A scoping review. World J. Pediatr. 2020, 17, 63–70.
- Kalach, N.; Kapel, N.; Waligora-Dupriet, A.-J.; Castelain, M.-C.; Cousin, M.O.; Sauvage, C.; Ba, F.; Nicolis, I.; Campeotto, F.; Butel, M.J.; et al. Intestinal permeability and fecal eosinophil-derived neurotoxin are the best diagnosis tools for digestive non-IgE-mediated cow’s milk allergy in toddlers. Clin. Chem. Lab. Med. 2012, 51, 351–361.
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.; Santoro, A.; et al. Non–IgE- or Mixed IgE/Non–IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226.
- Winberg, A.; Nagaeva, O.; Nagaev, I.; Lundell, C.; Arencibia, I.; Mincheva-Nilsson, L.; Rönmark, E.; West, C.E. Dynamics of cytokine mRNA expression and fecal biomarkers in school-children undergoing a double-blind placebo-controlled food challenge series. Cytokine 2016, 88, 259–266.
- Mehl, A.; Rolinck-Werninghaus, C.; Staden, U.; Verstege, A.; Wahn, U.; Beyer, K.; Niggemann, B. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J. Allergy Clin. Immunol. 2006, 118, 923–929.
- Syrigou, E.I.; Pitsios, C.; Panagiotou, I.; Chouliaras, G.; Kitsiou, S.; Kanariou, M.; Roma-Giannikou, E. Food allergy-related paediatric constipation: The usefulness of atopy patch test. Eur. J. Pediatr. 2011, 170, 1173–1178.
- Luo, Y.; Zhang, G.-Q.; Li, Z.-Y. The diagnostic value of APT for food allergy in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2019, 30, 451–461.
- Mowszet, K.; Matusiewicz, K.; Iwańczak, B. Value of the Atopy Patch Test in the Diagnosis of Food Allergy in Children with Gastrointestinal Symptoms. Adv. Clin. Exp. Med. 2014, 23, 403–409.
- De Boissieu, D.; Waguet, J.; Dupont, C. The atopy patch tests for detection of cow’s milk allergy with digestive symptoms. J. Pediatr. 2003, 142, 203–205.
- Cudowska, B.; Kaczmarski, M. Atopy patch test in the diagnosis of food allergy in children with gastrointestinal symptoms. Adv. Med. Sci. 2010, 55, 153–160.
- Kokkonen, T.R.J.; Ruuska, T.; Karttunen, T.; Niinimäki, A. Mucosal pathology of the foregut associated with food allergy and recurrent abdominal pains in children. Acta Paediatr. 2001, 90, 16–21.
- Turjanmaa, K.; Darsow, U.; Niggemann, B.; Rancé, F.; Vanto, T.; Werfel, T. EAACI/GA2LEN Position paper: Present status of the atopy patch test. Allergy 2006, 61, 1377–1384.
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e1112.
- Ikeda, K.; Ida, S.; Kawahara, H.; Kawamoto, K.; Etani, Y.; Kubota, A. Importance of evaluating for cow’s milk allergy in pediatric surgical patients with functional bowel symptoms. J. Pediatr. Surg. 2011, 46, 2332–2335.
- Matsumoto, K. Non-IgE-Related Diagnostic Methods (LST, Patch Test). Chem. Immunol. Allergy 2015, 101, 79–86.
- Yagi, H.; Sato, K.; Arakawa, N.; Inoue, T.; Nishida, Y.; Yamada, S.; Ishige, T.; Yamada, Y.; Arakawa, H.; Takizawa, T. Expression of Leucine-rich Repeat-containing Protein 32 Following Lymphocyte Stimulation in Patients with Non-IgE-mediated. Gastrointest. Food Allerg. 2020, 93, 645–655.
- Vandenplas, Y.; A Brough, H.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current Guidelines and Future Strategies for the Management of Cow’s Milk Allergy. J. Asthma Allergy 2021, 14, 1243–1256.
- National Institute for Health and Care Excellence. NICE Guideline: Food Allergy in under 19s: Assessment and Diagnosis (CG116); NICE: London, UK, 2011.
- NICE Quality Standard ; Quality Statement 1: Allergy-Focused Clinical History; National Institute for Health and Care Excellence: London, UK, 2016.
- Vandenplas, Y.; Mukherjee, R.; Dupont, C.; Eigenmann, P.; Høst, A.; Kuitunen, M.; Ribes-Koninkx, C.; Shah, N.; Szajewska, H.; Von Berg, A.; et al. Protocol for the validation of sensitivity and specificity of the Cow’s Milk-related Symptom Score (CoMiSS) against open food challenge in a single-blinded, prospective, multicentre trial in infants. BMJ Open 2018, 8, e019968.
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the Cow’s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2014, 104, 334–339.
- Salvatore, S.; Bertoni, E.; Bogni, F.; Bonaita, V.; Armano, C.; Moretti, A.; Baù, M.; Luini, C.; D’Auria, E.; Marinoni, M.; et al. Testing the Cow’s Milk-Related Symptom Score (CoMiSSTM) for the Response to a Cow’s Milk-Free Diet in Infants: A Prospective Study. Nutrients 2019, 11, 2402.
- Vandenplas, Y.; Salvatore, S.; Ribes-Koninckx, C.; Carvajal, E.; Szajewska, H.; Huysentruyt, K. The Cow Milk Symptom Score (CoMiSSTM) in presumed healthy infants. PLoS ONE 2018, 13, e0200603.
- Iacono, G.; Cavataio, F.; Montalto, G.; Soresi, M.; Notarbartolo, A.; Carroccio, A. Persistent cow’s milk protein intolerance in infants: The changing faces of the same disease. Clin. Exp. Allergy 1998, 28, 817–823.
- Carroccio, A.; Montalto, G.; Custro, N.; Notarbartolo, A.; Cavataio, F.; D’Amico, D.; Alabrese, D.; Iacono, G. Evidence of very delayed clinical reactions to cow’s milk in cow’s milk-intolerant patients. Allergy 2000, 55, 574–579.
- Carroccio, A.; Iacono, G. Review article: Chronic constipation and food hypersensitivity? An intriguing relationship. Aliment. Pharmacol. Ther. 2006, 24, 1295–1304.
- Vanderhoof, J.A.; Perry, D.; Hanner, T.L.; Young, R.J. Allergic Constipation: Association with Infantile Milk Allergy. Clin. Pediatr. 2001, 40, 399–402.
- Kokkonen, J.; Haapalahti, M.; Tikkanen, S.; Karttunen, R.; Savilahti, E. Gastrointestinal complaints and diagnosis in children: A population-based study. Acta Paediatr. 2004, 93, 880–886.
- Royal College of Paediatrics and Child Health. Taking an Allergy Focused Clinical History. Available online: https://www.rcpch.ac.uk/sites/default/files/Taking_an_Allergy_Focused_Clinical_History_-_Allergy_Care_Pathways_Project.pdf (accessed on 15 January 2022).
- Tabbers, M.M.; DiLorenzo, C.; Berger, M.Y.; Faure, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A.; European Society for Pediatric Gastroenterology, H.; et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274.
- Shah, N.; Lindley, K.; Milla, P. Cow’s milk and chronic constipation in children. N. Engl. J. Med. 1999, 340, 891–892.
- Borrelli, O.; Barbara, G.; Di Nardo, G.; Cremon, C.; Lucarelli, S.; Frediani, T.; Paganelli, M.; De Giorgio, R.; Stanghellini, V.; Cucchiara, S. Neuroimmune Interaction and Anorectal Motility in Children With Food Allergy-Related Chronic Constipation. Am. J. Gastroenterol. 2009, 104, 454–463.
- Singh, H.; Connor, F. Paediatric constipation: An approach and evidence-based treatment regimen. Aust. J. Gen. Pract. 2018, 47, 273–277.
- Gelsomino, M.; Del Vescovo, E.; Bersani, G.; Sopo, S.M. Functional constipation related to cow’s milk allergy in children. A management proposal. Allergol. Immunopathol. 2021, 49, 17–20.
- Sopo, S.M.; Arena, R.; Scala, G. Functional Constipation and Cow’s-Milk Allergy. J. Pediatr. Gastroenterol. Nutr. 2014, 59, e34.
- Bourkheili, A.M.; Mehrabani, S.; Esmaelidoohi, M.; Ahmadi, M.H.; Moslemi, L. Effect of Cow’s-milk–free diet on chronic constipation in children; A randomized clinical trial. Casp. J. Intern. Med. 2021, 12, 91–96.
- Daher, S.; Tahan, S.; Sole, D.; Naspitz, C.K.; Da Silva Patricio, F.R.; Neto, U.F.; De Morais, M.B. Cow’s milk protein intolerance and chronic constipation in children. Pediatr. Allergy Immunol. 2001, 12, 339–342.
- Dehghani, S.M.; Ahmadpour, B.; Haghighat, M.; Kashef, S.; Imanieh, M.H.; Soleimani, M. The Role of Cow’s Milk Allergy in Pediatric Chronic Constipation: A Randomized Clinical Trial. Iran. J. Pediatr. 2012, 22, 468–474.
- Iacono, G.; Carroccio, A.; Cavataio, F.; Montalto, G.; Cantarero, M.; Notarbartolo, A. Chronic constipation as a symptom of cow milk allergy. J. Pediatr. 1995, 126, 34–39.
- Iacono, G.; Bonventre, S.; Scalici, C.; Maresi, E.; Di Prima, L.; Soresi, M.; Di Gesù, G.; Noto, D.; Carroccio, A. Food intolerance and chronic constipation: Manometry and histology study. Eur. J. Gastroenterol. Hepatol. 2006, 18, 143–150.
- El-Hodhod, M.A.; Younis, N.T.; Zaitoun, Y.A.; Daoud, S.D. Cow’s milk allergy related pediatric constipation: Appropriate time of milk tolerance. Pediatr. Allergy Immunol. 2010, 21, e407–e412.
- Simeone, D.; Miele, E.; Boccia, G.; Marino, A.; Troncone, R.; Staiano, A. Prevalence of atopy in children with chronic constipation. Arch. Dis. Child. 2008, 93, 1044–1047.
- Sopo, S.M.; Arena, R.; Greco, M.; Bergamini, M.; Monaco, S. Constipation and Cow’s Milk Allergy: A Review of the Literature. Int. Arch. Allergy Immunol. 2014, 164, 40–45.
- Salvatore, S.; Abkari, A.; Cai, W.; Catto-Smith, A.; Cruchet, S.; Gottrand, F.; Hegar, B.; Lifschitz, C.; Ludwig, T.; Shah, N.; et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants. Acta Paediatr. 2018, 107, 1512–1520.
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086.
- Irastorza, I.; Ibáñez, B.; Delgado-Sanzonetti, L.; Maruri, N.; Vitoria, J. Cow’s-Milk–free Diet as a Therapeutic Option in Childhood Chronic Constipation. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 171–176.
- Ball, H.B.; Luyt, D. Home-based cow’s milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow’s milk allergy. Clin. Exp. Allergy 2019, 49, 911–920.
- Van Wering, H.M.; Tabbers, M.M.; A Benninga, M. Are constipation drugs effective and safe to be used in children?: A review of the literature. Expert Opin. Drug Saf. 2011, 11, 71–82.
- Gordon, M.; Macdonald, J.K.; E Parker, C.; Akobeng, A.K.; Thomas, A.G. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst. Rev. 2016, 2016, CD009118.
- Pijpers, M.A.M.; Tabbers, M.M.; Benninga, M.A.; Berger, M.Y. Currently recommended treatments of childhood constipation are not evidence based: A systematic literature review on the effect of laxative treatment and dietary measures. Arch. Dis. Child. 2008, 94, 117–131.
- Tappin, D.; Grzeda, M.; Joinson, C.; Heron, J. Challenging the view that lack of fibre causes childhood constipation. Arch. Dis. Child. 2020, 105, 864–868.
- Pijpers, M.; Bongers, M.; Benninga, M.; Berger, M. Functional Constipation in Children: A Systematic Review on Prognosis and Predictive Factors. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 256–268.
- Van Ginkel, R.; Reitsma, J.B.; A Büller, H.; van Wijk, M.; Taminiau, J.A.; Benninga, M.A. Childhood constipation: Longitudinal follow-up beyond puberty. Gastroenterology 2003, 125, 357–363.
- Bongers, M.E.J.; van Wijk, M.P.; Reitsma, J.B.; Benninga, M.A. Long-Term Prognosis for Childhood Constipation: Clinical Outcomes in Adulthood. Pediatrics 2010, 126, e156–e162.
- Crowley, E.T.; Williams, L.T.; Roberts, T.K.; Dunstan, R.H.; Jones, P.D. Does Milk Cause Constipation? A Crossover Dietary Trial. Nutrients 2013, 5, 253–266.
- Vandenplas, Y.; Alturaiki, M.A.; Al-Qabandi, W.; Alrefaee, F.; Bassil, Z.; Eid, B.; El Beleidy, A.; AlMehaidib, A.I.; Mouawad, P.; Sokhn, M. Middle East Consensus Statement on the Diagnosis and Management of Functional Gastrointestinal Disorders in <12 Months Old Infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2016, 19, 153–161.
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein–induced enterocolitis syndrome: Executive summary—Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4.
- Dupont, C.; Chouraqui, J.-P.; Linglart, A.; Bocquet, A.; Darmaun, D.; Feillet, F.; Frelut, M.-L.; Girardet, J.-P.; Hankard, R.; Rozé, J.-C.; et al. Nutritional management of cow’s milk allergy in children: An update. Arch. Pédiatrie 2018, 25, 236–243.
- Jaime, B.E.; Martín, J.J.D.; Baviera, L.C.B.; Monzón, A.C.; Hernández, A.H.; Burriel, J.I.G.; Mérida, M.J.G.; Fernández, C.P.; Rodríguez, C.C.; Riechmann, E.R.; et al. Alergia a las proteínas de leche de vaca no mediada por IgE: Documento de consenso de la Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP), la Asociación Española de Pediatría de Atención Primaria (AEPAP), la Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (SEPEAP) y la Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica (SEICAP). An. Pediatría 2019, 90, 193.e111–193.e191.
- D’Auria, E.; Salvatore, S.; Acunzo, M.; Peroni, D.; Pendezza, E.; Di Profio, E.; Fiore, G.; Zuccotti, G.V.; Verduci, E. Hydrolysed Formulas in the Management of Cow’s Milk Allergy: New Insights, Pitfalls and Tips. Nutrients 2021, 13, 2762.
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Pract. 2018, 6, 383–399.
- Host, A.; Halken, S. Hypoallergenic formulas—When, to whom and how long: After more than 15 years we know the right indication! Allergy 2004, 59, 45–52.
- American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000, 106, 346–349.
- Kemp, A.S.; Hill, D.J.; Allen, K.; Anderson, K.; Davidson, G.P.; Day, A.S.; Heine, R.G.; E Peake, J.; Prescott, S.L.; Shugg, A.W.; et al. Guidelines for the use of infant formulas to treat cows milk protein allergy: An Australian consensus panel opinion. Med. J. Aust. 2008, 188, 109–112.
- Merritt, R.J.; Fleet, S.E.; Fifi, A.; Jump, C.; Schwartz, S.; Sentongo, T.; Duro, D.; Rudolph, J.; Turner, J. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper: Plant-based Milks. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 276–281.
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A Comprehensive Review of Sensitization and Allergy to Soy-Based Products. Clin. Rev. Allergy Immunol. 2014, 46, 272–281.
- Meyer, R.; Foong, R.-X.M.; Thapar, N.; Kritas, S.; Shah, N. Systematic review of the impact of feed protein type and degree of hydrolysis on gastric emptying in children. BMC Gastroenterol. 2015, 15, 137.
- Dalziel, J.; Peters, J.; Dunstan, K.; McKenzie, C.; Spencer, N.; Haggarty, N.; Roy, N. Alteration in propagating colonic contractions by dairy proteins in isolated rat large intestine. J. Dairy Sci. 2019, 102, 9598–9604.
- Wu, S.-L.; Ding, D.; Fang, A.-P.; Chen, P.-Y.; Chen, S.; Jing, L.-P.; Chen, Y.-M.; Zhu, H.-L. Growth, Gastrointestinal Tolerance and Stool Characteristics of Healthy Term Infants Fed an Infant Formula Containing Hydrolyzed Whey Protein (63%) and Intact Casein (37%): A Randomized Clinical Trial. Nutrients 2017, 9, 1254.
- Kearsey, I.; Hutson, J.M.; Southwell, B.R. The effect of food withdrawal in children with rapid-transit constipation. Pediatr. Surg. Int. 2016, 32, 683–689.
- D’Auria, E.; Pendezza, E.; Leone, A.; Riccaboni, F.; Bosetti, A.; Borsani, B.; Zuccotti, G.; Bertoli, S. Nutrient intake in school-aged children with food allergies: A case-control study. Int. J. Food Sci. Nutr. 2021, 1–8.
- Pensabene, L.; Salvatore, S.; D’Auria, E.; Parisi, F.; Concolino, D.; Borrelli, O.; Thapar, N.; Staiano, A.; Vandenplas, Y.; Saps, M. Cow’s Milk Protein Allergy in Infancy: A Risk Factor for Functional Gastrointestinal Disorders in Children? Nutrients 2018, 10, 1716.
- Saps, M.; Lu, P.; Bonilla, S. Cow’s-Milk Allergy Is a Risk Factor for the Development of FGIDs in Children. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 166–169.
- Olén, O.; Neuman, A.; Koopmann, B.; Ludvigsson, J.F.; Ballardini, N.; Westman, M.; Melen, E.; Kull, I.; Simren, M.; Bergström, A. Allergy-related diseases and recurrent abdominal pain during—A birth cohort study. Aliment. Pharmacol. Ther. 2014, 40, 1349–1358.
- Di Nardo, G.; Cremon, C.; Frediani, S.; Lucarelli, S.; Villa, M.P.; Stanghellini, V.; La Torre, G.; Martemucci, L.; Barbara, G. Allergic Proctocolitis Is a Risk Factor for Functional Gastrointestinal Disorders in Children. J. Pediatr. 2018, 195, 128–133.e121.
- Tan, T.-K.; Chen, A.-C.; Lin, C.-L.; Shen, T.-C.; Li, T.-C.; Wei, C.-C. Preschoolers With Allergic Diseases Have an Increased Risk of Irritable Bowel Syndrome When Reaching School Age. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 26–30.
- Koloski, N.; Jones, M.; Walker, M.M.; Veysey, M.; Zala, A.; Keely, S.; Holtmann, G.; Talley, N.J. Population based study: Atopy and autoimmune diseases are associated with functional dyspepsia and irritable bowel syndrome, independent of psychological distress. Aliment. Pharmacol. Ther. 2019, 49, 546–555.
- Nocerino, R.; Di Costanzo, M.; Bedogni, G.; Cosenza, L.; Maddalena, Y.; Di Scala, C.; Della Gatta, G.; Carucci, L.; Voto, L.; Coppola, S.; et al. Dietary Treatment with Extensively Hydrolyzed Casein Formula Containing the Probiotic Lactobacillus rhamnosus GG Prevents the Occurrence of Functional Gastrointestinal Disorders in Children with Cow’s Milk Allergy. J. Pediatr. 2019, 213, 137–142.e2.
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: A randomized trial. J. Allergy Clin. Immunol. 2012, 129, 580–582.e5.
- Paparo, L.; Nocerino, R.; Bruno, C.; Di Scala, C.; Cosenza, L.; Bedogni, G.; Di Costanzo, M.; Mennini, M.; D’Argenio, V.; Salvatore, F.; et al. Randomized controlled trial on the influence of dietary intervention on epigenetic mechanisms in children with cow’s milk allergy: The EPICMA study. Sci. Rep. 2019, 9, 2828.
- Sánchez-Valverde, F.; Etayo, V.; Gil, F.; Aznal, E.; Martínez, D.; Amézqueta, A.; Mendizábal, M.; Galbete, A.; Pastor, N.; Vanderhoof, J. Factors Associated with the Development of Immune Tolerance in Children with Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2019, 179, 290–296.
- Carroccio, A.; Di Prima, L.; Iacono, G.; Florena, A.M.; D’Arpa, F.; Sciumè, C.; Cefalu, A.B.; Noto, D.; Averna, M. Multiple food hypersensitivity as a cause of refractory chronic constipation in adults. Scand. J. Gastroenterol. 2006, 41, 498–504.
- Ercan, N.; Adıgüzel, K.T. Effect of early childhood cow’s milk elimination diet on eating behaviours, nutrition and growth status at age 2–6 years. J. Hum. Nutr. Diet. 2021.
- Meyer, R.; De Koker, C.; Dziubak, R.; Skrapac, A.; Godwin, H.; Reeve, K.; Chebar-Lozinsky, A.; Shah, N. A practical approach to vitamin and mineral supplementation in food allergic children. Clin. Transl. Allergy 2015, 5, 11.
- Canani, R.B.; Leone, L.; D’Auria, E.; Riva, E.; Nocerino, R.; Ruotolo, S.; Terrin, G.; Cosenza, L.; Di Costanzo, M.; Passariello, A.; et al. The Effects of Dietary Counseling on Children with Food Allergy: A Prospective, Multicenter Intervention Study. J. Acad. Nutr. Diet. 2014, 114, 1432–1439.
- D’Auria, E.; Pendezza, E.; Zuccotti, G.V. Personalized Nutrition in Food Allergy: Tips for Clinical Practice. Front. Pediatr. 2020, 8, 113.
- Wróblewska, B.; Szyc, A.M.; Markiewicz, L.H.; Zakrzewska, M.; Romaszko, E. Increased prevalence of eating disorders as a biopsychosocial implication of food allergy. PLoS ONE 2018, 13, e0198607.
- Wiklund, C.A.; Kuja-Halkola, R.; Thornton, L.M.; Hübel, C.; Leppä, V.; Bulik, C.M. Prolonged constipation and diarrhea in childhood and disordered eating in adolescence. J. Psychosom. Res. 2019, 126, 109797.
- Ms, H.K.M.; Wagner, C.; Becker, K.; Bühren, K.; Correll, C.U.; Egberts, K.M.; Ehrlich, S.; Fleischhaker, C.; Föcker, M.; Hahn, F.; et al. Incontinence and constipation in adolescent patients with anorexia nervosa—Results of a multicenter study from a German web-based registry for children and adolescents with anorexia nervosa. Int. J. Eat. Disord. 2019, 53, 219–228.
