Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nezar H Khdary and Version 4 by Amina Yu.
Metal oxides are essential in determining the overpotential and product selectivity in the CO2reduction reactions (CO2RR). In contrast, developing efficient and stable metal oxides is a significant challenge that must be achieved to lower the production cost of fuels in the practical implementation of CO2RR technologies. Earlier, several efforts have been made to develop metal oxides to convert CO2into value-added chemicals and fuels.
- CO
- capturing
- conversion
- carbon dioxide capture technologies
- metal oxide
1. Carbon Dioxide Capturing Processes
Post-combustion is the most suitable method harnessed for capturing CO2 because it involves the ready retrofitting of existing infrastructure. Researchers have developed four different CO2 capture techniques for post-combustion (Figure 1).
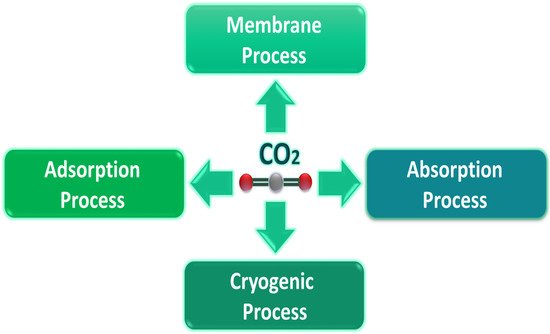
Figure 1.
Post-combustion carbon dioxide capture processes.
2. Carbon Dioxide Absorption
The absorption phenomenon occurs when the particles of a substance are distributed evenly over all parts of the substance (Figure 2A), whether liquid or solid. The difference in the adsorption of gas or liquid molecules and their adherence to solid material surface is diagrammatically represented (Figure 2B). In some reactions, absorption and adsorption coincide as the particles are absorbed, then the substance is removed. Subsequently, particles get adsorbed on the surface of the material, and this phenomenon is called sorption (Figure 2C) [1][27]. The absorption method is one of the most suitable methods for trapping CO2 because it has a high CO2 loading capacity, reasonable absorption rate, and low cost.
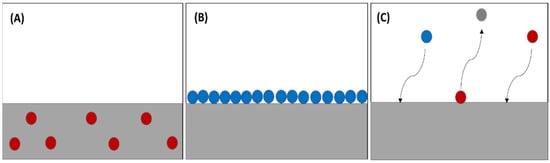
Figure 2.
(
A
) Absorption process, (
B
) adsorption process, (
C
) sorption process.
32. Metal Oxides for CO2 Uptake
Many sorbent materials have been used to increase the efficiency of capturing and absorbing carbon dioxide, including zeolite, activated carbon, and silica. Zeolite contains compounds of crystalline aluminosilicates that are characterized by chemical and physical properties, such as selectivity and high thermal stability [2][28]. Zeolite 13X (a bench-mark zeolite) showed absorption of up to 7.4 mmolg−1 and higher selectivity than activated carbon. However, zeolite consumes higher temperatures for regeneration [3][29]. Metal oxides were the most cost-effective and had lesser toxicity than zeolites and MOFs among the absorbents. In addition, the oxide-based sorbents can trap carbon dioxide with high selectivity when exposed to high temperatures [4][30]. Usually, alkaline earth metal oxide sorbents, for example.g., magnesium oxide (MgO) and calcium oxide (CaO), are utilized in the absorption CO2 process. However, these methods offer a few disadvantages [5][31]. The main drawbacks associated with the application of solid absorbents are the fast saturation and consuming energy for activation after several cycles [6][32]. Magnesium oxide was an excellent candidate for CO2 absorption performance [7][8][33,34] because it had a suitable surface morphology for oxygen generation that improved absorption performance and low regeneration energy consumption [9][10][35,36]. The incorporation of MgO-activated carbon nanofibers (ACNFs) for CO2 absorption was successfully integrated by electrospun activated carbon nanofibers (ACNFs) using the simple volumetric method. The fusion of thermally stable metal oxides contributes to the absorption of CO2 at its lowest temperature. The addition of ACNFs increases the production fusion of thermally stable, contributing to the absorption of CO2 at its lowest temperature. The incorporation of MgO-ACNFs showed a higher CO2 absorption capacity of 2.72 mmolg−1 [11][12][37,38]. Another study investigated magnesium oxide (MgO) nanoparticles (NPs) and MgO nanoparticles supporting activated carbon-based bamboo (BAC) for CO2 adsorption. The MgO nanoparticles that supported BAC had surface areas of 297.1 m2 g−1. ItThe was showedstudy shows that the physical adsorption of CO2 on MgO(NPs)-BAC was improved by 112% (39.8 mg g−1), compared to activated carbon-BAC (18.8 mg g−1) or MgO nanoparticles (12.8 mg g−1) [13][39].
MgO with fibrous silica was utilized to improve the performance of CO2 absorption via ultrasound-assisted impregnation [14][40]. MgO-fibrous silica showed the highest absorption of 9.77 mmolg−1, while fibrous silica without MgO showed an absorbance of 0.52 mmolg−1.
One of the most extensively utilized is calcium oxide for its distinguishing features for CO2 as a solid adsorbent. However, it has limited drawbacks, including excessive sintering and mechanical failure. The carbonation process and interaction between CaO and CO2 slow down once the initial layer of calcium carbonate is created [15][41]. Several studies have attempted to improve the stability and reusability of CaO as an adsorbent by employing various strategies. CaO derived from nanosized CaCO3 was studied by Florin et al. [16][42]. For the carbonation of CaO derived from nanosized CaCO3, the sorbent material was subjected to five carbonation cycles (24 h per cycle). They concluded that there was no morphological impediment when given enough time. After 100 CO2 capture-and-release cycles of 20 min, they found that the residual conversion capacity was 20% higher than previously reported for bulk CaO, demonstrating the potential of nanosized CaO. The increase of the reactivity of the adsorbent was investigated by Li et al. using an ethanol/water mixture to hydrate CaO. They found that the spent catalyst’s sorption capacity increased by two times as much as initially, and the sorption capacity of CaO could be improved by changing the synthesis method [17][43]. Belova et al. [18][44] used the strategy of dispersing CaO on an inert support, such as ɣ-Al2O3, to improve the sorption capabilities of CaO and minimize sintering. A layer of carbonate restricts the diffusion of CO2 and slows down the initial carbonation reaction, as previously stated. Increased dispersion reduces the possibility of sintering by increasing the surface area of the CaO particles. CaO dispersed on high surface area ɣ-Al2O3 resulted in a stable sorbent that could overcome the difficulties associated with limited long-term stability, slow uptake kinetics, and energy intensive regeneration [19][45]. Thermal gravimetric analysis (TGA) was used to investigate CO2 uptake kinetics and capacities, while multicycle experiments evaluated long-term stability. In comparison to bulk CaO powder, they found that dispersed CaO was a more effective low-temperature sorbent, with up to 1.7 times the capacity to bind CO2. It was found that CaO dispersed on ɣ-Al2O3 had better long-term stability than bulk CaO, which was tested for 84 CO2 capture-and-release cycles at 650 °C. While the bulk CaO’s adsorption capacity dropped below 50% after 20 cycles, the CaO/ɣ-Al2O3 adsorbent maintained 90% of its efficiency, with no sintering after the same number of cycles [20][46].
Adding metal oxides to an amino acid, such as lysine, for CO2 capturing is a promising process. Some groups had knownstudied the catalytic effect of metal oxides on the salts of amino acids, which have many advantages that make them suitable alternative absorbers for carbon dioxide [21][47] Some of these advantages are their high cyclic loading and good oxygen stability. It also allows the salt formation in potassium or lithium hydroxide, which makes the amino acid salt non-volatile in a stripper. The metal oxide catalytic activity depends on the defect sites present on the metal oxide surface [22][48] This metal oxide surface is exposed to water, which generates metallic hydroxides, suggesting that the metal oxides can enhance the carbon dioxide absorption kinetics of the lysine salt absorbent liquid, as shown in Figure 3.
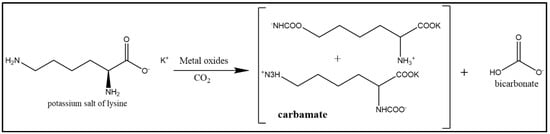
Figure 3. Structure of potassium salt of lysine (Adapted with permission from Ref. [47]. Copyright 2018 Elsevier).
Nanoparticles, such as zinc and cobalt, have been used to increase the absorption rate of carbon dioxide, which helps reduce operating costs [24][25][51,52]. The catalytic performance of Ni (NPs), as additives to optimize CO2 absorption in an MEA under different mixing conditions (limited- and high-mixing), was examined using two microfluidic platforms that effectively control polyphase flows. NPs (Ni) can increase CO2 absorption by 34% and 54% in limited- and high-mixing conditions, respectively. In addition, Ni (NPs) can reduce the amount of MEA needed in the system by speeding the lengthening time to achieve equilibrium with carbon dioxide absorption. ItThe wasstudy found that Ni (NPs) could still perform its function for more than 140 h [26][27][53,54].
The addition of TiO2, ZnO, and ZrO2 (NPs) were also tested with diethanolamine solution to investigate the effect on the absorption of carbon dioxide in a continuously stirred system using a stirrer bubble column, the influence of TiO2, ZnO, and ZrO2 nanoparticles, in aqueous piperazine solution, on the hydrodynamic and CO2 absorption rate was examined experimentally. The absorption performance of TiO2 and ZrO2 nanoparticles improves with solid-loading, up to a maximum value, then declines, and the TiO2, ZnO, and ZrO2 nanoparticles had optimal values of 0.05, 0.1, and 0.05 (wt%), respectively. The inclusion of ZrO2 nanoparticles had the minimum effect on the performance, compared to TiO2 and ZnO nanoparticles, and the maximum absorption rates of TiO2, ZnO, and ZrO2 were 14.7%, 16.6%, and 3.7%, respectively [28][55].
A nanocomposite of metal-organic–silica (MOS) was synthesized and functionalized, with propyl-ethylenediamine as a metal linker, for chemically attaching Cu2+ and Ag1+ to Cu and Ag nanoparticles for CO2 uptake. The diameter of Cu and Ag nanoparticles ranged from 5–20 nm. ItThe wasstudy showed that the composite gradually absorbed the carbon dioxide, with a saturation peak after 25 min, and incorporated metal nanoparticles with modified silica increased CO2 uptake from 20 to 100%. In addition, silica decorated with Cu nanoparticles showed the maximum CO2 uptake compared to Ag, Au, and Fe [29][60]. ItThe wasstudy, also noticed that the effect of nanoparticles is superior to that of metal oxide nanoparticles, especially in the case of copper.
To effectively trap carbon dioxide, the surface of silica particles was chemically modified with the amine. The particles were implanted on the membranes of polyvinylidene fluoride–hexafluoropropylene (PVDF–HFP). The results demonstrated that adding amino–silica particles improved the properties of the membrane and increased the CO2 uptake (0.8 mmol g−1) compared to the pane membrane [30][63].
The method of using a catalyst to capture and convert carbon dioxide is an important topic. In this Wcontext, work has been done utilizing a ZnO catalyst, integrated with Ru, with amine as a novel catalyst for CO2 capture and conversion to methanol. The ZnO catalyst assisted the reaction in a moderate condition, due to forming a ZnO–amide bond on its surface. As a result, a 30% yield has been achieved [31][64].
43. CO2 Conversion
4.1. CO
3.1. CO
2
Conversion Using Catalyst-Supporting Materials
Metal oxide interactions are widely used to improve catalytic capabilities, but they are frequently limited to instances where only one component is assisted by the other. The surface of metal oxides is a crucial factor for effective interaction with target molecules. It can be engineered to improve activity and sensitive properties. Thanks to recent breakthroughs in nanotechnology, metal oxides can now be used in novel ways [32][65]. Several transition metal oxides have electrical conductivity, a broad bandgap, and stability under reaction circumstances, making outstanding catalysts for turning carbon dioxide into valuable compounds using diverse processes [33][34][66,67]. Many factors affect carbon conversion reaction using metal oxides, including the catalyst surface reaction conditions and supporting material. The CO2 conversion process and conversion rate are greatly influenced by the catalyst, catalyst carrier, and integration strategies. Through the study of the influence of the carrier material, it is clear that the distance between the sites of the active metal oxide and carrier can be engineered to improve the interaction of the surface metal support by adapting the support integration method [35][68]. In this context, Bachar Alrafei et al., studied the conversion of CO2 to methane using Ni and (Ni–Co) catalysts supported on alumina in different ratios of nickel from 5% to 20 wt% and cobalt from 3% to 10 wt%. The optimum ratio was Ni 20 wt% and Co 10 wt%. These catalysts showed high activity at low temperatures. In addition, the selectivity was 100%, and it showed a high rate of methane production and carbon dioxide hydrogenation compared to other catalysts. In another perspective, nickel oxide, supported by titanium, showed more activity in CO oxidation than the catalysts of alumina and silica [36][37][69,70].
From another viewpone, int, a study on increasing the conversion rate of CO2 and methane through Co–Pt/MgO–Al2O3 bimetallic aerogel catalysts found that it had significant activity and improvement compared to Co/ Pt. The conversion rate of carbon dioxide reached 40%, while Co/Pt was less than 1%. Furthermore, the addition of Pt enhanced the ability to reduce CO, due to the electron transfer process between the active sites of metals [38][71].
For CO2 reduction into syngas, the Pt/TiO2 was utilized with photoelectrochemical assistance. The solar-to-syngas efficiency was 0.87%, and the stability was 10 h. The role of the metal/oxide catalyst in this process is to activate CO2 molecules and stabilise the primary chemical intermediates, whereas the function of the supporting surface is electronic induction [39][72]. Silver nanoparticles on MOFs, Ag@MIL-100(Fe), and Ag@UIO-66(Zr) have been tested to capture and convert CO2 into carboxylic acids. The Ag@MOF, under mild conditions, showed excellent activity and reusability for CO2 capture and conversion [40][73]. Pd/Ce-MOF have been developed for CO oxidation and CO2 uptake. The combination of Pd nanoparticles within Ce-MOF results in a unique catalyst capable of both CO oxidation at modest temperatures (370 K) and efficient uptake of the CO2 (at 273 K) [41][74]. A thin film of iron oxide has been employed as a supporter, and the conversion of CO2 to hydrocarbon has been achieved using copper tetramers (Cu4) on a thin film of iron oxide (Fe2O3) at atmospheric pressure, as the Cu4 on Fe2O3 could enhance methanol synthesis at low temperatures and has a selectivity of 63%. However, at temperatures above 325 °C, the reaction pathway shifted from methanol to methane (CO2 + 4H2 → CH4 + 2H2O), and methanol production deteriorated, whereas when the reaction was performed at 425 °C, the selectivity for methane reached 98% [42][75].
The interaction and activation of CO2 on the Bi2O3-TiO2 was examined. The results showed that the properties of bismuth-containing oxides could be utilized to adsorb and excite carbon dioxide, which is the crucial first step in the reductive conversion of CO2 to valuable molecules. This combination of Bi3+ in TiO2 species offers a fascinating composite that can stimulate CO2 by strong adsorption and alteration or electron transfer to produce a carboxylate or by direct breaking of the C−O bond [43][76]. The use of metal oxides to load the noble elements in carbon dioxide conversion reactions was carried out using different oxides (TiO2, SiO2, γ-Al2O3, ZrO2, and CeO2). The noble metals (Ru, Rh, and Pd) are effective catalysts in converting carbon dioxide [44][77]. Under moderate reaction conditions, the alkaline metal oxide (MgO) supported by (Pd) has been investigated. Pd/MgO was examined with commercial noble metal catalysts to verify its efficacy in converting carbon dioxide to methanol, using 5% wt of the metal, at 150 °C and 70 bars for 15 h, by adding K3PO4. The addition of K3PO4 to Pd/MgO showed a negative effect on converting carbon dioxide to methanol, as it was 0.3%, while, in the absence of K3PO4, it was 4.63% [45][78]. The productivity of the Pd/MgO catalyst is low compared to the Pd-Z1FO catalyst, which gave a 14% conversion rate to CO2. However, the linear association between methanol and the amount of PdZn shows that the PdZn increased the active sites, and the selectivity was 52% [46][79].
Among the metals, nickel is a promising metal due to its high activity and the fact that it is relatively cheaper than noble metals. However, Ni-based catalysts tend to be deactivated during the methanation reaction due to coke formation and sintering of Ni particles at high temperatures [47][80]. It has been suggested that the addition of ceria can enhance the catalyst performance and coke resistance; therefore.
4.2. CO
3.2. CO
2
Conversion Using Single Metal Oxide
Several studies have been conducted to separately evaluate the effect of metal oxides on the conversion process. For instance, five metal oxides (ZnO, SnO2, Fe2O3, La2O3, and CeO2) were synthesized using the sol−gel process in polyvinyl alcohol, and their catalytic activity was tested in direct carbonization of glycerol with CO2. The results showed that the best reaction condition was 180 °C, 150 bar, and 12 h of reaction time, and zinc oxide showed the best performance among other oxides, with a yield of 8.1% of glycerol carbonate [23][7]. Highly active Cox(CoO)1−x catalysts are used as catalysts for CO2 hydrogenation reaction. The association between catalytic reactivities and metal/metal oxide ratios and the functions of catalytic activities were examined. Metal ratios of x = 0.2, 0.5, and 0.8 were applied in the samples. The reaction was knownstudied using a Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) chamber, from a low temperature of 25 to a high temperature of 350 °C. The result demonstrated that the C–O bond could be easily broken on the cobalt catalyst to produce hydrocarbons. The maximum conversion was obtained at 350 °C, with a conversion rate of 98, 99, and 93% on Co(0.2), Co(0.5), and Co(0.8) samples, respectively [48][85].
4.3. Binary Metal Oxides for CO
3.3. Binary Metal Oxides for CO
2
Conversion
Binary metal oxides (BMOs) contain two metal ions that are either electrochemically active or inactive. BTMOs are characterized by their high conductivity and abundant active sites, making them more stable. BMOs are synthesized in several ways, including the template, electrodeposition, microwave-assisted, and solvothermal methods [49][86]. Zhou et al. used Fe3O6H6/Cu (111) as an analogue model for CuFe electrocatalyst by taking a CO2 aqueous environment, and a clear improvement was observed, compared to iron and copper separately because of a more suitable for electron-hole reinforcement than a copper catalyst, in addition to providing active centers to enhance kinetic properties [50][87]. Calculations of Density Functional Theory (DFT) revealed that the copper-iron binary system could operate in synergy to distort the linear formation of carbon dioxide, resulting in a significant reduction in activation energy and, thus, aiding in methanolic synthesis, with a 51% faradaic efficiency and more than twice that of 20% Cu and 0% Fe.
On the other hand, a two-metal, Zn–Cu electric catalyst introduced selectivity and high-efficiency activity to convert CO2 to CO, where DFT calculations demonstrated that the fusion of copper on the zinc surface reduced the activation capacity from 0.44 to 0.15 volts, increasing the selectivity of Zn–Cu to be 97% faradaic efficiency. In contrast, zinc selectivity was only 30% faradaic efficiency, indicating that incorporating copper into zinc generates a synergistic effect that improves the conversion of CO2 [51][88]. The highly cooperative win-win metal-oxide interaction that enables unprecedented catalytic functionalities for electrochemical CO2 reduction reactions has been investigated in a single SnOx/Ag catalyst. In the CO production mode, the oxide promotes the metal. In the HCOOH production mode, the metal promotes the oxide, resulting in potential-dependent bifunctional CO2 conversion to fuels and chemicals, with H2 evolution repressed throughout the potential window. The electron transfer from Ag to SnOx and dual-site cooperative binding for reaction intermediates at the SnOx/Ag interface are responsible for stabilizing the key intermediate in the CO pathway, changing the potential-limiting step in the HCOOH pathway and increasing the kinetic barrier in the H2 evolution pathway, all of which lead to highly synergistic CO2 electroreduction, as per spectroscopic studies and computational simulations [52][89].
Mixtures of two or more metal oxides, or with heterogeneous materials, may enhance the effectiveness of the catalyst in converting carbon dioxide to other materials because they contain oxygen in varying proportions, where can be divalent, trivalent, or tetravalent, which leads to the synergistic effect. For instance, knownstudying the hydrogenation of carbon dioxide to methanol while adding graphene oxide to CuO–ZnO–ZrO2 catalysts significantly enhanced the methanol selectivity and production rate of the catalysts. There are more active sites for CO2 and H2 adsorption when added 0.5–2.5 wt% (GO). When graphene oxide content exceeded 2.5 wt%, the size of the CuO particles grew significantly, resulting in a lower rate of methanol production. The graphene oxide-free CuO–ZnO–ZrO2 catalyst was compared to the graphene oxide-coated CuO–ZnO–ZrO2 catalyst at different temperatures [53][90].
4.4. Metal Oxide Nanoparticles for CO
3.4. Metal Oxide Nanoparticles for CO
2
Conversion
Nanoparticles (NPs) are a broad class of materials that include particulate substances, with one dimension less than 100 nm. This broadly divided NPs into various categories, depending on their morphology, size, and chemical and physical properties [55][56][92,93]. Copper nanoparticles (NPs) were able to selectively transform C2–C3 products (ethylene, ethanol, and propanol) with low redundant capacities. Some researchers found that, when making copper NPs at high temperatures, the copper NPs transformed into cubic-like particles mixed with smaller nanoparticles.
These structures can selectively generate C2 and C3 products together, C2–C3 faradaic efficiency (FE), reaching 50%, at only −0.75 V, illustrating the importance of on-site structural evolution. In contrast, it was concluded that catalyst support plays an essential role in the high selectivity of the multiple carbons. Furthermore, they found that the structural transformation can occur not only under low bias conditions but is controlled by the initial arrangement of NPs. Thus, the catalytic behaviour can be significantly improved [57][94].
