SOur planet urgently needs sustainable solutions are needed to avoid to alleviate the anthropogenic global warming and climate change. Homogeneous catalysis mighthas the potential to play a fundamental role for this. Pin this process, providing novel, efficient, and at the same time eco-friendly routes for both chemicals and energy production. In particular, pincer-type complexes are ligation shows promising iproperties in terms of long-term stability, and selectivity, efficiency and the use ofas well as allowing for mild reaction conditions. P and low catalyst loading. Indeed, pincer complexes have been used in manyapplied to a plethora of sustainable chemical reactions, for exampleprocesses, such as hydrogen release and up, CO2 capture and conversion of CO2, N2, N2 fixation, and biomass valorization for the synthesis of high-value chemicals and fuels.
1. Introduction
PDuring the last 15 years, organometallic pincer
-type complexes
arehave emerged as a highly promising
group of catalysts for numerous
sprocesses within sustainable
processes. High catalchemistry. They have been applied in energy production through hydrogen generation, dehydrogenative synthesis of high-value chemicals, as well as CO2 and N2 hy
drogenati
c activity under milons for carbon dioxide capture and recycling and a more sustainable ammonia production, respectively. As such, the use of this family of homogeneous catalysts enhances the sustainability of an incredible number of chemical processes. High catalytic activity at mild reaction conditions, low catalyst loading,
and hcombined with high selectivity a
nd excellent atom efficiency are the general main advantages
important for . Notably, all these aspects are crucial when considering the sustainab
le reactions as guidility of chemical processes, as dictated by the green chemistry guidelines
[1].
IUn
creased robustness because of the pincer stabilization results in increasinly stable fortunately, catalyst deactivation and/or degradation are usually the main drawbacks of homogeneous catalysis, otherwise excellent systems in terms of activity, selectivity, and reaction conditions. The currently employed heterogeneous alternatives are more robust and with an established know-how on the processes, but they usually require high temperatures and hence are high-energy demanding. Pincer-type ligations provides increased robustness because of the stabilization of the tridentate coordination, resulting in homogeneous catalytic systems
[2][3][4]with increased chemical and thermal stability [2,3,4].
The
ligand design of pincer complex
es offers numerous possibilities as well as potential catalytic applications [5,
6]. Fo
f the r example, the pincer arm can bear an array of different heteroatoms and functionalities. In addition to affording chemical stability, the pincer ligand can take active part of the catalytic cycle by providing a suitable coordination site for the substrate, weakening selected bonds (H-X bonds), or accepting/donating electrons and protons. Moreover, the cooperation between the central metal atom and the ligand is tunable based on the desired steric/electronic environment and catalytic application.After the first family of PCP
tycomp
e,lexes was synthetized by Shaw in 1976
[5].[7], Nnume
ous pincer comrous research groups have applied this concept for almost all types of homogeneously catalyzed chemical reactions. A myriad of novel complexes
have since with different pincer arms have been synthetized
as and characterized, including a vast family of carbene pincers [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23], PNP [24,25,26,27,28,29], PNN [30,31], POP [32], PCP [33,34,35,36,37,38], SNS [39,40], NCN [41], NSiN [42], CNC [43], CNN [44], NNN [45], as well as
sulfu
r- [46,47,48], s
ilicon- [49,50], sele
nium- [51,52], and
boron-functi
n onalized [53,54] pinc
er liga
tands. Indeed, this topic represents one of the most attractive areas in homogeneous catalysis
[6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127] [55,56,57,58,59,60,61,62,63].
ExMa
mples areny of the most promising results in sustainable transformations have been achieved with second and third-row transition metals, such as Ru [40,64,65,66,67,68,69,70,71,72], [128][129][130]Os [73,74,75,76], Ir [77,78,79,80,81], Rh [82,83], and Pd [84,85,86,87,88,89]. Nevertheless,
th
ydroame current trend in the scientific community is to identify cheaper alternatives based on earth-abundant metals such as Fe [90,91,92,93,94,95,96], Mn [97,98,99,100], Ni
[101,102,103,104,105], V [106,107,108], an
d Co [109,110,111,112,113,114]. In pa
rti
cular, iron
[131][132][133],and manganese PNP pincer complexes sh
ydrocarboxylationow optimal performance in many relevant sustainable transformations [134],in the optic of the hydro
gen economy. Sev
ineral excellent reviews cover this relevant transition toward first-row metals for a more sustainable chemical production [115,116,117,118,119,120,121,122,123,124].
More recently
, the incorporation of pincer compl
atioexes into porous materials acting as supports has been
[135]investigated using the supported (ionic) liquid phase catalysis (SILP or SLP) [125,
126]. The idea
minomethylation is to combine the excellent activity of homogeneous systems with the robustness given by the heterogeneous nature of the support. Important examples using pincer-type homogeneous catalysts can be found in aldehyde hydrogenation [136]using Fe(II)-PNP complexes [127,128], a
nd continuous-fl
ow alkane dehydrogenation
[137][138][139][140][141][142][129].
Furthermore,
several
kane metathetis groups have been exploring the use of pincer complexes for a wide series chemical transformations, further expanding the applicability of this family of catalysts. Representative examples include [143]olefination [130,131,132], hydroamination [133,134,135], hydrocarboxylation [136], hydrovinylation [137], amin
ome
thylation [138], dehydrogenation of alkanes [139,140,141,142,143,144], alkane metathetis [145], N-formylation
[144][145]of amines [146,147],
C-alkylation of secondary alcohol
Cs [148,149], α-alkylation
[146][147],of ketone
s α-[150,151], and alkylation
[148][149]of amines [152,153,154,155,
156] and a
nilines [157].
The deoxydehydration (DODH) of biom
ass-derived vi
ne alkylcinal diols and polyols has also been explored by employing metal pincer complexes. The reaction proceeds in the presence of a sacrificial reducing agent and results in the formation of alkenes, relevant building blocks for the polymer industry. Some examples using pincer ligation
[150][151][152][153][154]are reported with vanadium [106,107], rhenium [158], a
s well as molybden
ilum pincer catalysts [159]. The fi
eld is relatively immature an
e alkylationd further optimization is necessary. A comprehensive overview of the best catalytic systems for DODH reactions, including the aforementioned [155]pincer complexes,
is provi
cinalded in the detailed reviews of Fristrup [160] and
i Monbaliu [161].
Remarkably, there are several repo
rts in l
deoxydehydrationiterature using pincer-type metal complexes as suitable catalysts for water splitting reactions. The process is key for the development of the hydrogen economy that requires green and sustainable hydrogen produced via solar or wind [104][105][156][157][158][159],energy. Many groups explored various combinations of metals and
wpincer ligation [14,162,163]; in pa
rt
er splitticular, several works report the use of Milstein Ru-PNP catalyst 3 for thi
s tran
gsformation [12][160][161][162][163][164][165][166][167][164,165,166,167,168,169].
2. Dehydrogenation Reactions
2.1. Early Works
In tThe
1960s, Charman showed the first example of
acceptorless alcohol dehydrogenation by homogeneou AAD by homogeneous catalysis dates back to 1960s with the work by Charman, using rhodium chloride as catalys
ist [168][198]. In the mid-1970s, Robinson d
escribe
mod the ruthenium complex [Ru(OCOCF3)2(CO)(PPh3)2] in
s combination with tr
ated ifluoroacetic acid for the dehydrogenation of isopropanol, 1-butanol, ethanol, methanol, and glycerol
[169][170][171][172][199,200,201,202].
Several improvements were achieved in the subsequent 20 years, using various type of homogeneous systems in combinations with a range of additives, including light irradiation.
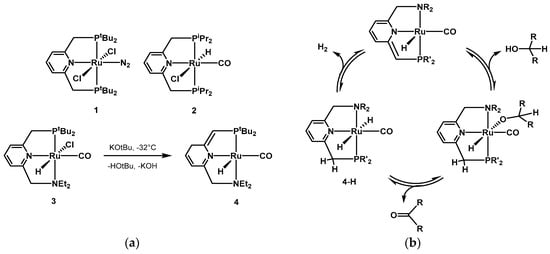
In 2004, Milstein presented the first example of metal–ligand cooperating pincer ligands in AAD for synthetic purposes
[173][174][203,204]. In
2015,a Li reporteseries of ruthenium(II)-based comp
utational mechanistic studies on several reactions using the lexes, the PNP pincer bearing a pyridine moiety and various phosphine substituted side arms was found to be very active for the dehydrogenation of simple secondary alcohols. Some examples of the first generations of Milstein
PNP’s catalysts can be found in Scheme 1a. In most cases, there is a direct participation of the pincer ligand
PNNs in the cataly
ststic [175]cycle.
The Simultaneouslypyridine moiety rearranges by aromatization-dearomatization [64,
177,205,206,207,208,209], facilitat
he Beller group starteding the coordination of the alcoholic substrate and the subsequent hydrogen release from the dihydride species, as depicted in the catalytic cycle in Scheme 1b. The
xploring the in situ involvement of the pincer moiety in the catalytic cycle has been investigated by many groups [210,211,212,213,214,215]. In 2015, Li
reported computation
fluence of various phosphines and nial mechanistic studies on several reactions using the Milstein PNP and PNN catalysts [216]. The aut
hor
ogen contas recalculated rate-determining
ligands mixed with ruthenium catalyst precursorsteps and investigated the aromatization-dearomatization equilibria. It was found that aromatic PNP and PNN ligands often provide the lowest activation energy for some steps, whereas for
the dehydrogenation of isopropanolother steps, the aromatization–dearomatization process was not involved [176]. Iin
athe following work from 2011, Beller tested both known catalystlowest energy pathway. Very recently, Gusev investigated the mechanism of AAD of alcohols, as well as
the in sester hydrogenation, using catalyst 4, and i
dent
u formation ofified the dihydrido complex 4-H (Scheme 1b) as the active species
usfor both dehydrogenati
ng coon and hydrogenation reactions [217].
Scheme 1. (a) Examples of Milstein’s first generation pincer catalysts [204]; (b) example of acceptorless alcohol dehydrogenation (AAD) reaction mechanism using Milstein’s type PNN pincer complexes based on aromatization/dearomatization of the pyridine moiety.
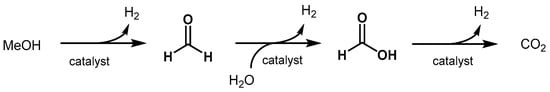
2.2.1. Methanol Dehydrogenation
The hom
biogeneous dehydrogenation
s of Ru-precursors of methanol to H2 and
CO2 Nin the optic of a methanol-
based econ
taining pincer ligands.omy has been intensively studied over the past decade. The topic has been reviewed by Alberico and Nielsen in 2015 [320], by Prakash Iin 201
38 [321],
Band very rece
ller showedntly by Araya, Liso, Cui, and Knudsen Kær [322]. Importantly, meth
e efficient converanol is currently produced from fossil fuels through syngas, hence a sustainable production from biomass or/and atmospheric CO2 is
hi
ghly desirable (see Section 3.1.2). The aqueo
us reformin
of g of methanol in
to ethyl acetavolves three consecutive steps yielding three molecule of hydrogen for each molecule of methanol (Scheme 9). The first
ste
using Ru-MACHO,p is the dehydrogenation of methanol to affor
eported in 2012 to efficiently catalyze ester d formaldehyde and the first equivalent of hydrogen. Then, formaldehyde reacts with water to form methanediol that can undergo a second dehydrogenation
[177].resulting Iin
2014, Beller showed that it is feformic acid. The latter is further dehydrogenated to finally produce CO2 a
snd the thi
ble to genrd molecule of hydrogen.
Scheme 9. Reaction pathway of aqueous methanol reforming.
The
r
ateforming of methanol to produce hydrogen
from ethanol/water mixtures as well as from industrial bio-is currently carried out at elevated temperatures (>200 °C) and pressures (>25 bar) by means of heterogeneous catalysts such as Pt/ Al2O3, as we
ll as th
anoe less expensive Cu/ZnO/Al
2O3 [323,324,325,326,327,328]. As discussed belo
btained from fermentation processes, without prior removal of the water content (5%) [178]w, pincer complexes allow the direct release of hydrogen gas from aqueous methanol at temperatures below 100 °C, with low catalyst loadings as well as promising stability properties.
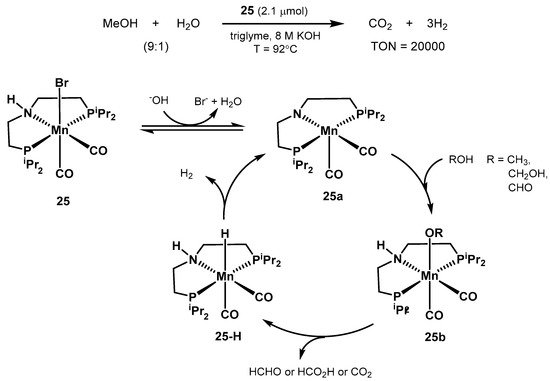
2.2.1. Methanol Dehydrogenation
In 2017, Beller proposed
the structura
lly defined manganese c
omplex 25 a
s an act
aive catalyst for aqueous methanol dehydrogenation
[179](Scheme 16) [346]. The optimized conditions resulted in 20,000 turnovers after 900 h at 92 °C, starting from a 9:1 CH3OH/H2O mixture in triglyme, with 8M KOH, and in the presence of 10 equivalents of the PNP ligand to the catalyst (2.1 μmol).
EMoreover, other organic carriers such as ethanol, paraformaldehyde, and formic acid were
alsosuccessfully dehydrogenated
. Bel as well. Unlike the previous work by Bernskoetter, Hazari, and Holthausen [336], the presence of Lewis acid additives resul
te
r ald in no observable improved catalytic activity.
Scheme 16. Manganese-catalyzed methanol reforming and proposed catalytic cycle proposed by the group of Beller [346].
The s
ame gro
up showed that
an ir, similar to the PNP complexes of ruthenium, iron, and manganese, also the iridium-PNP catalyst
8 is able to promote
s methanol dehydrogenation under mild conditions
[180]albeit with lower catalytic activity [347]. Complex 8 afforded a TON of 1900 after 60 h at 92 °C, showing promising stability over time. In
this case too, highly basic conditions were required (8M KOH) for consistent catalytic activity starting from a 9:1 mixture of CH3OH/H2O.
In 2019, Beller improved the
performa
ctivitynce of Ru-PNP catalysts for methanol dehydrogenation using a
nother bi-catalytic system
[181]formed by the catalysts 10/10-Me (Scheme 13b) [335]. Based on observations on the formic acid dehydrogenation step [348], the addition of catalyst 10-Me promotes the rate of hydrogen release from formic acid in the last step. The combination of the two catalysts together was 1.5 times more active than individually. Employing 8.56–9.62 μmol of 10 + 10-Me in a 9:1 MeOH:H2O mixture in triglyme, with 40 mmol KOH at 92.
5 °C, afforded a clean 3:1 H2/CO2
mixture with TOF = 1063 h−1 and a TON = 3189 (calculated based on total amount of catalysts present) after 3 h. This work further shows that applying bi-catalytic systems in cascade reactions is a promising solution to improve the catalytic performance, taking advantage of synergetic effects between two active catalytic species.
2.2.2. Formic Acid Dehydrogenation
FThe use of formic acid as hydrogen carrier and storage system has been
widely investigated and reviewed in
various works [299,300,350,351,352,353,354,355,356,357,358,359]. A variety of both heterogeneous and hom
ogeneous ca
ntalytic systems have been applied for its decomposition, including the use of light irradiation [360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381].
In 2011, following previously
reported works
on iron-catalyzed formic acid [182][183][184][185][186][187][188][189][190][191][192][193]dehydrogenation [382,383]. Beller reported the remarkable activity of an iron complex bearing the tetradentate tripodal ligand tris[(2-diphenylphosphino)ethyl]phosphine [P(CH2CH2PPh2)3 (PP3) [384].
FThe authors tested both the in situ formation of the active species, as well as various synthesized iro
r examn hydride complexes bearing the same PP3 ligand. The activity of the synthetized catalysts was found to be comp
arable
with that of the in situ formed systems in the presence of 2 equivalents of PP3 ligand. After optimization,
simply applying 5 mmol of Fe(BF4)2·6H2O and 2 equivalents of PP3 to a solution of formic acid in propylene carbonate, afforded a TOF of 9425 h−1 and a surprising TON of 92,000 at 80 °C.
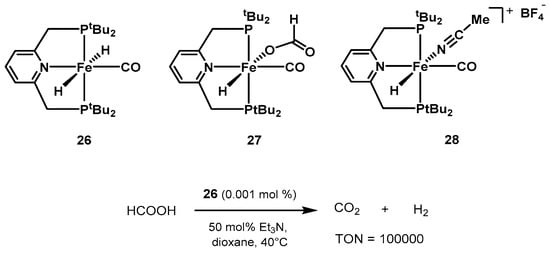
Contemporarily, Milstein
was als
hoo investigating the performance of iron pincer complexes based on the lutidine moiety, reporting suitable catalysts for the hydrogenation of ketones [385,386], as we
ll as CO2 hyd
rogenation to formate [387]. In 2013, the group showed a seri
es of iron pincer complexes
as active catalysts for formic acid dehydrogenation (Scheme 17) [388]. The dihydrido complex trans-[Fe-(tBuPNP)(H)2(CO)] 26 show
ed the best performance, reachi
th a lng TON values up to 100,000 at 40 °C in the presence of trialkylamines. 0.001 mol% of 26 in 1,4-dioxane, in the presence of 50 mol% NEt3, resu
lt
ided in full conversion of formic acid after 10 days.
Scheme 17. PNP pincer catalysts screened by Milstein for low-temperature formic acid dehydrogenation [388].
In 2018, Beller reported that also the known rutheni
um dihydride [RuH2(PPh3)4] is a suitable catalyst for the dehydrogen
ation of aque
moous formic acid at low temperature (TOF up to 36,000 h−1 at 60 °C i
n THF) [407]. The
cat
y as cataalyst was active for 120 days and it does not require basic additives.
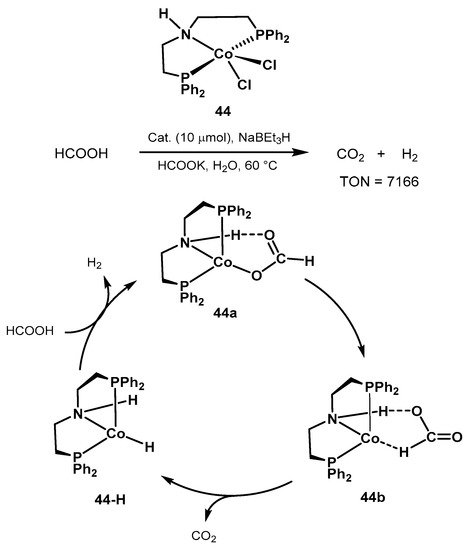
The same group al
so proposed the cobalt catalyst
s precursor 44 shown in Scheme 29 for
formic acid dehydrogenation
oin aqueous media and at mild conditions [408]. Reactions were perf
ormed ketonesat 60–80 °C in the presence of HCOOK. Since the catalytically active species are air-sensitive, the authors investigated the in situ activation of the pre-catalyst 44 in the [194][195]presence of NaBEt3, resulting in the active hydrido complex 44-H (Scheme 29). Importantly, under optimized conditions,
COthe benchmark Ru-MACHO resulted in scarce H2 evolution, wh
yereas the manganese catalyst 25 showed
no activity in aqueous conditions. The author
ogenation to s proposed an outer-sphere mechanistic cycle similar to the classic Ru-PNP catalyst, with the amine proton taking active part in the catalytic cycle. The authors concluded that the rate-determining step is the C-H activation resulting in CO2 release and forma
tion of t
he amine
[196],complex. The work provides useful informa
nd fortion for the development of non-noble metal catalysts for formic acid dehydrogenation
[197].
Scheme 29. Novel cobalt-PNP pincer complex for low-temperature formic acid dehydrogenation proposed by Beller [408].
3. Hydrogenation Reactions
PFor case of brevity, the focus will be on processes involving carbon dioxide and dinitrogen as the main substrates of interests, as well as chemical transformations promoting the valorization of biomass-derived molecules. Nevertheless, pincer complexes have
been usedachieved remarkable results in the (transfer) hydrogenation of
manya wide series of substrates
, such as ketones
[194][198][199][200][201][202][203][385,457,458,459,460,461,462], esters
[38][204][177][195][205][206][207][208][209][210][211][212][213][214][215][216][217][218][219][220][40,179,220,386,400,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477], aldehydes
[221][222][223][478,479,480], amides
[65][224][225][226][227][228][67,481,482,483,484,485], and imines
[229][230][486,487].
C
O2arbon dioxide is
sadly known to potentiallbe the main responsible for the anthropogenic climate change and global warming [488,489,490,491]. At the same time, CO2 represents an easily a
ccessible C1 building block
, i with the potential to replace the commonly used petrochemical carbon sources in a plethora of useful chemical transformations, dramatically increasing
stheir intrinsic sustainability
[231][232][233][492,493,494].
CO2Several approaches have been investigated for its capture from the atmosphere
or, as well as from localized emission sources
[495,496,497,498,499,500,501,502,503,504]. Th
ave
been studidesorbed
[234][235][236][237][238][239][240][241][242][243]. CO
2 is subsequently
scompressed and stored i
or n underground rock formations or utilized in
the direct synthesis of value-added products
[244][245][246][247][248][249][505,506,507,508,509,510].
TIndeed, the industry
already uses several million tons of CO
2 for
the produc
ing e.g.tion of e.g., urea, salicylic acid, cyclic carbonates, and polypropylenecarbonate
[250][251][252][511,512,513].
CIn the past decades, the catalytic hydrogenation of CO
2 has gained attention
for storingas a powerful tool to store green hydrogen
with, thereby electrical energy, as introduced by the seminal works by Asinger
[253][514], Leitner
[254][255][515,516], Noyori
[256][517], a
nds well as Olah
[257][258][259][260][301,518,519,520]. The
process
ynthesi, combined with the aforementioned methanol/formic acid dehydrogenation reactions, has the potential to close the ideal cycle of CO2-free energy releas
e ofand storage. Currently, both methanol
from and formic acid are industrially produced using fossil feedstock via carbon monoxide, which has lower kinetic and thermodynamic stability compared to CO
2. is The direct synthesis from CO2 is tradit
ypi
conally carried out at high temperatures and pressures
bywith heterogeneous
metal catalysts such as Cu/ZnO/Al
2O
3 [261][262][263][264][521,522,523,524]. Thus, the sustainability of the direct CO2-route is strictly dependent on the use of green hydrogen produced without contemporary CO2 release in the atmosphere, as well as catalytic systems operating efficiently under milder reaction conditions.
HA variety of homogeneous catalytic systems ha
ves been
usedemployed for the
direct hydrogenation of CO
2 into green fuels [213,214,517,525,526,527,528,529,530,531,532,533,534,535], in
cluding first
o -row metal complexes [536,537,538,539,540,541,542,543,544]. Pincer-type lig
ation shows again ver
een fuelsy encouraging features in terms of stability and mild reaction conditions, with promising possibility of further optimization. In addition, often the same catalyst is active [265][266][256][267][268][269][270][271][272][273][274][275][276][277][278][279][280][281][282][283][284][285][286]in both directions of hydrogenation and dehydrogenation, expanding the applicability and robustness of this family of catalysts.
3.1. CO
2 Hydrogenation
Hydrogenation
3.1.1. Early Works
The hydrogenation of carbon dioxide by means of homogeneous catalysis has grown extensively in the last decade. An overview of the best performing systems for CO
2 hydrogenation up to 2010 can be found in the work of Beller
[287][557], while in 2018 and 2019 Prakash reviewed the topic in depth including the use of pincer type complexes
[288][289][558,559].
In this review, we will focus mostly in CO2
conversion to formic acid (and/or formate salts) as well as to methanol, both of them representing accessible green fuel and hydrogen carriers.Up to 2010, the best performing catalytic system was represented by the iridium-PNP catalyst
9 (shown in Scheme 2), reported by Nozaki in 2009, which overcame previously reported Ru
[290][291][292][293][560,561,562,563], Rh
[294][295][296][564,565,566], and Ir
[297][567] homogeneous systems. In
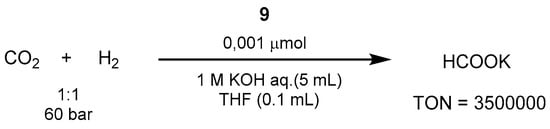
2011, Milst
ein and co-workers published the first example of hydrogenation of carbonates into alcohols and carbamates into alcohol and amines as an indirect route forhe work by Nozaki, the reactions were carried out in aqueous KOH, resulting in potassium formate (HCOOK) as the product [568]. tTh
e synthesis of meus, using the trihydridoiridium(III)-PNP complex 6 at
h 120 °C an
ol fromd 60 bar of 1:1 CO
2/H2 [298]in 1.
0 The same year, Sanford proposed a cascadeM aqueous KOH it was possible to achieve excellent TON and TOF values of 3,500,000 and 150,000 h−1, re
aspecti
vely (Scheme 37).
Scheme 37. CO2 hydrogenation with Ir-PNP catalyst reported by Nozaki in 2009 [568].
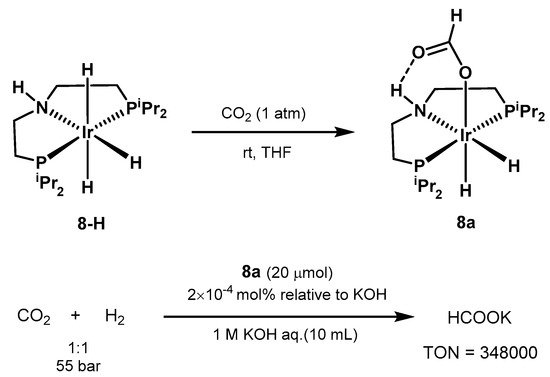
The use o
f iridium pin
mechanism for CO2 hcer complexes was further explored by
d Hazar
i in 2011 [569]. The autho
grs were
nation using the Milstein able to isolate the air and moisture stable catalyst
248a, obtained from 8-H in
the presenc
oe of CO2. Under optim
bi
nation with other two homogeneous catalysts, i.e., (PMe3)4Ru-(Cl)(zed conditions, the system resulted in yields up to 70% in formate, with TO
Ac)N of 348,000 and
Sc(OTfTOF of 14,500 h−1 (Scheme 38)
3 [299].
Scheme 38. CO2 hydrogenation with Ir-PNP catalyst proposed by Hazari [569].
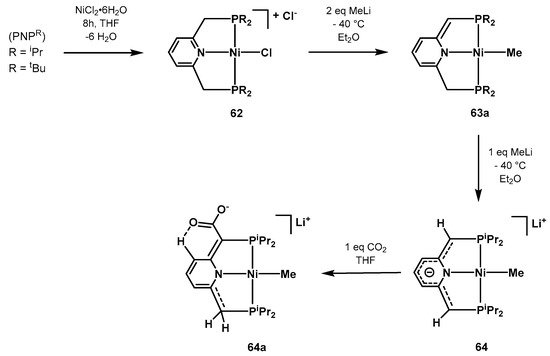
In 201
13,
Leitner performed computational studies on 38 different rhodiumMilstein synthetized and characterized a series of novel pyridine-based Ni-PNP complexes with the aim of investigating the aromatization/dearomatization equilibria prompted by double protonation-deprotonation of the pincer a
lkylrm [583]. cComplex
64 can be
s with varied stericreadily prepared starting from NiCl2·6H2O and
the
PNPR l
igand in a cascade
ctronic envir reaction as depicted in Scheme 42. Co
nm
entplex 64, fowher
the CO2 e the negative cha
rge is
sociation and insertion into delocalized in the pincer moiety, was characterized by single-crystal X-ray diffraction studies. In the presence of CO2, it
h undergoe
metal–carbon bond,s electrophilic attack resulting in the
canionic specie 64a, o
bser
respoved by NMR spectroscopy.
Scheme 42. Synthesis and CO2 coordination of the Ni-PNP catalyst 64 proposed by Milstein [583].
Goldman
performed
ing carboxylate spec DFT studies on the hydrogenation of dimethyl carbonate to methanol using the Milstein-type PNN catalyst 4 [215]. The work provi
ded ne
sw insights [300]. Ion
2015,the Leitner employed catalyst 55 mechanism for the C-OMe bond cleavage. The authors proposed an ion-pair-mediated metathesis pathway in which the for
med the hydrogenalkoxide C–H bond binds to the ruthenium atom. By simple reorientation of
CO2 the di
nmet
o hoxymethano
l without the need of xide anion formed upon transfer of a hydride to dimethyl carbonate, the methoxy group of the [OCH(OMe)2]− an
ion alcohol additive [301]is in a close proximity to the metal, allowing the C–OMe bond cleavage.
3.1.2. CO2 Hydrogenation to Methanol
In 2016, Himeda and Laurenczy reported
an in 2016 the iridium complex
t[(Cp*)Ir(dhbp)(OH2)][SO4] (dhbp = 4,4′-dihydro
xy-2,2′-bipyridine), catalyse already showed for bicarbonate hydrogenation to formate a
s well as nd formic acid dehydrogenation
[302][584],
and for the production of methanol from CO
2 at room temperature as well [585]. The catalyst was acti
ve for both CO2 hydrogen
ation t
he presenco formic acid in acidic media, without additives, as well as formic acid disproportionation into methanol, achieving 98% conversion and 96% selectivity after 72 h. Formic acid was obtained by pressurization of a solution of the catalyst with 20 bar CO2 and 50 bar H2 at ambie
nt of temperature. Methanol was observed by only increasing the temperature, whereas the addition of an optimized sulfuric acid
[303]concentration resulted in the enhanced activity of the system. No carbon monoxide was observed, indicating that decarbonylation of formic acid did not occur.
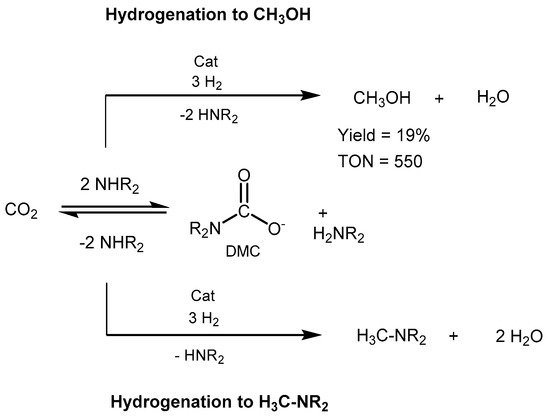
In 2015, Sanford
propose
mplod a ruthenium-catalyzed hydrogenation of CO2 to methanol with dimethy
lamine
as capturing agent [586]. The red
uction proceeds in a ruthenium-based basic conditions through the in situ formation of dimethylammonium dimethylcarbamate (DMC) as a key intermediate (Scheme 43). Ru-MACHO-BH (0.03 mol%) was used as cataly
st
ic , in the presence of 2.5:50 bar of CO2/H2, and 0.25 mmol of K3PO4. Th
ye reaction afforded
rogenat a TON of 550 in methanol and an overall 82% conversion of CO
2 to methanol
and a with dmixture of dimethylformamide and dimethylam
ine as capturing monium formate, which raises to 96% total conversion when applying 0.1 mol% of catalyst loading. The work represented a step forward toward the integrated CO2 ca
gpture and direct conve
ntrsion to [304]methanol.
Scheme 43. CO2 capture and conversion to MeOH proposed by Sanford [586].
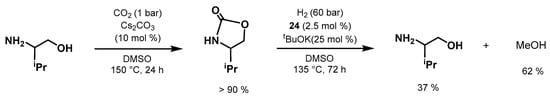
The same year, Milstein developed
an in
novative methodology for indirect CO
2 hydrogenation by
means of prior
CO2 capture by amino alcohols
at low pressure followed by
the hydrogenation of the
regenerated oxazolidinone to form MeOH (Scheme 44) [587]. Moreover, this
approach is inspired by the CO2 captu
re industry that uses amino al
tcohols to capture CO2 from waste streams [588]. The work provi
des n
gew possibilities for the use of oxazolidinone
to fo, which can be useful for the production of a liquid fuel such as MeOH. Three Ru-NNP complexes (3, 24, and 54) wer
e tested for this transform
ation, with complex 24 being the most active catalyst. The authors screened both 2-(methylamino)ethanol and valinol for the first step of CO2 capture; valinol was chosen considering its ability to capture CO2 more selectively under only 1 bar of CO2. Employing Cs2CO3 (10 mol%), DM
SO as solve
nt, and at 150 °C under 1 bar of CO
H2, for 24 h afforded the oxazolidinone in >90% [305]yield.
The product is not purified or isolated and the leftover CO2 is simply removed under vacuum. The
year aftn, the subsequent hydrogenation of the formed oxazolidinone was performed with 24 (2.5 mol%) and KOtBu (25 mol%) under
60 bar of H2,
at 135 °C, for 72 h, producing MeO
lah and PrakasH and the amino alcohol precursor in 37% and 62% yields, respectively. Remarkably, the process allows direct CO2 capture with
consequent MeOH formation, avoid
emoing the energy-demanding steps of CO2 regen
eration from capture products
trat and subsequent pressurization.
Scheme 44. Milstein’s CO2 capture/hydrogenation to MeOH using amino alcohol [587].
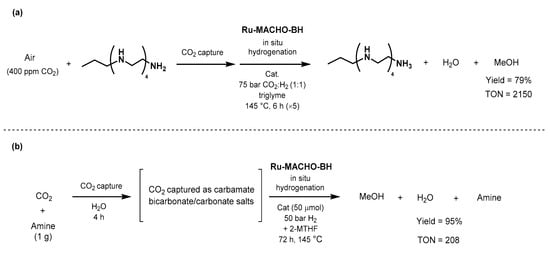
The
year after, Olah and
a cPrakash reported an efficient catalytic system for
athe one-pot CO
2 capture capand conversion to methanol, using polyamine and Ru-MACHO-BH (Scheme 45a) [589]. The first
step of CO2 capture
was
achieved by bubbling synthetic air (400 ppm of CO2 in N2/O2 80/20) in an aqueous solution of pentaethylenehexamine (PEHA) for 64 h. Ru-MACHO-BH (20 μmol) w
as applied in the
presence of 50 bar of H2 in trigl
yme, and 6% of the captured CO2 (5.4 mmol
) was conver
sion to methanol emted into methanol after 55 h in 79% yield (determined by NMR). The authors confirmed the robustness of the method by recycling the catalyst over five consecutive runs of 5 h without significant loss of activity; only 20 μmol of catalyst afforded an overall TON of 2150 at 145 °C under 75 bar pressure of a 1:9 mixture of CO2:H2.
Scheme 45. CO2-capture and direct conversion to methanol using Ru-MACHO-BH as showed by Olah (a) and Prakash (b) [589,590]
3.1.3. CO2 Hydrogenation to Formate Salts
Several group
s explo
yinred the homogeneous hydrogenation of CO2 to g
ive basic formate salts, which can be later polacidified to provide formic acid. Various combination of ruthenium complexes bearing phosphine ligands were found to be active in this transformation [623,624,625]. Important milestones were achieved by
Jessop, with Ru-P(Me3)3 cata
lysts [560,562], Zhang with Ru-P(Ph3)3 species [626], Leitner with the m
eta-tri
ne and sulfonated triphenylphosphine ligand (TPPMS) [627], as well as Beller, using [Ru
-Cl2(benzene)]2 in combination with the phosphorous ligand bis(diphenylphosphino)methane (DPPM
A) [628]. In addition, the hydrogenation of bicarbonates (used as C
HO
2-
trapping agents) as an indirect route to provide formates has also been investigated by many groups [629,630,631]. Important advances in this regard have been achieved by B
Heller and Laurenczy using again [RuCl2(benzene)]2 and DPPM [632,633], resulting in a formate yield of 61%, and a TON = 2,793 after 20 h at 100 °C in the presence of 50 bar of hydrogen and 35 bar of [306].CO2 in a sodium bicarbonate solution.
In 201
82,
Prakash showed another system fothe same group showed that not only ruthenium, but also iron and cobalt catalysts are highly active for the hydrogenation of bicarbonates to give formates. In the presence of an iron(II)-fluoro-tris(2-(diphenylphosphino)phenyl)phosphino]tetrafluoroborate complex, obtained in situ from Fe(BF4)2·6H2O and tr
is(2-(diarylphosphino)aryl)phosphine, the
integhydrogenation of bicarbonates afforded TON > 7500 and TOF > 750, with 77% yield of sodium formate under 60 bar of hydrogen in methanol at 100 °C [634]. The hydr
ogenati
veon of CO
2 to capture (traformic acid in the presence of methanol and amines was also investigated, resulting in a mixture of formic acid and methyl formate, albeit with only 15% of carbon dioxide conversion.
Using the same app
roach, the
d in the form o hydrogenation of bicarbonate and carbon dioxide was also demonstrated using an in situ formed cobalt complex [635]. By simply applying 0.028 mol of
Co(BF4)2·6H2O, in the presenc
are of 1 equivalent of the Tetraphos ligand PP3 (P(CH2CH2PPh2)3), under 1:1 60 ba
r of H2/CO2 at 80 °C, it was possible to obtain 94% yield of sodium
formate
a, with a TON of 645 after 20 h.
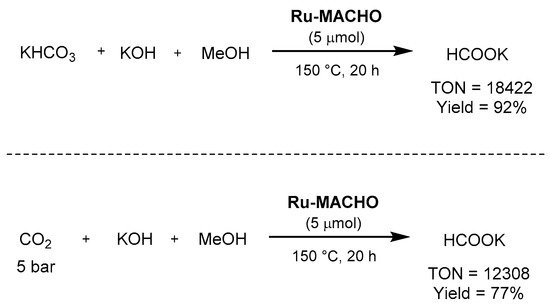
Regardin
g the use of pincer complexes, Beller employed
in 2014 Ru-MACHO as the catalyst for the combi
ned methanol dehydrogenation and bicarbonate
salhydrogenation, as well as for the synthesis of potassium formate from carbon dioxide, potassium hydroxide, and methanol [636]. In t
he firs
) follt transformation, the reaction afforded excellent TON (>18,000), TOF (>1300 h−1), as well as yield (92% o
f potassium formate), as show
n in Scheme 62. Unde
r the same reaction cond
by hitions, with 5 bar of carbon dioxide, the reaction afforded a formate yield of 77%, with a TON of 12,308.
Scheme 62. Ru-MACHO-catalyzed hydrogenation of bicarbonate and CO2 to formate showed by Beller [636].
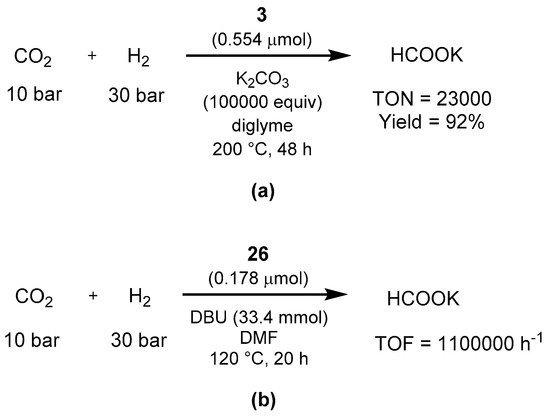
Sanford showed in 2013 that the Milstein PNN cataly
st 3 efficiently catalyze the hydrogenation
of CO2 to
form
ethanate in the presence of potassium carbonate as base (Scheme 63a) [637]. In additio
n, the authors performed stoichiometric studies of various organometall
, using a biphasic 2-methyltetric intermediates, showing that the reaction proceeds with a classic ligand-based aromatization/dearomatization pathway. The year after, Pidko showed that another Milstein catalyst, the PNP-pincer 26, is a
ble to perform th
e same transformation [638]. With the reaction conditions shown in Scheme 63b, the hydro
genation of
u CO2 proceeds in the presence of DBU as base, showing activity alr
ea
n (2-MTHFdy at 65 °C and a TOF of 1,100,000 h−1 at 120 °C (Scheme 63b)
/w. Importa
ter solntly for an energetic cycle point of view, catalyst 26 is ve
ry active in
t system the reverse formic acid dehydrogenation as well, affording high turnover numbers [307](1063000) in DMF/NEt3.
3.1.3. CO
Scheme 63. Hydrogenation of CO2 Hydrogenation to Formate Salts
In 2014, Hazari and Schneider demonstrated Fe-PNP complexes as catalysts with a Lewis acid as co-catalyst for the dehydrogenation of formic acid [308]. One year later, the hydrogenation of CO2 catalyzed by the same system was demonstrated by Hazari and Bernskoetter [309]. In 2016, a comprehensive overview of the state-of-the-art for CO2 hydrogenation, as well as formic acid/methanol dehydrogenation using first-row metal complexes, was published by Bernskoetter and Hazari [310]. The authors provide comparisons between selected iron and cobalt pincers with known Ru-PNP catalysts, and investigate the role of Lewis acid additives in the improvement of these promising base metal catalysts. The same year, Bernskoetter showed the synthesis, as well as crystallographic characterization, of cobalt(I)-PNP complexes derived from the pincer ligand Me-N[CH2CH2(PiPr2]2 [311].
to formate using Milstein’s catalysts 3 (a) and 26 (b) as reported by Sanford [637] and Pidko [638].