Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Eslam El-Sawy.
Mercapto (or sulfanyl)-coumarins are heterocycles of great interest in the development of valuable active structures in material and biological domains. They represent a highly exploitable class of compounds that open many possibilities for further chemical transformations.
- mercapto-coumarins
- Newman–Kwart
- fluorescence
- 7-mercapto-4-methyl coumarin
1. Introduction
Coumarins (2H-1-benzopyran-2-ones) are an elite class of compounds present in various natural products, and they have wide applications, viz., as additives in food [1,2][1][2], perfumes [3], cosmetics [4], and pharmaceuticals [5[5][6],6], as well as in the preparation of optical brighteners [7], dispersed fluorescent [8,9,10][8][9][10] and laser dyes [11], and useful medicinal products [12,13][12][13]. On the other hand, the carbon–sulfur bond formation plays an important role in organic synthesis [14,15,16,17][14][15][16][17]. The introduction of the thiol group to organic structures has emerged as an important tool in medicinal chemistry and chemical biology [18,19,20,21][18][19][20][21]. It plays a distinguished role in the fabrication of applicable substances in the field of advanced functional materials [22], structural frameworks of natural products [23], and the pharmaceutical industry [24,25,26][24][25][26]. Therefore, there is an increasing demand to investigate thiol-based coupling reactions focusing on their chemoselectivity and their tolerance of various functional groups in order to provide feasible access to new chemical architectures [27,28][27][28].
The incorporation of a thiol functional group into coumarin results in mercapto-coumarins. Although mercapto-coumarins have been relatively less extensively studied [18[18][19][20][21],19,20,21], their chemistry and bioactivity appear to be interesting. This functionalization of coumarin allows a special reactivity due to the implication of the thiol group in different types of organic reactions. This facilitates access to various series of derivatives that may have special applications or biological activities.
By exploring mercapto-coumarin derivatives, wresearchers found that four common forms of functional thiol group integrate into the coumarin moiety that occupies different positions, either on the pyrone ring or on the benzene ring. The common four mercapto-coumarins are 3-mercapto-coumarin, 4-mercapto-coumarin, 6-mercapto-coumarin, and 7-mercapto-coumarin (Figure 1).
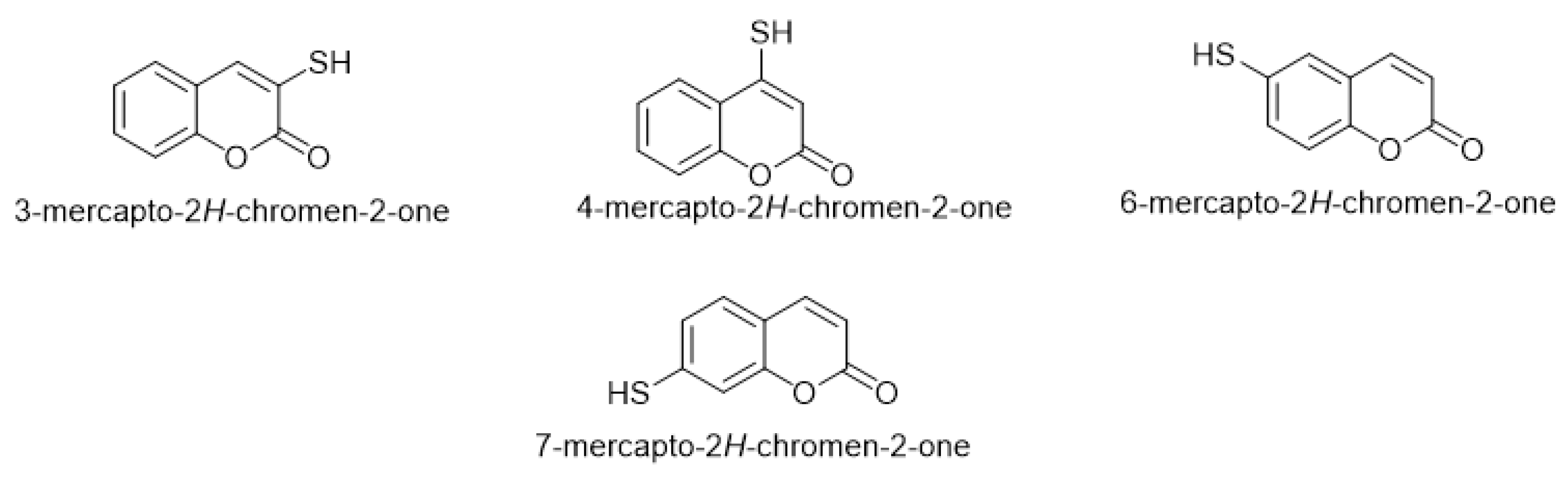
Figure 1. The four common mercapto-coumarin derivatives.
2. 3-Mercapto-Coumarin
By analyzing the synthesis of 3-mercapto-coumarin, wresearchers found that the source of sulfur was a heterocyclic compound not an inorganic reagent. In addition, the coumarin was formed in situ from primary sources, which were salicylaldehydes.
Qiyi et al. reported the synthesis of 3-mercapto-coumarin (4) from 2-hydroxybenzylidenerhodanine (3). The latter was produced in situ from salicylaldehyde (1) and 2-thioxothiazolidin-4-one (2). The reaction proceeded to the final target by refluxing of compound 3 in diluted ethanolic sodium hydroxide solution (Scheme 1) [18].
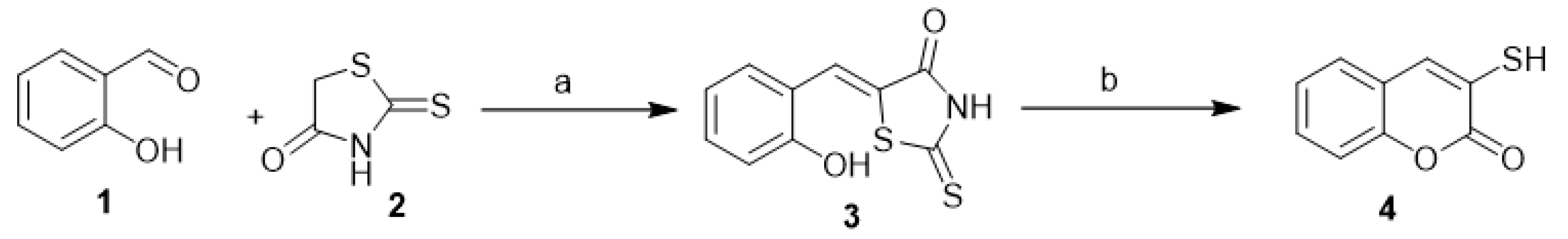
Scheme 1.
The synthesis of 3-mercapto-coumarin (
4
). Reagents and conditions: (
a
) EtOH, reflux, TEA, 80% yield; (
b
) EtOH, NaOH, reflux, 88% yield.
In 2009, a green catalyst-free synthetic protocol for synthesizing varieties of the target 3-mercapto-coumarins was reported. In this protocol, refluxing of 2-methyl-2-phenyl-1,3-oxa-thiolan-5-one (5) and salicylaldehyde derivatives in water afforded the formation of corresponding 3-mercapto-coumarins (4) in excellent yields (82–97%) (Scheme 2) [22].
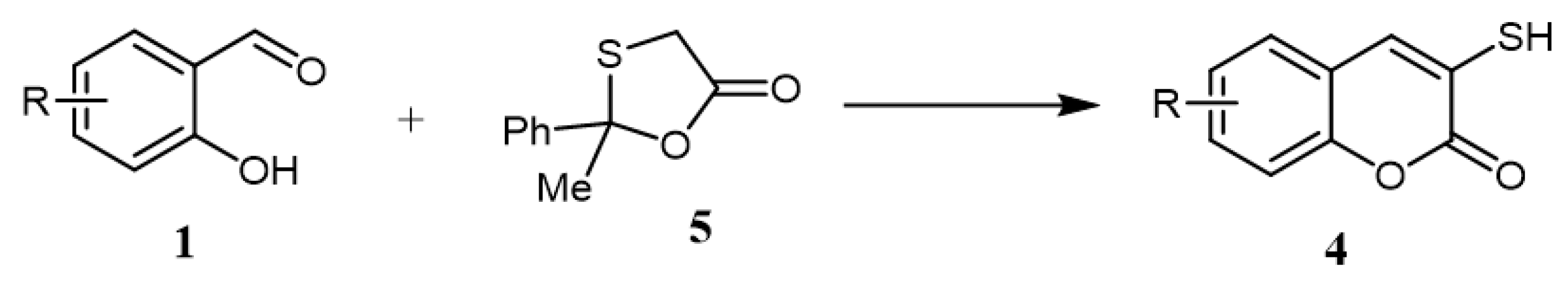
Scheme 2.
Catalyst-free conditions for the synthesis of 3-mercapto-coumarins (
4
): Reagents and conditions: water; reflux; 8–10 h; 8 outputs with 82–97% yield.
3. Reactivity of 3-Mercapto-Coumarin
3-Mercapto-coumarin (4) contributed to the synthesis of many chain and fused compounds. Accordingly, the reaction of 3-mercapto-coumarin (4) with some acrylonitriles and acrylates under the Michael addition condition created S-acetonitrile 6a, S-propanenitrile 6b, S-ethayl acetate 6c, and S-propanoate 6d coumarin derivatives, respectively (Scheme 3) [18]. On the other hand, Mannich reaction of 3-mercapto-coumarin (4) with formaldehyde produced 3-hydroxy-methylthio-coumarin (7). The latter reacted with diphenylamine to give the corresponding α-aminomethylated thioether (8) (Scheme 3) [23].
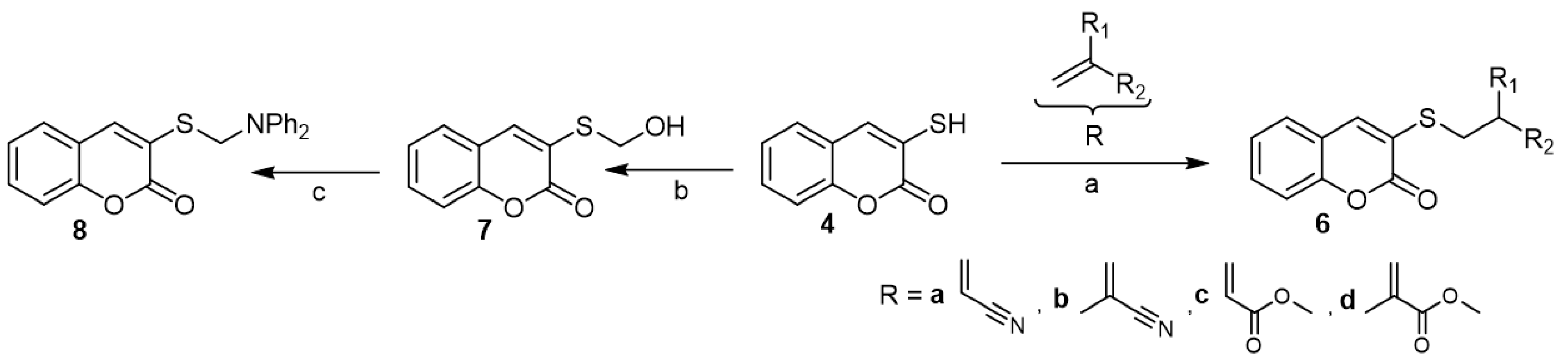
Scheme 3. Michael addition and Mannich reaction of 3-mercapto-coumarins (4). Reagents and conditions: (a) water, NaOH 10%, 50–56 °C, 4–12 h, four outputs with 40–92% yield; (b) formaldehyde, ethanol; (c) diphenyl amine, AcOH.
In a Chinese patent (2016), the author disclosed a method to fabricate benzothiophene-2-carboxylic acid (9) via a phase-transfer catalyst of 3-mercapto-coumarin (4) under high-pressure, 0.8–1.2 MPa (Scheme 4) [24].
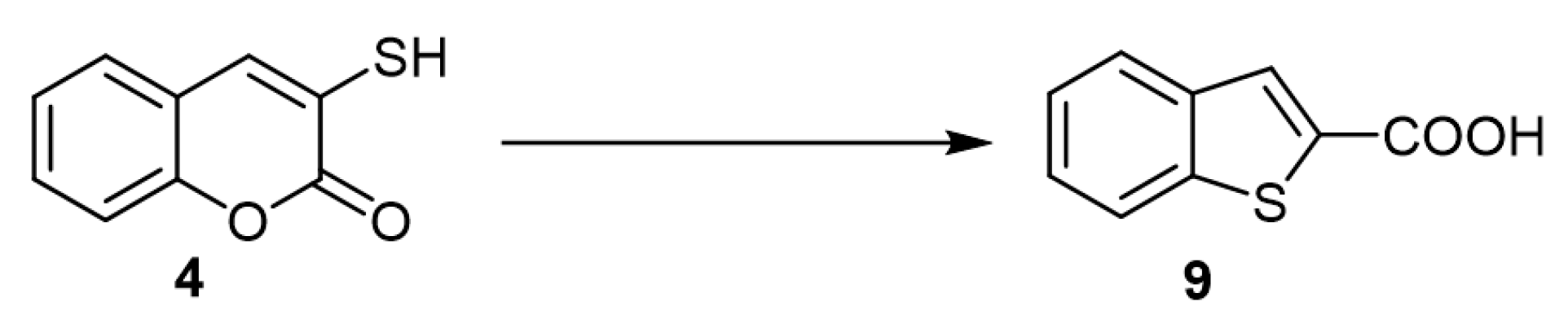
Scheme 4. Benzothiophen-2-carboxylic (9) acid via phase-transfer catalyst: Reagents and conditions: high-pressure vessel, 0.8–1.2 MPa; aq. KOH 37%; tetrabutyl ammonium hydroxide; 135 °C, 10 h; conc. HCl; 41.6% yield.
N-Acetyl-S-(3-coumarinyl)cysteine (11), which could be isolated from rat urine [25], was synthesized by the reaction of 3-mercapto-coumarin (3) and N-acetyl-3-chloro-D,L-alanine methyl ester (10) (Scheme 5) [26].
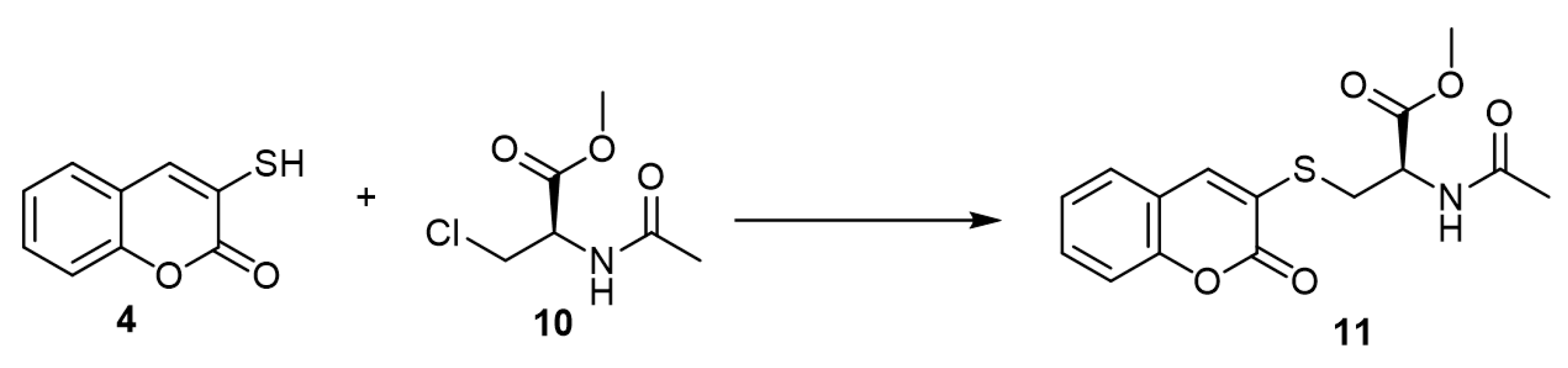
Scheme 5.
Synthesis of
N
-acetyl-S-(3-coumarinyl)cysteine. Reagents and conditions: TEA, acetonitrile, N
2
, 4 h, stirring, 47% yield.
4. 4-Mercapto-Coumarin
The synthesis of 4-mercapto-coumarin by methods based on 4-hydroxycoumarin has already been discussed [19,27,28,29,30][19][27][28][29][30].
In 1970, Peinhardt and Reppel allowed 4-hydroxycoumarin (12) to react with phosphorus oxychloride to get 4-chlorocoumarin (13). The latter, under reaction with potassium hydrosulfide in situ prepared from potassium hydroxide with methanol saturated with hydrogen sulfide (H2S), gave the corresponding 4-mercapto-coumarin (14) in a good yield, 90% (Scheme 6) [27].
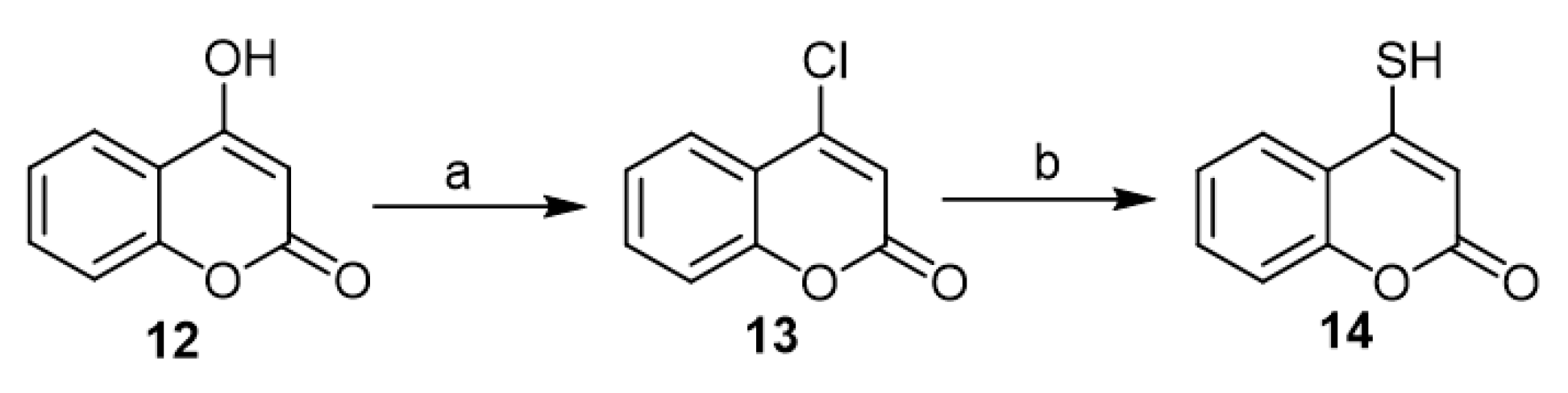
Scheme 6. The synthesis of 4-mercapto-coumarin (14). Reagents and conditions: (a) POCl3, reflux, 2 h, 55% yield; (b) KSH generated from (KOH, MeOH saturated with H2S), reflux, 90% yield.
Recently, the synthesis of 4-mercapto-coumarin (14) occupied the scope of interest of Ghosh’s work as an in situ transformed intermediate to synthesize different coumarin-fused heterocycles via 4-hydroxycoumarin (12) [19,28,29,30][19][28][29][30]. Dissolving the 4-hydroxycoumarin (12) in pyridine followed by the addition of toluene-4-sulfonyl chloride led to the formation of the tosyl derivative (15). Treatment of the latter with NaSH in ethanol furnished the corresponding 4-mercapto-coumarin (14), which succeeded by transformation to the final product (Scheme 7).
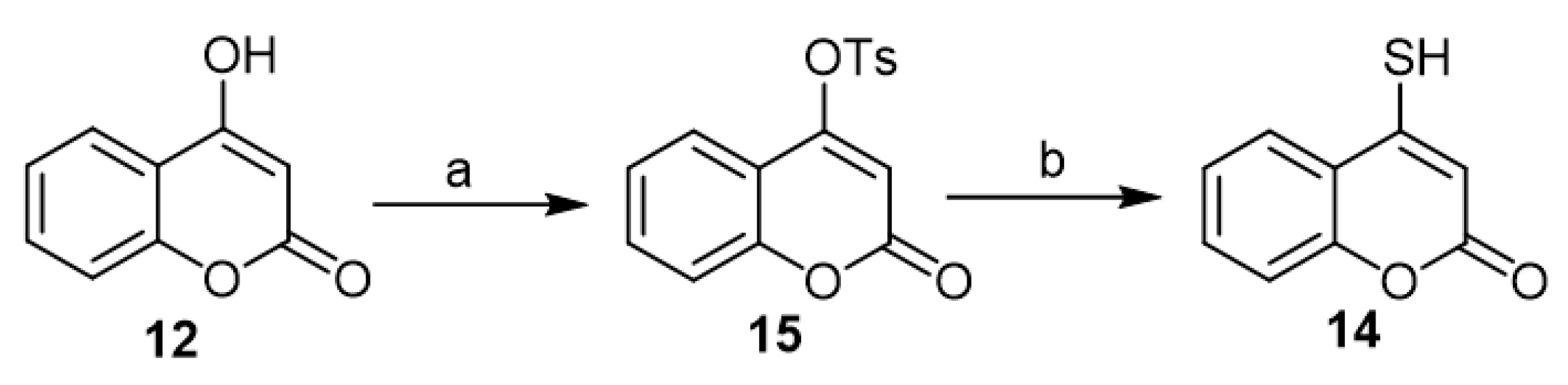
Scheme 7. The synthesis of 4-mercapto-coumarin (14) according to Ghosh’s work. Reagents and conditions: (a) TsCl, pyridine, 30 min, stirring, 90% yield; (b) NaSH, EtOH, 0–10 °C, 2 h, stirring, no yield was recorded as the product was used in the subsequent reaction without further purification.
5. Reactivity of 4-Mercapto-Coumarin
In 1975, Eiden and Zimmermannhe synthesized diphenylacetyl thioester (16) and biscoumarinyl sulfide (17) via the reaction of 4-mercapto-coumarin with 2,2-diphenylethen-1-one according to Scheme 8 [31].
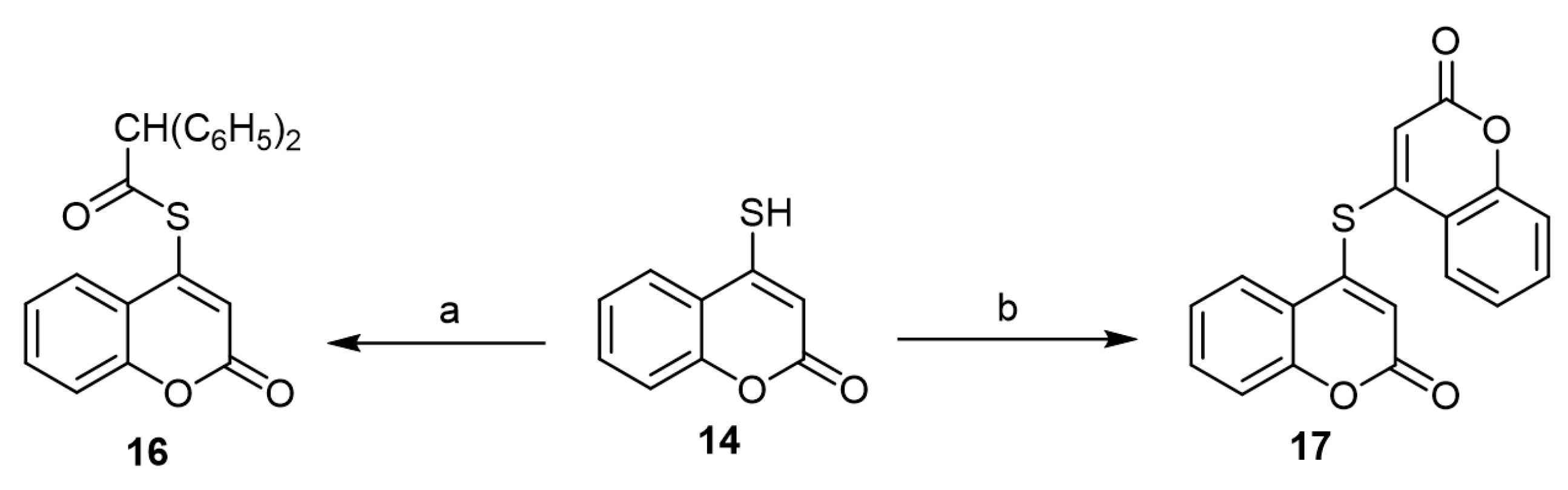
Scheme 8. The reaction of 4-mercapto-coumarin (14) with 2,2-diphenylethen-1-one. Reagents and conditions: (a) 2,2-diphenylethen-1-one, benzene, 16 h, reflux, 59% yield; (b) 2,2-diphenylethen-1-one, benzene, 5 h, 6.2% yield.
In the previous example of Ghosh’s work, 4-mercapto-coumarin served as a transitional compound to produce different coumarin-fused heterocycles employing 4-hydroxycoumarin (12) as a starting reactant. As the compound (14) was produced, it converted immediately to the final products (Scheme 7).
Accordingly, various 2H-thiopyrano[3,2-c][1]benzopyran-5-ones (19) [28,29][28][29] and 4-aryloxymethylthiopyrano[3,2-c][1]benzopyran-5(2H)-ones (21) [19,30][19][30] were prepared through the thio-Claisen rearrangement of 4-propargylthio[1]benzopyran-2-ones (18) and 4-[4-aryloxybut-2-ynylthio][1]benzopyran-2-ones (20) (Scheme 9). Compounds 18 and 20 were prepared based on a two-phase mixture of 4-mercapto-coumarin (14) with propargyl halides and 1-chloro-4-aryloxybut-2-yne, respectively (Scheme 9).
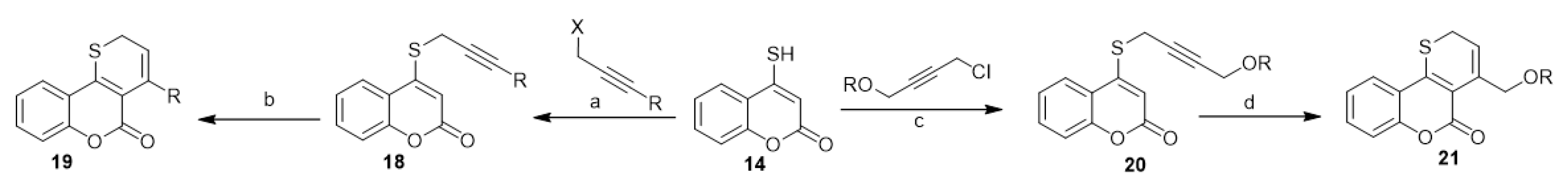
Scheme 9. Utilization of 4-mercapto-coumarin (14) in the synthesis of 2H-thiopyrano[3,2-c][1]benzopyran-5-ones. Reagents and conditions: (a,c) CHCl3, 1% aqueous NaOH, r.t., stirring, benzyltriethyl ammonium chloride (BTEAC) or tetrabutylammonium bromide (TBAB); (b,d) chlorobenzene, reflux, 30 min–4 h, six derivatives of 79–85% yield.
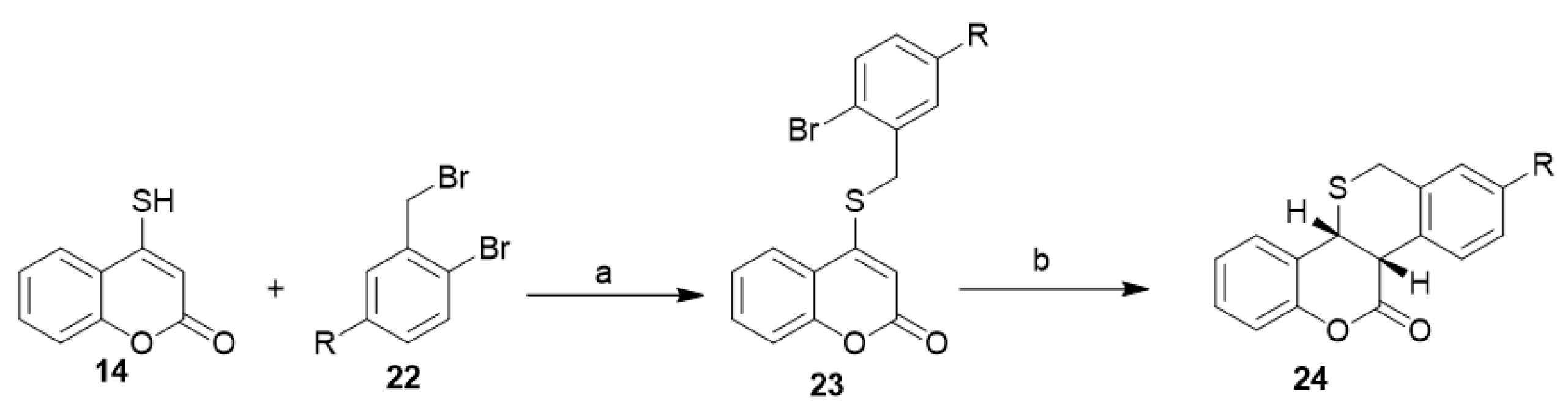
Regioselective synthesis of coumarin-annulated sulfur heterocycles, cis-benzothiopyrano[3,2-c]benzopyran-7(2H)-ones (24), was reported through aryl radical cyclization. The corresponding 4-[(2-bromobenzyl)sulfanyl]-2H-chromen-2-ones (23) was in situ prepared from a reaction between 4-mercapto-coumarin (14) and tributyltin hydride (22) in the presence of a radical initiator (AIBN) (Scheme 10) [32].
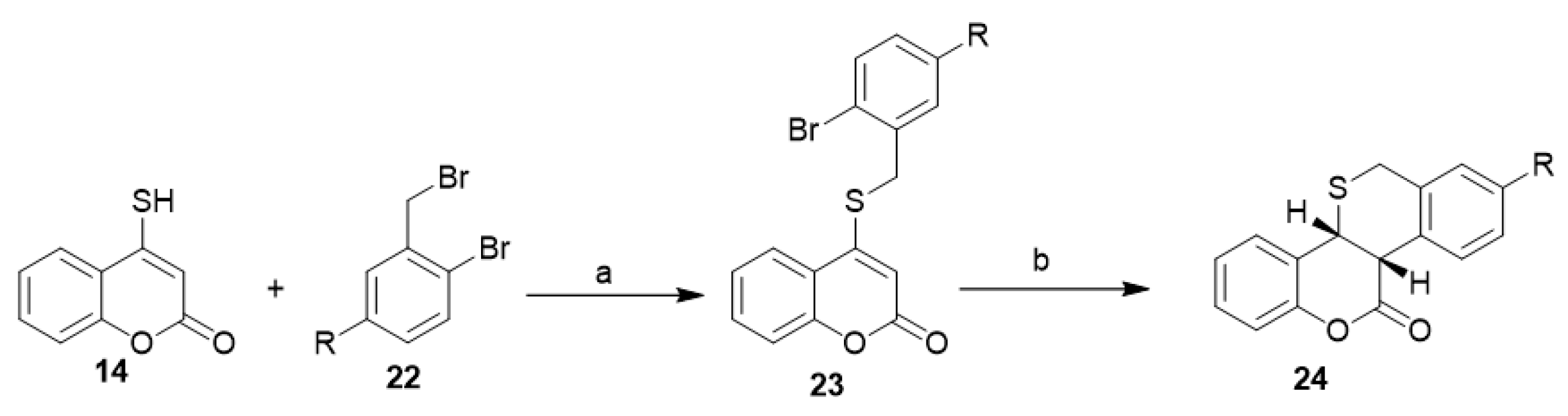
Scheme 10. Regioselective synthesis of coumarin-annulated sulfur heterocycles. Reagent and conditions: (a) 1% aq. NaOH–CHCl3, benzyltriethyl ammonium chloride (BTEAC), 1 h, r.t., R=H 83% yield, R=OCH3 85% yields; (b) Bu3SnH, AIBN, benzene, N2, reflux, 1 h, R=H 72% yield, R=OCH3 75% yield.
In another publication, some thieno[3,2-c][1]benzopyran-4-ones (27) were synthesized by thermal thio-Claisen rearrangement of 4-allylthio[1]benzo-pyran-2-ones (26) (Scheme 11). Compounds 26 resulted from a basic catalyzed reaction between 4-mercapto-coumarin (8) and different allylic halides (25). Without being separated from the reaction medium, compounds 26a–d ended in four different derivatives via phase-transfer-catalyzed alkylation using TBAB or BTEAC as a catalyst. The differentiation of the end products depended on the alkyl substitutions (R1, R2) on the allyl halide, which influenced the mechanism of the cyclization during the final step (Scheme 11) [33].
Scheme 11. Regioselective synthesis of thieno[3,2-c][1]benzopyran-4-ones (27). Reagent and conditions: (a) 1% aq. NaOH-CHCl3, benzyltriethyl ammonium chloride (BTEAC), stirring, 4 h, r.t., four products, 75–85% yield; (b) reflux, 0.5 h, HCl, four products, 65–80% yield.
Nematollahi et al. investigated the electrochemical oxidation of catechols (28) in the presence of 4-mercapto-coumarin (14) as the nucleophile in water/acetonitrile (50/50) solution. Through an EC mechanism and in a one-pot process, 4-(dihydroxyphenylthio)-2H-chromen-2-one derivatives 29a and 29b were afforded (Scheme 12) [34]. In another work of the same group, they explored the reactivity of catechol (28) and 4-mercapto-coumarin (14) in the presence of potassium ferricyanide as an oxidizing agent (decker oxidation) to develop thieno[3,2-c]chromen-6-onederivatives (30) (Scheme 12) [35].
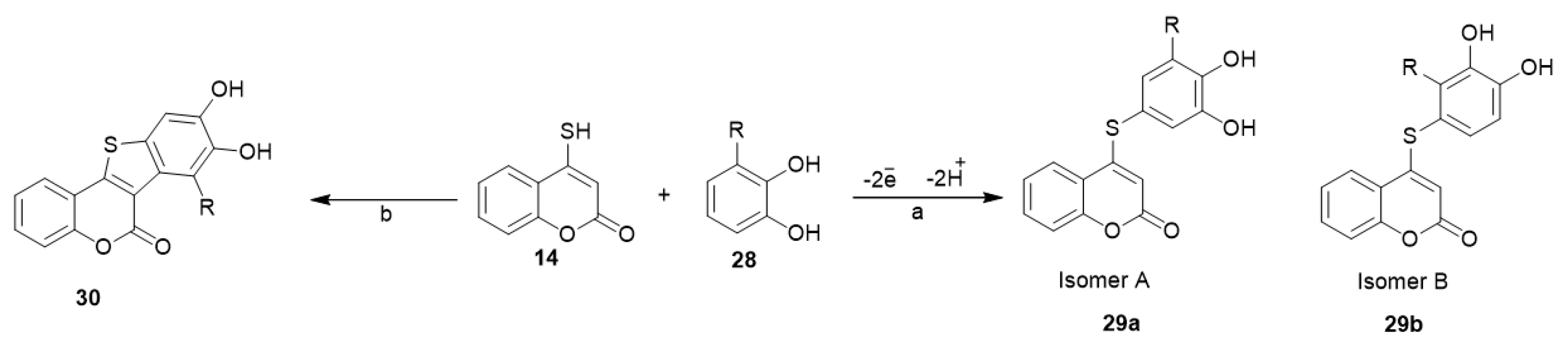
Scheme 12. Synthetic pathways for the reaction of catechol with 4-mercapto-coumarin. Reagents and conditions: (a) sodium acetate solution (c = 0.2 M) in water/acetonitrile (50/50), undivided cell equipped with graphite anode, a large stainless steel gauze cathode, 25 °C, R=CH3 isomer ratio with 52.5%/47.5% yield, R=OCH3 isomer ratio with 96.5%3.5% yield; (b) sodium acetate solution (0.2 M)/acetonitrile (70/30), stirring, r.t., 20–30 min, R=CH3 75% yield, R=OCH3 70% yield.
A series of 3-chloro-1-(5-((2-oxo-2H-chromen-4-yl)thio)-4-phenyl thiazol-2-yl)-4-substituted phenyl azetidin-2-ones (36) were synthesized in five sequential steps with the participation of 4-mercapto-coumarin (14) in addition to acetophenone (31), thiourea, and chloroacetyl chloride [36] (Scheme 13). The synthesized compounds showed potent antimicrobial activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus pyogenes, Aspergillus niger, Aspergillus clavatus, and Candida albicans [36].
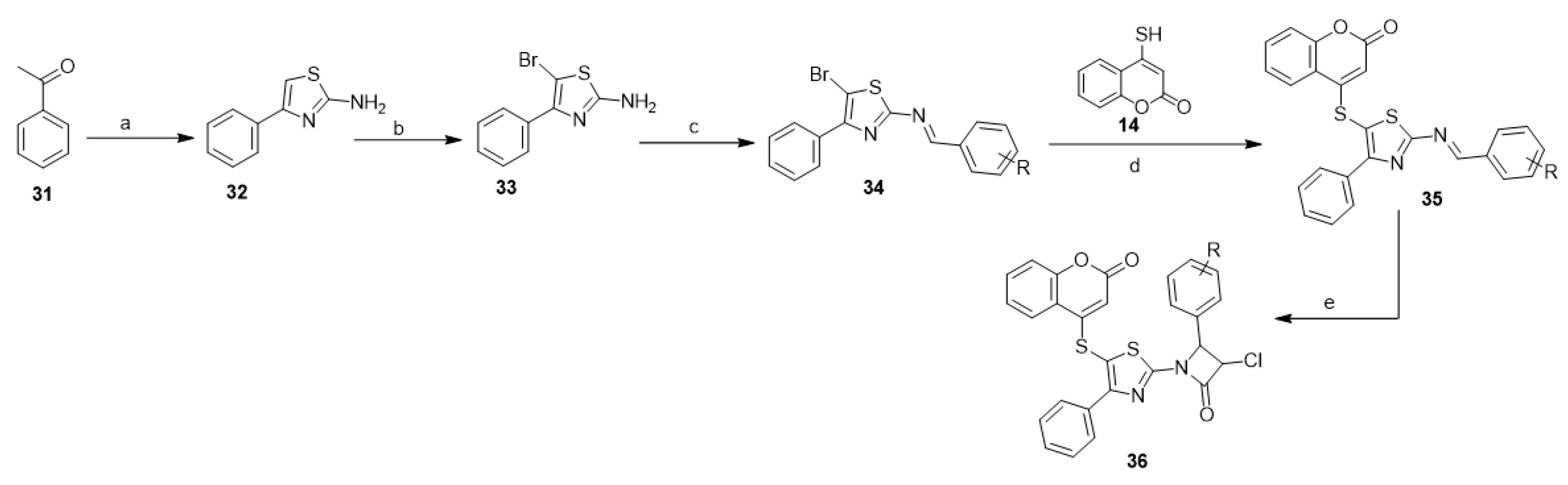
Scheme 13. 4-Mercapto-coumarin in the synthesis 3-chloro-azetidin-2-one derivatives (36). Reagents and conditions: (a) iodine, thiourea, ethanol, 2–3 h; (b) bromine, acetic acid reflux, 2 h; (c) aromatic aldehyde, acetic acid, ethanol, reflux, 6 h; (d) ethanol, reflux, 5 h; (e) chloroacetyl-chloride, triethylamine, 1,4-dioxane, reflux 7 h, 10 outputs 70–78% yields.
