You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Azzurra Stefanucci.
Elastases are a broad group of enzymes involved in the lysis of elastin, the main component of elastic fibres. They are produced and released in the human body, mainly by neutrophils and the pancreas.
- elastase inhibitors
- human neutrophil elastase
- pancreatic elastase
1. Introduction
Elastic fibres compose an essential part of the extracellular matrix of connective tissue, where they allow its reversible and repetitive deformation. Each elastic fibre is mainly constituted by an amorphous core of elastin, which occupies more than 90% of the fibre, surrounded by an external envelope of microfibrils [1,2][1][2]. Fibrillin microfibrils ensure extensibility and elasticity during the deformation of tissues. A huge variety of fibrillin-binding proteins occurs for the assembly of elastic fibres [3], which play an important role in ensuring mechanical resilience, durability and cell interactivity within tissues [4].
Elastin is a hydrophobic protein that displays a huge cross-linking among Lys residues following the oxidation performed by the lysil-oxidase enzyme, which gives elastin a high level insolubility [1]. This protein contains a large quantity of hydrophobic amino acids. In particular, higher vertebrates’ elastin is composed of more than 30% Gly and about 75% Val, Ala and Pro residues [1,5][1][5]. The formation process of elastic fibres is called elastogenesis, comprising tropoelastin (elastin’s subunits) synthesis, coacervation, cross-linking and deposition [4].
Elastases are a group of enzymes that selectively lyse the amorphous component of elastic fibre, namely elastin [2]. Elastases are produced and released in different locations of the human body. Among them, human neutrophil elastase and pancreatic elastase show an important physiological and pathological role. In detail, pancreatic elastase measurement in faeces (faecal elastase-1) is used as an indirect test to determine the onset of the exocrine pancreatic insufficiency (EPI). Pancreatic elastase is secreted from pancreatic acinar cells and after binding to the bile salts goes through a tiny degradation into the gut. Clinicians can make use of two possible ELISA tests to measure faecal elastase-1 through monoclonal or polyclonal antibodies [6]. For example, the ScheBo Pancreatic Elastase 1 Stool Test (ScheBo Biotech, Giessen, Germany) is one of the most-used tests based on two monoclonal antibodies that measure the isoforms chymotrypsin-like elastase 3B (CELA3B) and, with lower efficiency, CELA3A [7].
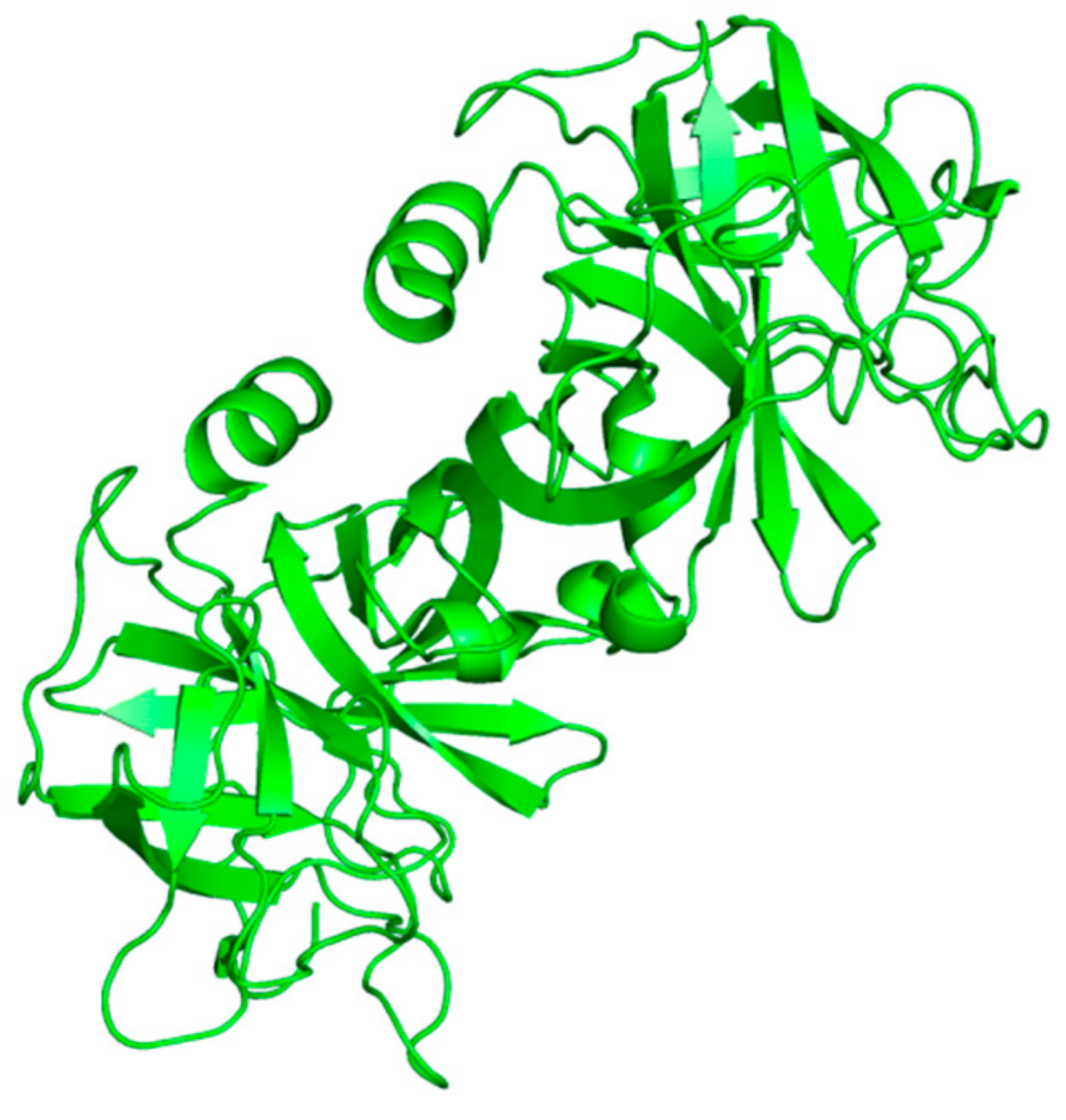
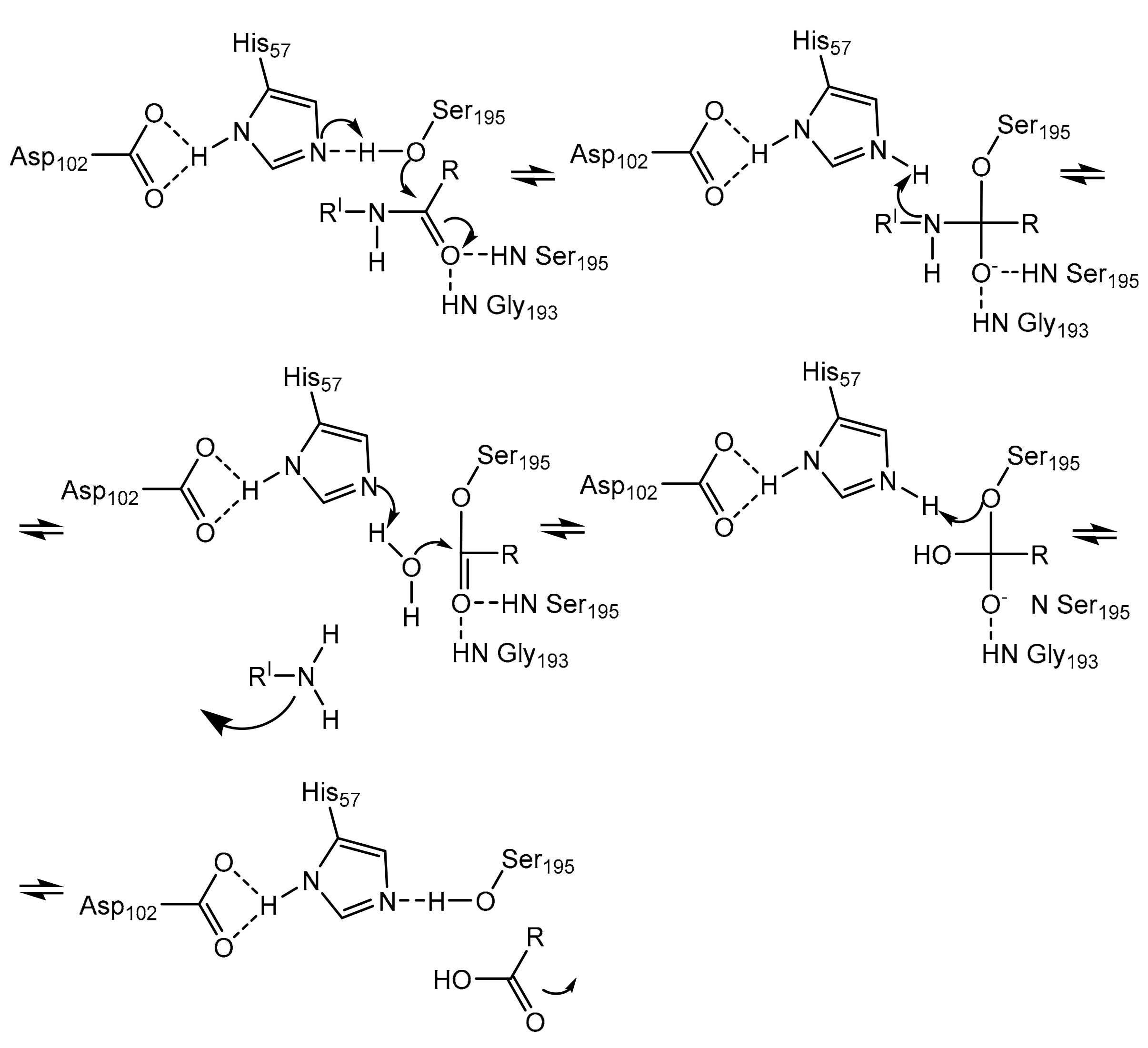
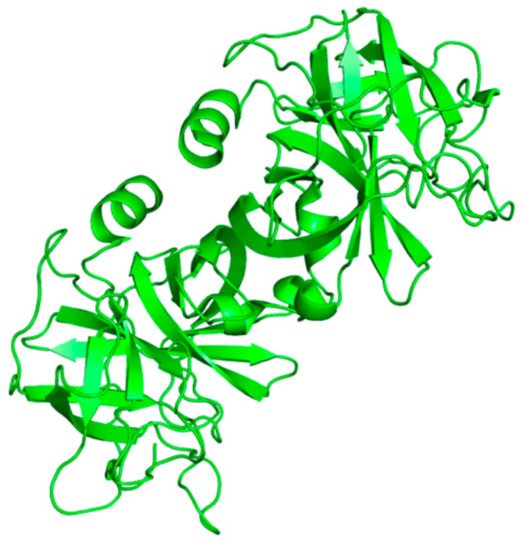
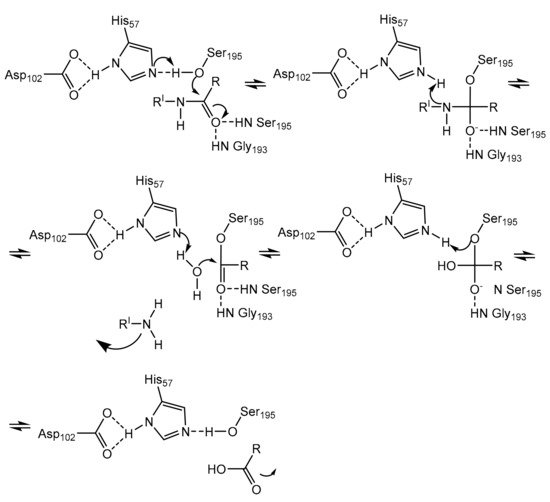
Neutrophils, a large population of leukocytes, play an important role in the regulation of the innate and adaptive immune responses, quickly achieving the inflammation/infection site [8]. During the degranulation process, neutrophils release human neutrophil elastase (HNE) (Figure 1), a glycoprotein composed of 218 amino acids, through extracellular neutrophil traps (NETs) [8,9][8][9]. NETs are composed of decondensed chromatin, neutrophil elastase and other enzymes [8]. HNE is a serine protease belonging to the chymotripsin family that is produced in the bone marrow during the stage of promyelocytes [9]. Neutrophil serine proteases are involved in inflammation and the immune response [10]. HNE is deposited as an active enzyme in the azurophilic granules of polymorphonuclear neutrophils. This enzyme has a catalytic site of three amino acids, called the catalytic triade: Asp102, His57 and Ser195. The mechanism of action of serine proteases, which includes neutrophil elastase, is illustrated in Scheme 1; when the target ligand achieves the enzymatic catalytic site, a proton transfer happens among the three amino acids, causing an increased nucleophile activity of Ser195 with a concomitant peptide bond cleavage [9,11][9][11]. In regular conditions, a balance between human neutrophil elastase activity and its endogenous inhibitors (e.g., elafin, serpins, α1-antitrypsin and secretory leukocyte proteinase inhibitor) is guaranteed, while the imbalance between elastase activity and its endogenous inhibitors can cause a variety of illnesses, such as chronic obstructive pulmonary disease, acute lung injury, acute respiratory distress syndrome and pulmonary fibrosis [12,13][12][13]. It has recently been hypothesized that neutrophil elastase inhibitors could be used to treat acute respiratory distress syndrome (ARDS) caused by COVID-19 infection, but their effective use is yet unproven, and more clinical studies should be conducted to evaluate the response and effectiveness of these types of inhibitors in COVID-19 patients [14].
2. Natural Peptide Elastase Inhibitors Isolated from Animal Sources
2.1. ShSPI from Scolopendra Hainanum
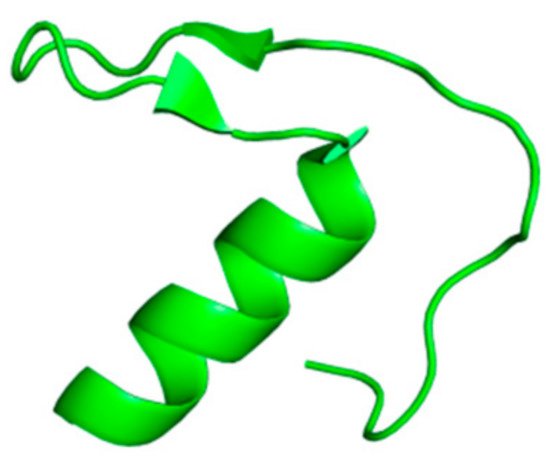
Luan et al. discovered a new serine protease inhibitor from the cDNA library of the venom glands of centipede Scolopendra Hainanum. This compound is called ShSPI, and it has been identified as an atypical kazal-type protease inhibitor that could be developed as a novel drug for the treatment of illnesses caused by human elastase. ShSPI is a bioactive peptide composed of 34 amino acids whose primary sequence is: CPQVCPAIYQPVFDEFGRMYSNSCEMQRARCLRG. The three-dimensional structure (Figure 2) displays a cystine-stabilized α-helix formed by Ser23 and Arg33 and a two-stranded anti-parallel β-sheet (Pro11 to Asp14 and Gly17 to Tyr20); the folding is guaranteed by the tertiary structure and the presence of two disulfide bonds among Cys1-Cys31 and Cys5-Cys24 [12]. Its inhibitory activity was compared to Sivelestat (ONO-5046), a human neutrophil elastase inhibitor tested as clinical drug for the treatment of ARDS and recently taken in consideration for the management of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) or ARDS with coagulopathy caused by COVID-19 infection [12,17,18][12][17][18]. Both compounds inhibit HNE in a dose-dependent manner, but ShSPI exhibits a more effective activity than Sivelestat at the same concentration. ShSPI acts with a non-competitive inhibition mechanism against elastase, displaying a Ki value of 12.6 nM, and its equilibrium dissociation constant (KD) to HNE is 4.2 × 10−8; furthermore, ShSPI shows a huge stability after co-incubation with human plasma over 48 h [12].
2.2. AvKTI from Araneus Ventricosus
Peptide AvKTI has been isolated from a spider (Araneus ventricosus); it has the ability to inhibit trypsin, chymotrypsin, plasmin and human neutrophil elastase. It is composed of 170 amino acids and comprises a signal peptide of 19 units, a pro-peptide of 94 building blocks and a mature peptide of 57 amino acids that represents a Kunitz domain. The mature AvKTI has a peptide sequence (KDRCLLPKVTGPCKASLTRYYYDKDTKACVEFIYGGCRGNRNNFKQKDECEKACTDH) similar to other Kunitz-type serine protease inhibitors. This compound is the first Kunitz-type serine protease inhibitor isolated from spiders to be known for its antifibrinolytic and antielastolytic activity [19]. Kunitz-type inhibitors are usually composed of a single inhibitory domain or a multi-domain included in a single-chain compound [20]. It is produced in the spider’s epidermis; however, the physiological role of this compound in spiders is not yet clear [19,20][19][20]. AvKTI inhibits neutrophil elastase with an IC50 value of 446.93 nM and plasmin with an IC50 value of 10.07 nM, showing a lower neutrophil elastase inhibitory activity of about 44.4-fold. The Ki value of AvKTI against neutrophil elastase is 169.07 nM, while its Ki value against plasmin is 4.89 nM [19].
2.3. Gaumerin from Hirudo Nipponia
Guamerin is a cysteine-rich polypeptide composed of 57 amino acids (VDENAEDTHGLCGEKTCSPAQVCLNNECACTAIRCMIFCPNGFKVDENGCEYPCTCA) with a molecular weight of 6.110 that was isolated from a native Korean leech Hirudo nipponia by Jung et al. Its structure is characterized by ten cysteine residues that confer a high rigidity due to the formation of disulfide bridges. Guamerin showed stability at 25 °C in a range of pH varying from 1 to 11. It has the ability to inhibit human leukocyte elastase (HLE) with a Ki value of 8.1 × 10−14 M [21].
3. Natural Peptide Elastase Inhibitors Isolated from Fungi
3.1. Desmethylisaridin C2, Isaridin E, Isaridin C2 and Roseocardin from Beauveria Felina
Eight cyclodepsipeptides have been produced by the filamentous fungi Beauveria felina after the addition of suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor, to the culture medium; three of them are new compounds, e.g., desmethylisaridin C2, desmethylisaridin E and isaridin F [22]. This is an example of an epigenetic tool used for obtaining inhibitor peptides from fungal sources [23]. Among them, Desmethylisaridin C2, isaridin E, isaridin C2 and roseocardin (Figure 3) are able to inhibit elastase release induced by formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP) in human neutrophils and to express an anti-inflammatory action without toxicity for human neutrophils. The IC50 values of these compounds are displayed in Table 1 [22].
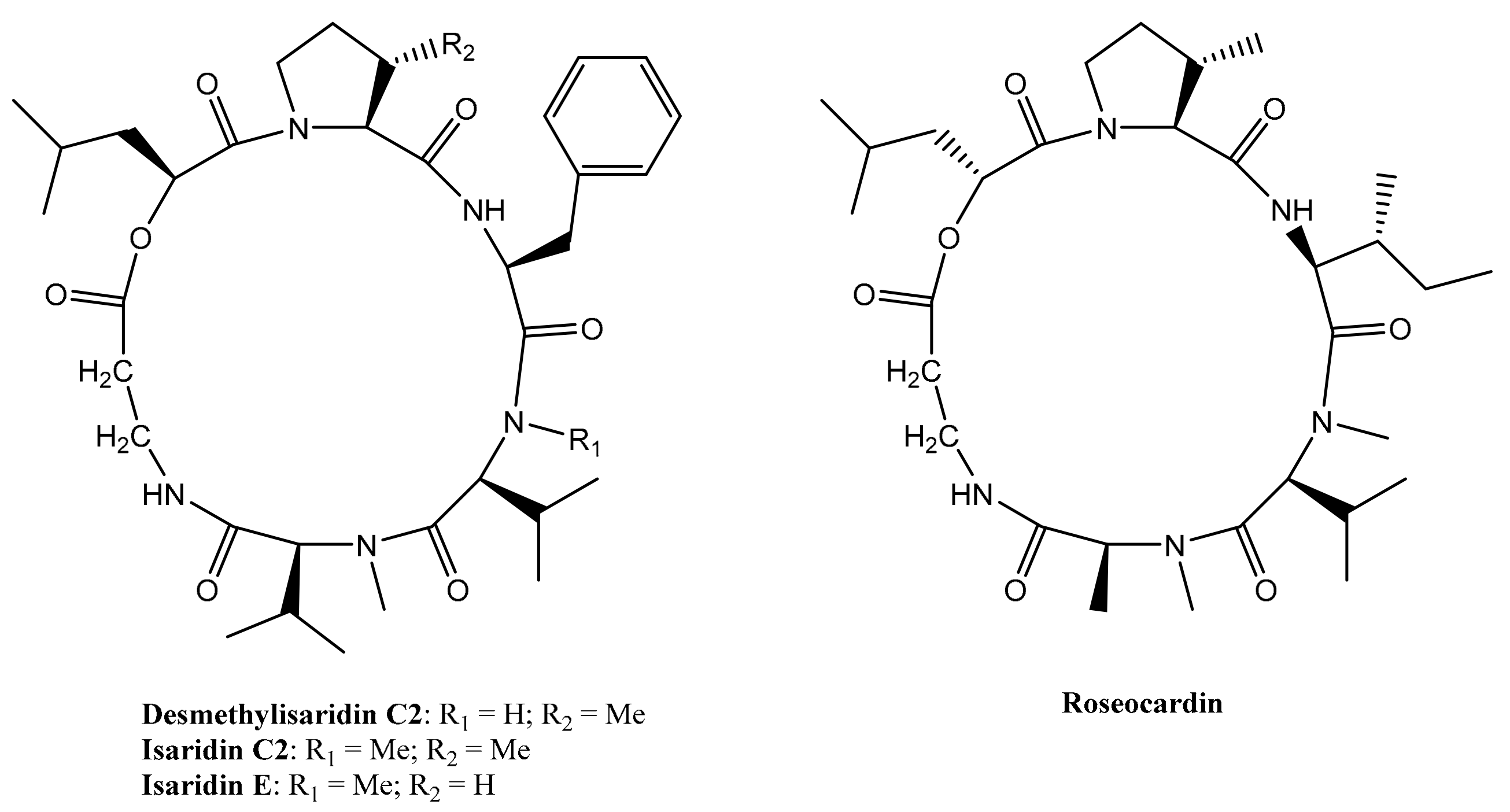 Figure 3. Structures of Desmethylisaridin C2, isaridin E, isaridin C2 and roseocardin.
Figure 3. Structures of Desmethylisaridin C2, isaridin E, isaridin C2 and roseocardin.Table 1. IC50 values of Desmethylisaridin C2, Isaridin E, Isaridin C2 and Roseocardin in vitro on formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP)-induced human neutrophils [22].
| Compounds | IC50 (μM) |
|---|---|
| Desmethylisaridin C2 | 10.01 ± 0.46 |
| Isaridin E | 12.76 ± 1.00 |
| Isaridin C2 | 12.12 ± 0.72 |
| Roseocardin | 15.09 ± 0.28 |
3.2. AFUEI from Aspergillus fumigatus
AFUEI is an elastase inhibitor purified by Okumura et al. from Aspergillus fumigatus strain AFU-12 that came from the sputum of a patient afflicted with allergic bronchopulmonary aspergillosis. Its primary amino acid structure is composed of 68 residues (DPATCEKEAQFVKQELIGQPYTDAVANALQSNPIRVLHPGDMITMEYIASRLNIQVNENNEIISAHCA), and the molecular mass of its protein portion is 7526.2 Da. AFUEI could be effective against inflammatory diseases since it is able to inhibit human leukocyte elastase with an inhibitory activity of 83.8% [24]. Aspergillus fumigatus synthesizes A. fumigatus elastase (AFUE) and native A. fumigatus elastase inhibitor (N-AFUEI) in liquid medium, but N-AFUEI is barely produced. Therefore, for medical purposes, synthetic-AFUEI (S-AFUEI) has been produced by Peptide Institute Inc. (Osaka, Japan) since 2015. Further investigations about S-AFUEI’s biological and physiological activities have been reported by Fukui et al. [25].