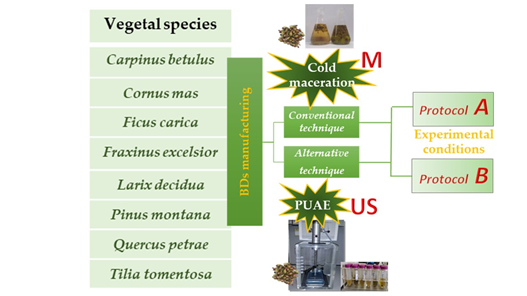
The use of herbal food supplements, as a concentrate form of vegetable extracts, increased so much over the past years to count them among the relevant sources of dietetic polyphenols. Bud-derivatives are a category of botanicals perceived as a “new entry” in this sector since they are still poorly studied.
Due to the lack of a manufacturing process specification, very different products can be found on the market in terms of their polyphenolic profile depending on the experimental conditions of manufacturing
.
In this research
two different manufacturing processes, using two different protocols, and eight species (
Carpinus betulus
L.,
Cornus mas
L.,
Ficus carica
L.,
Fraxinus excelsior
L.,
Larix decidua
Mill.,
Pinus montana
Mill.,
Quercus petraea
(Matt.) Liebl.,
Tilia tomentosa
Moench), commonly used to produce bud-derivatives, have been considered as a case study. An untargeted spectroscopic fingerprint of the extracts, coupled to chemometrics, provide to be a useful tool to identify these botanicals. The targeted phytochemical fingerprint by HPLC provided a screening of the main bud-derivatives polyphenolic classes highlighting a high variability depending on both method and protocol used. Nevertheless, ultrasonic extraction proved to be less sensitive to the different extraction protocols than conventional maceration regarding the extract polyphenolic profile.
- bud-derivatives
- botanicals
- polyphenols
- UV-Visible spectroscopic fingerprint
- chemometrics
- targeted chromatographic fingerprint
1. Introduction
In recent decades, food supplements have an important impact on the consumers showing a significant expectation for their health and well-being
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29]. They are concentrated sources of nutrients or bioactive compounds endowed with nutritional or physiological effects and, due to their presumed health benefits, they can supplement the common diet
[30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][1]. They are concentrated sources of nutrients or bioactive compounds endowed with nutritional or physiological effects and, due to their presumed health benefits, they can supplement the common diet
. In particular, the interest in herbal food supplements (botanicals) is exponentially grown and consequently the relative market has increased in all the world
[4]
. Botanicals are become among the most popular into the food supplements category, due to the general belief which “natural” is better, healthier and safer than synthetic drugs, although this is not always true
[4]
.
Bud-derivatives (BDs) are a relatively new category of herbal food supplements and they represent one of the supply chains investigated in the FINNOVER project (Innovative strategies for the development of cross-border green supply chains), an European Interreg Alcotra Italy/France project (2017–2020) whose aim is the green innovation of several agro-industrial chains
[5]
. BDs are conventionally produced, according to the European Pharmacopoeia VIII edition
[6]
, by cold maceration of the fresh meristematic tissues of trees and herbaceous plants (i.e., buds and young sprouts) using as extraction solvent mixtures of water, ethanol and glycerol
. These natural products are already marketed, and a long history of use as dietary supplements for human well-being and health is reported in traditional
medicine. No health claims are yet approved by the European Food Safety Authority (EFSA) and just for some of these botanicals pharmacognostic findings supported their use as adjuvants in several diseases. Although gemmotherapy has been used since ancient times because of the peculiar content of buds in bio-active compounds, especially polyphenols, nowadays BDs are still a little studied “niche” production
. The lack of detailed scientific information and a clear and unique regulation, as well as for the category of herbal food supplements in overall
[11]
, it makes these products high risk and there is an increase request for efficient quality control to ensure the proper identification of the botanical source and their content
[12]
.
With regards to BDs, a first problem it is accidentally confusing the raw material: fresh buds must be collected, generally from spontaneous grown, in a very limited period in the late winter and/or in the early spring, corresponding to the annual germination of the plant . During this period, plants may not show their distinctive characteristics and sometimes the attribution of the botanical species may be difficult for the collector. A second problem concerns the manufacturing process and the extraction protocols whose parameters are not strictly defined, and production rules are often loose and deficient
.
In recent years, many health benefits of dietetic polyphenol supplementation have been described in humans i.e., against aging and cardiovascular disease
, to prevent obesity and diabetes
, to modulate human gut microbiota
[20]
and to improve the brain cognition skills
. This knowledge guides the choice of consumers not only towards plant foods but also towards herbal food supplements, whose polyphenol content is often even more concentrated and responsible for their bioactivity
. Nevertheless, polyphenols content is strongly influenced by the manufacturing methods whose parameters are often not strictly defined (e.g., solvent ratios in the extraction mixtures, raw material/extraction mixture ratios, extraction time) and thus they could affect the final compositions
[25]
.
In previous articles, the polyphenolic pattern of some BDs prepared starting from different botanical species have been studied
.
In this research, eight species spontaneously grown and commonly used to produce BDs, i.e.,
Carpinus betulus
L.,
Cornus mas
L.,
Ficus carica
L.,
Fraxinus excelsior
L.,
Larix decidua
Mill.,
Pinus montana
Mill.,
Quercus petraea
(Matt.) Liebl.,
Tilia tomentosa
Moench, have been taken into account as case study. Two different manufacturing methods, one conventional (maceration) and one innovative (direct sonication), as well as two different extraction protocols have been taken into account and the corresponding polyphenolic extracts’ profiles have been investigated.
A strategy based on the untargeted UV-Visible fingerprinting coupled to chemometrics (Principal Component Analysis—PCA) has been proposed for the screening of the polyphenolic BDs profile in order to obtain a rapid control tool
[27]
. Finally, HPLC methods were used to obtain a targeted chromatographic profile
of the main polyphenol classes (i.e., flavonols, benzoic acids, catechins, cinnamic acids). Polyphenols are correlated with their potential health-promoting activity
[29]
, even if they are strongly influenced both by the methods and protocols used
[25]
.
Figure 1. The global scheme of this research.
2. Experimental
2.1. Bud-derivatives
Buds, belonging to eight different vegetable species (
Carpinus betulus
L.,
Cornus mas
L.,
Ficus carica
L.,
Fraxinus excelsior
L.,
Larix decidua
Mill.,
Pinus montana
Mill.,
Quercus petraea
(Matt.) Liebl.,
Tilia tomentosa
Moench) were collected from plants spontaneously grown in the Turin Province (Italy) and were immediately authenticated by an agronomist.
Table 1.
The collection sites, the corresponding geo-localization coordinates, and the scientific naturalistic illustrations of the eight different bud species.
|
Vegetable Species |
Family (Order) |
Collection Site |
Geo-Localization Coordinates |
|
Carpinus betulus |
Betulacee (Fagales) |
Bricherasio Prarostino San Germano Rostino |
44.821,7.285; 44.831,7.272;
44.825,7.275 44.913,7.237 44.868,7.253 |
|
Cornus mas |
Cornaceae (Cornales) |
Bricherasio Torre Pellice Villar Pellice |
44.854,7.250; 44.855,7.250; 44.823,7.307 44.813,7.181 44.804,7.154 |
|
Ficus carica |
Moraceae (Rosales) |
Brondello Pagno |
44.604,7.422; 44.603,7.419; 44.603,7.418 44.598,7.424; 44.597,7.424; 44.598,7.425 |
|
Fraxinus excelsior |
Oleacee (Lamiales) |
Angrogna Bricherasio Massello Paesana Pagno San Germano Chisone |
44.869,7.173 44.822,7.284 44.964,7.031 44.656,7.261; 44.651,7.257 44.597,7.424; 44.598,7.425; 44.598,7.424 44.888,7.261
|
|
Larix decidua |
Pinacee (Pinales) |
Praly |
44.902,7.055 |
|
Pinus montana |
Pinacee (Pinales) |
Masello Pramollo |
44.948,7.065; 44.948,7.068; 44.947,7.063 44.918,7.193 |
|
Quercus petraea |
Fagaceae (Malvales) |
Bricherasio |
44.848,7.275; 44.850,7.274; 44.842,7.282; 44.831,7.270 |
|
Tilia tomentosa |
Malvaceae (Malvales) |
Angrogna Bobbio Pellice Bricherasio Perrero |
44.849,7.223 44.799,7.131 44.832,7.265; 44.816,7.282; 44.821,7.273; 44.821,7.285; 44.822,7.283; 44.818,7.279 44.936,7.139 |
2.2. Conventional Cold Maceration (M) as Traditional Method and Pulsed Ultrasound-Assisted Extraction (US) as Alternative Method to produce Bud-derivatives
BDs were prepared both using a cold maceration (M) and by Pulsed Ultrasound-Assisted Extraction (US), following two different experimental manufacturing protocols, reported in Table 2.
(A) A 21 days maceration of buds in glycerol/ethanol 96% (1/1
w
/
w
) with a 1:20 bud/solvent ratio (considering the dry weight) has been performed, according to the official method of glyceric macerates reported in the European Pharmacopoeia VIII edition
[9]
(“M_A”).
(B) A 3 months maceration of buds in a mixture of water/glycerol/ethanol 96% (50/20/30
w
/
w
/
w
) as extraction solvent with a bud/solvent ratio variable (considering the fresh weight) depending on the botanical species (see Table 2) has been used, according to the method optimized and used by the Company to produce glyceric macerates (“M_B”).
Detailed experimental extraction procedures are reported in the original article available online
https://www.mdpi.com/2304-8158/9/10/1343/htm
.
Table 2.
BDs obtained starting from the eight vegetable species (raw materials). Two different methods (cold maceration - M and Pulsed Ultrasound-Assisted Extraction US) and two different experimental protocol (Protocol A and B) are taken into account.
|
|
Sample Identification Code |
Vegetable Species |
Extraction Method |
Experimental Protocol |
Bud/Solvent Ratio |
|
1 |
Cb_M_A |
Carpinus betulus |
M |
Protocol A |
1/20 DW |
|
2 |
Cb_US_A |
Carpinus betulus |
US |
Protocol A |
1/20 DW |
|
3 |
Cb_M_B |
Carpinus betulus |
M |
Protocol B |
1/15 FW |
|
4 |
Cb_US_B |
Carpinus betulus |
US |
Protocol B |
1/15 FW |
|
5 |
Cm_M_A |
Cornus mas |
M |
Protocol A |
1/20 DW |
|
6 |
Cm_US_A |
Cornus mas |
US |
Protocol A |
1/20 DW |
|
7 |
Cm_M_B |
Cornus mas |
M |
Protocol B |
1/20 FW |
|
8 |
Cm_US_B |
Cornus mas |
US |
Protocol B |
1/20 FW |
|
9 |
Fc_M_A |
Ficus carica |
M |
Protocol A |
1/20 DW |
|
10 |
Fc _US_A |
Ficus carica |
US |
Protocol A |
1/20 DW |
|
11 |
Fc _M_B |
Ficus carica |
M |
Protocol B |
1/10 FW |
|
12 |
Fc_US_B |
Ficus carica |
US |
Protocol B |
1/10 FW |
|
13 |
Fe_M_A |
Fraxinus excelsior |
M |
Protocol A |
1/20 DW |
|
14 |
Fe_US_A |
Fraxinus excelsior |
US |
Protocol A |
1/20 DW |
|
15 |
Fe_M_B |
Fraxinus excelsior |
M |
Protocol B |
1/10 FW |
|
16 |
Fe_US_B |
Fraxinus excelsior |
US |
Protocol B |
1/10 FW |
|
17 |
Ld_M_A |
Larix decidua |
M |
Protocol A |
1/20 DW |
|
18 |
Ld_US_A |
Larix decidua |
US |
Protocol A |
1/20 DW |
|
19 |
Ld_M_B |
Larix decidua |
M |
Protocol B |
1/20 FW |
|
20 |
Ld_US_B |
Larix decidua |
US |
Protocol B |
1/20 FW |
|
21 |
Pm_M_A |
Pinus montana |
M |
Protocol A |
1/20 DW |
|
22 |
Pm_US_A |
Pinus montana |
US |
Protocol A |
1/20 DW |
|
23 |
Pm_M_B |
Pinus montana |
M |
Protocol B |
1/10 FW |
|
24 |
Pm_US_B |
Pinus montana |
US |
Protocol B |
1/10 FW |
|
25 |
Qp_M_A |
Quercus petraea |
M |
Protocol A |
1/20 DW |
|
26 |
Qp_US_B |
Quercus petraea |
US |
Protocol A |
1/20 DW |
|
27 |
Qp_M_B |
Quercus petraea |
M |
Protocol B |
1/15 FW |
|
28 |
Qp_US_B |
Quercus petraea |
US |
Protocol B |
1/15 FW |
|
29 |
Tt_M_A |
Tilia tomentosa |
M |
Protocol A |
1/20 DW |
|
30 |
Tt_US_A |
Tilia tomentosa |
US |
Protocol A |
1/20 DW |
|
31 |
Tt_M_B |
Tilia tomentosa |
M |
Protocol B |
1/15 FW |
|
32 |
Tt_US_B |
Tilia tomentosa |
US |
Protocol B |
1/15 FW |
* DW: dry weight; FW: fresh weight.
2.3. Spectroscopic and chromatographic analysis: UV-Visible and HPLC Fingerprints of Bud-derivatives
UV–Visible absorption spectra (200 nm–900 nm) were recorded to obtain an untargeted fingerprint, instead HPLC–DAD methods were used for fingerprint analysis and phytochemical identification of samples. Four polyphenolic classes were considered: benzoic acids (ellagic and gallic acids), catechins ((+)catechin and (-)epicatechin), cinnamic acids (caffeic, chlorogenic, coumaric, and ferulic acids), and flavonols (hyperoside, isoquercitrin, quercetin, quercitrin, and rutin). Total bioactive compound content (TBCC) was determined as the sum of the most important bioactive compounds with positive effects on human organism (“multimarker approach”)
[30]
.
Detailed experimental procedures are reported in the original article available online
https://www.mdpi.com/2304-8158/9/10/1343/htm
.
2.4. Chemometric Analysis and Data matrices Organization
Multivariate data analysis has been performed by CAT (
Chemometric Agile Tool
) software, one advanced chemometric multivariate analysis tool based on R, developed by the Chemistry Group of the Italian Chemical Society
[31]
. PCA was applied as common multivariate statistical method of unsupervised pattern recognition. Its aim is extracting important information from the data and decreasing the high-dimensional dataset volume by maintaining the important information
.
A data matrix A
32,601
consisting of 32 rows (corresponding to the BDs analyzed, 4 samples for each of the eight botanical species investigated) and 601 columns (the absorbance values in the range of 200–500 nm of the UV-Visible spectra, with 0.5 nm of resolution) was prepared and further analyzed by PCA. Standard normal variate (SNV) transform and column autoscaling were previously performed on the spectral data to remove multiplicative effects of scattering and to scale the data, respectively
[34]
.
Available sample were divided in two different subsets: a calibration (or training) set and a test (or evaluation) set in order to build and validate the statistical model, respectively
[35]
. For a reliable validation strategy, it is important that data used as test set were not used to build the model in order to avoid the overestimations of the prediction ability
[35]
. 32 samples, previously reported in Table 2, were selected for the construction and identification of the model (Calibration set). The representative calibration data set consisted of 4 extracts (M_A, M_B, US_A, US_B) for each botanical species investigated (
Carpinus betulus
L.,
Cornus mas
L.,
Ficus carica
L.,
Fraxinus excelsior
L.,
Larix decidua
Mill.,
Pinus montana
Mill.,
Quercus petraea
(Matt.) Liebl.,
Tilia tomentosa
Moench). Furthermore 16 BDs, obtained both by conventional maceration and ultrasound extraction respectively from the same eight vegetal species, were randomly selected and used as an independent set to test the model and assess its validity (Test set, Table 3).
Table 3.
External test set. 16 BDs obtained starting from the eight vegetable species using two different methods (cold maceration M and Pulsed Ultrasound-Assisted Extraction US) and two different experimental protocol (Protocol A and B) are taken into account as independent set to test the statistical model.
|
|
Sample Identification Code |
Vegetable Species |
Extraction Method |
Experimental Protocol |
|
1 |
Cb_TS |
Carpinus betulus |
US |
Protocol A |
|
2 |
Cb_TS2 |
Carpinus betulus |
US |
Protocol B |
|
3 |
Cm_TS |
Cornus mas |
US |
Protocol A |
|
4 |
Cm_TS2 |
Cornus mas |
US |
Protocol B |
|
5 |
Fc_TS |
Ficus carica |
US |
Protocol A |
|
6 |
Fc _TS2 |
Ficus carica |
US |
Protocol B |
|
7 |
Fe_TS |
Fraxinus excelsior |
M |
Protocol A |
|
8 |
Fe_TS2 |
Fraxinus excelsior |
US |
Protocol A |
|
9 |
Ld_TS |
Larix decidua |
US |
Protocol A |
|
10 |
Ld_TS2 |
Larix decidua |
US |
Protocol B |
|
11 |
Pm_TS |
Pinus montana |
M |
Protocol A |
|
12 |
Pm_TS2 |
Pinus montana |
US |
Protocol A |
|
13 |
Qp_TS |
Quercus petraea |
M |
Protocol A |
|
14 |
Qp_TS2 |
Quercus petraea |
US |
Protocol A |
|
15 |
Tt_TS |
Tilia tomentosa |
US |
Protocol A |
|
16 |
Tt_TS2 |
Tilia tomentosa |
US |
Protocol B |
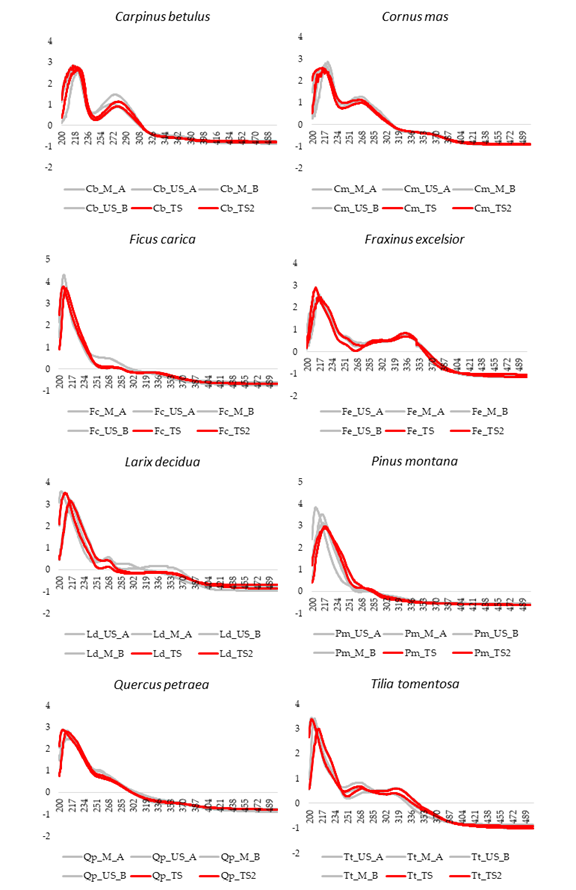
All the pre-treated UV-Visible absorption spectra, in the range 200–500 nm, are reported in Figure 2. For each species, the four averaged spectral profiles corresponding to the Calibration set (Table 2) are highlighted in grey while in red have been reported the Test set samples (TS/TS2) belonging to the same class.
Then, a data matrix B
32,620
consisting of 32 rows and 620 columns was prepared and analogously analyzed by PCA. B
32,620
rows correspond to the 32 BDs analyzed (Calibration set), and columns are the absorbance values of the UV-Visible spectra after SNV in the range 200–500 nm coupled to the chromatographic quantifications by HPLC (4 polyphenolic classes and 13 bioactive compounds). The data set was previously scaled by using a block scaling procedure
[36]
, with the aim to give to the spectroscopic and chromatographic variables a comparable influence in the data analysis. In fact, this pretreatment allows to divide variables in different blocks whose values will be scaled to attain the same block-variance after pretreatment. Moreover, the variables belonging to the same block are equally weighted.
Figure 2.
Averaged UV-Visible spectra of the 8 botanical species after SNV pre-treatment of data. For each species, the four averaged spectral profiles of the Calibration set (Table 2) are highlighted in grey while in red are reported the External Test set samples (Table 3).
3. Results and Discussion
The quality control of vegetal material is critical both if the botanical product is to be used as a drug or as an herbal food supplement. For consumer safety and the protection of who operate in this industrial field, quality control should be applied throughout the different processing steps, from the raw material to the final product. Scientific-naturalistic illustrations of the most common buds used in BDs production (Table 1) have been realized within the Finnover project by an expert botanical graphic designer, in order to provide a useful first tool for the operators in the BDs manufacturing. In fact, this peculiar raw material is generally spontaneously collected and mistakes in the attribution of some botanical species may be possible. For this, bud illustrations could represent a preliminary control of these vegetable materials after their collection in the point of view of a controlled manufacturing chain of BDs.
Moreover, a strategy based on the untargeted UV-Visible fingerprinting coupled to chemometrics allows rapid screening of the polyphenolic BDs profile to obtain a preliminary control tool to identify the botanical species.
3.1. Bud-Derivatives Identification: UV-Visible fingerprint
Figure 2 show the UV–Visible spectral profiles, after SNV pretreatment of the data, recorded for the eight vegetable species investigated:
Carpinus betulus
L.,
Cornus mas
L.,
Ficus carica
L.,
Fraxinus excelsior
L.,
Larix decidua
Mill.,
Pinus montana
Mill.,
Quercus petraea
(Matt.) Liebl.,
Tilia tomentosa
Moench. The extracts were obtained by the conventional maceration and the innovative green extraction (M or US) respectively, using the two experimental protocols (A or B) as described in detail in Table 2. Ultrasounds represent one of the innovative processing techniques of officinal plants. In fact, several companies already exploit innovative applications of ultrasound to obtain liquid foods, beverages, and alcoholic drinks
. Previously, the Authors described PUAE as an alternative time-saving method to the conventional maceration for the extraction of the polyphenolic fraction from buds [8]. Particularly, PUAE on a lab pilot reactor demonstrated to be an excellent approach for a rapid (20 min vs. 21 days or 3 months of maceration, depending on the Protocol applied) and efficient extraction of phenolic compounds.
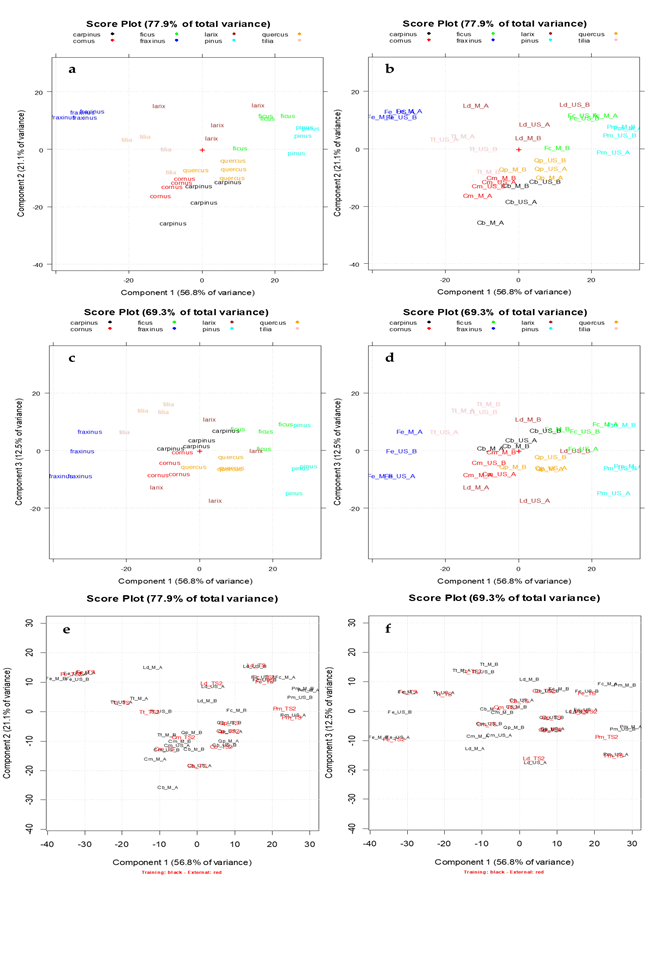
Looking at Figure 2, the spectra of the different vegetable species are quite different, highlighting as the pattern of absorbances in the UV–Visible region is strictly connected with the botanical origin of the plants. On the contrary, for each botanical species the spectral differences due to the extraction method (M or US) and to the extraction solvent (Protocol A or B), are minimal. The 501–900 nm interval has been preliminarily removed because there were none interesting absorptions in this spectral region at the assayed concentrations. PCA, an unsupervised pattern recognition technique was applied in order to explore and to analyze the data set using a multivariate approach since the analytical information contained in each spectrum was considered as a multivariate fingerprint. Particularly, the data matrix A
32,601
,
whose rows are the extracts (Calibration set) and the columns are the absorbances recorded in the spectral range 200–500 nm, was considered. PCA was performed on the pretreated and autoscaled data matrix. The first two principal components (PCs) of the data set (A
32,601
), which together explained the 77.9% of the total information of the data set since they visualize almost the 80% of the total variance, were firstly taken into account. Figure 3a,b shows the PCA score plots on the 1st–2nd principal components (PC1-PC2) obtained from the above-mentioned data matrix. In Figure 3a the extracts are categorized according to the vegetable species and each one is visualized with a different color (
Carpinus betulus
L.: black,
Cornus mas
L.: red,
Ficus carica
L: green.,
Fraxinus excelsior
L.: blue,
Larix decidua
Mill.: brown,
Pinus montana
Mill.: light blue,
Quercus petraea
(Matt.) Liebl.: orange,
Tilia tomentosa
Moench: pink). In Figure 3b, for each vegetable class all the extracts belonging to the calibration set were indicated with their identification code (see Table 2). PC1, the direction of maximum variance which explains almost the 60% of the total information, allows good discrimination between the botanical class regardless of the extraction method (M or US) and the experimental preparation protocol (A or B). Particularly, the
Fraxinus
class (blue, lowest scores on PC1) separates from
Ficus
(green) and
Pinus
(light blue)
which have higher scores on PC1. PC2, which explains the 21.1% of the remaining variance, allows to mainly separate
Larix
class (brown, highest scores on PC2) from
Quercu
s (orange) and
Carpinus
(black, lowest scores on PC2).
Figure 3c,d show the PCA score plots on the PC1-PC3, which explain together the 69.3% of the total variance of the data set. A good separation among the above cited botanical classes is also highlighted except for
Larix
and
Carpinus
ones. In fact, these latter separate on PC2 (Figures 3a and 4b) and since PCs are orthogonal, they are uncorrelated and no duplicate information are shown in their plots
[32]
.
In Figure 3e,f, the projections of the external test set (red samples) were reported on the PC1-PC2 and PC1-PC3 score plots respectively, showing a good correspondence with the calibration set for each botanical species.
Figure 3.
The scores plots of the UV–Visible absorbances data matrix A
32,601
. Each vegetable species is reported with a different color (
Carpinus betulus
L.: black,
Cornus mas
L.: red,
Ficus carica
L: green.,
Fraxinus excelsior
L.: blue,
Larix decidua
Mill.: brown,
Pinus montana
Mill.: light blue,
Quercus petraea
(Matt.) Liebl.: orange,
Tilia tomentosa
Moench: pink). (
a
) PC1-PC2 score plot with BDs categorized according to the vegetable species; (
b
) PC1-PC2 score plot with BDs categorized according to their identification code (Table 2); (
c
) PC1-PC3 score plot with BDs categorized according to the vegetable species; (
d
) PC1-PC3 score plot with BDs categorized according to their identification code (Table 2); (
e
) PC1-PC2 score plot obtained projecting the external test set samples (highlighted in red); (
f
) PC1-PC3 score plot obtained projecting the external test set samples (highlighted in red).
The spectral variables having greater importance (loading values) on the first three PCs are represented by spectral areas near the following absorbances (in ascending order): 200 nm, 212 nm, 240 nm, 275 nm, 310 nm, 360 nm, 420 nm, as highlighted in the Loading plot on PC1-PC2-PC3. Detailed information are reported in the original article, available online
https://www.mdpi.com/2304-8158/9/10/1343/htm
.
Several of them could be related to some secondary metabolites largely distributed in plant material (even in buds) such as tannins, whose structural variability depends on the vegetal species and even among organs of the same plant species
[39]
. The chemotaxonomic values of tannins have been recognized in the literature for several botanical species
and, the distribution of hydrolysable tannins has been used as chemotaxonomic markers by several authors
[42]
.
It is well known that the different classes of tannins present characteristic absorption bands in the UV spectral region. Particularly as far as hydrolysable tannins are concerned, gallotannins show two characteristic absorption maximums, λ max around 212 nm and λ max around 275 nm, with an inflection point (λ min) around 242 nm; ellagitannins present strong absorption near 200 nm and a shoulder around 277 nm and another absorption near 360 nm. Instead condensed tannins (or proanthocyanidins), chemically defined as flavonoid polymers in which the phenolic hydroxyls are partially or totally esterified with gallic acid, present an absorption around 200 nm, a λ min between 258–259 nm and λ max between 279–281 nm
[39]
. Nevertheless, also other polyphenols, such as hydroxycinnamic acids and flavonoids, could contribute to the UV-Visible fingerprints, even if some of them are more ubiquitarians and lesser species-specific
. Furthermore, as far as flavonoids are concerned, it is important to underline that their absorptions in the Visible are almost negligible at the measured concentrations, which are instead useful to avoid saturation of the UV region.
The fingerprint UV-Visible, at least in a preliminary screening step, seems to discriminate the peculiar polyphenols composition of BDs and could be a simple and quick method to confirm the proper identification of the botanical source after the botanic check by a professional botanist.
3.2. Bud-Derivatives Identification: UV-Visible and HPLC Fingerprints
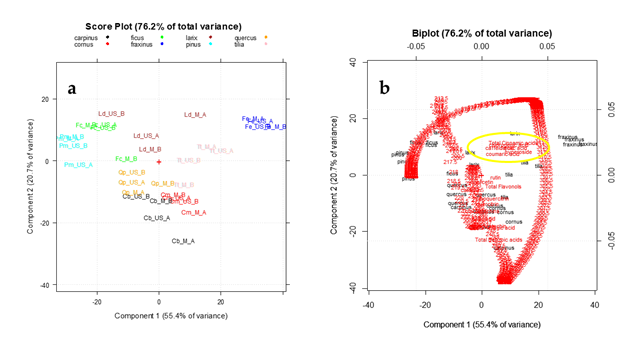
Figure 4
shows the PCA plots of the data matrix B
32,620
on PC1-PC2, which together explained the 76.2% of the total variance.
Figure 4.
The PC1–PC2 plots of the UV–Visible absorbances coupled to the HPLC data (data matrix B
32,620
): (
a
) Score plot; (
b
) Biplot.
PCA was performed on the pretreated and autoscaled data matrix, after the block scaling treatment in order to consider in the data analysis the same importance for the spectroscopic and chromatographic variables [45]. The PC1-PC2 score plot (Figure 4a) highlights a good separation between the vegetal species. Particularly PC1, which represents the direction of maximum variance explaining the 55.4% of the total information, allows good discrimination between
Fraxinus
class (blue, highest scores on PC1),
Ficus
(green) and
Pinus
(light blue)
classes,
which have lowest scores on this PC. As highlighted in the Biplot (Figure 4b) the variables having greater importance (loading value) on this separation are represented by total cinnamic acids, caffeic acid, coumaric acid and hyperoside content which are high in
Fraxinus
species and very low in
Pinus
one (as reported in Table 4). Instead PC2, which explains the 20.7% of the remaining information, allows mainly to separate
Carpinus
(black) and
Cornus
(red) classes from all the other ones. These species result particularly rich in tannins (catechins and benzoic acids).
Table 4. Bioactive classes and total phenolics in the analyzed samples.
|
Cinnamic Acids |
Flavonols |
Benzoic Acids |
Catechins |
Total Phenolics |
||||||
|
Sample ID |
Mean Value |
SD |
Mean Value |
SD |
Mean Value |
SD |
Mean Value |
SD |
Mean Value |
SD |
|
(mg/100 gFW**) |
(mg/100 gFW**) |
(mg/100 gFW**) |
(mg/100 gFW**) |
(mg/100 gFW**) |
||||||
|
Tt_M_A |
5.30 |
0.73 |
51.64 |
2.66 |
22.98 |
0.79 |
52.17 |
1.46 |
132.09 |
5.64 |
|
Tt_M_B |
23.87 |
1.06 |
90.79 |
5.02 |
6.62 |
1.04 |
50.68 |
1.03 |
171.97 |
8.16 |
|
Tt_US_A |
5.33 |
1.39 |
71.26 |
5.92 |
132.56 |
1.68 |
156.46 |
1.78 |
365.61 |
10.77 |
|
Tt_US_B |
12.43 |
5.20 |
100.23 |
14.84 |
96.28 |
8.41 |
81.15 |
10.16 |
290.10 |
38.61 |
|
Pm_M_A |
n.d. |
/ |
31.13 |
1.45 |
n.d. |
/ |
171.38 |
1.65 |
202.51 |
3.10 |
|
Pm_M_B |
n.d. |
/ |
n.d. |
/ |
n.d. |
/ |
49.36 |
2.29 |
49.36 |
2.29 |
|
Pm_US_A |
n.d. |
/ |
31.36 |
3.86 |
3.67 |
1.56 |
378.90 |
2.54 |
413.93 |
7.96 |
|
Pm_US_B |
n.d. |
/ |
38.74 |
4.35 |
n.d. |
/ |
325.88 |
4.77 |
364.62 |
9.12 |
|
Ld_M_A |
n.d. |
/ |
275.15 |
0.91 |
97.07 |
0.31 |
112.09 |
0.67 |
484.31 |
1.88 |
|
Ld_M_B |
n.d. |
/ |
151.57 |
2.23 |
137.23 |
0.88 |
70.90 |
2.62 |
359.70 |
5.72 |
|
Ld_US_A |
2.40 |
1.02 |
810.86 |
3.32 |
190.25 |
0.95 |
152.12 |
2.12 |
1155.63 |
7.42 |
|
Ld_US_B |
n.d. |
/ |
941.62 |
13.22 |
219.28 |
3.66 |
127.08 |
7.33 |
1287.98 |
24.21 |
|
Fe_M_A |
829.03 |
2.26 |
499.08 |
2.52 |
214.49 |
0.69 |
328.25 |
1.68 |
1870.85 |
7.15 |
|
Fe_M_B |
119.44 |
0.98 |
223.61 |
3.43 |
40.81 |
1.25 |
98.75 |
2.52 |
482.61 |
8.18 |
|
Fe_US_A |
151.00 |
2.32 |
378.93 |
4.62 |
115.82 |
0.93 |
225.26 |
2.21 |
871.01 |
10.07 |
|
Fe_US_B |
113.53 |
6.70 |
551.07 |
10.06 |
77.40 |
2.30 |
215.96 |
5.28 |
957.96 |
24.34 |
|
Cm_M_A |
23.97 |
0.40 |
1055.03 |
1.87 |
577.48 |
0.37 |
104.70 |
0.53 |
1761.19 |
3.18 |
|
Cm_M_B |
24.59 |
1.55 |
310.99 |
2.06 |
541.34 |
2.35 |
1161.65 |
2.48 |
2038.58 |
8.45 |
|
Cm_US_A |
14.87 |
1.04 |
672.04 |
3.57 |
276.38 |
1.33 |
98.83 |
1.21 |
1062.12 |
7.15 |
|
Cm_US_B |
n.d. |
/ |
784.79 |
12.98 |
329.55 |
2.85 |
167.03 |
4.67 |
1281.37 |
20.50 |
|
Cb_M_A |
47.04 |
0.83 |
442.45 |
2.04 |
286.40 |
1.25 |
523.93 |
1.14 |
1299.83 |
5.26 |
|
Cb_M_B |
n.d. |
/ |
203.20 |
1.18 |
418.85 |
2.56 |
248.73 |
2.73 |
870.78 |
6.47 |
|
Cb_US_A |
n.d. |
/ |
230.16 |
2.82 |
80.56 |
1.04 |
297.57 |
1.07 |
608.29 |
4.92 |
|
Cb_US_B |
n.d. |
/ |
198.98 |
5.89 |
206.42 |
4.05 |
227.60 |
3.00 |
633.00 |
12.95 |
|
Fc_M_A |
62.21 |
0.84 |
287.89 |
4.35 |
67.29 |
0.89 |
267.35 |
2.16 |
684.74 |
8.25 |
|
Fc_M_B |
n.d. |
/ |
123.28 |
3.65 |
45.86 |
1.08 |
68.42 |
2.11 |
237.57 |
6.83 |
|
Fc_US_A |
6.49 |
2.62 |
116.68 |
4.31 |
26.33 |
1.18 |
138.27 |
2.64 |
287.77 |
10.76 |
|
Fc_US_B |
10.77 |
5.54 |
155.02 |
11.39 |
52.18 |
3.49 |
183.91 |
7.34 |
401.88 |
27.76 |
|
Qp_M_A |
5.08 |
0.65 |
223.63 |
1.97 |
283.59 |
1.28 |
294.75 |
0.85 |
807.06 |
4.75 |
|
Qp_M_B |
n.d. |
/ |
59.40 |
2.75 |
84.02 |
2.16 |
109.81 |
2.18 |
253.23 |
7.09 |
|
Qp_US_A |
1.76 |
1.29 |
55.98 |
4.96 |
223.32 |
2.35 |
253.81 |
2.23 |
534.87 |
10.83 |
|
Qp_US_B |
n.d. |
/ |
72.09 |
8.50 |
58.43 |
5.70 |
161.81 |
4.89 |
292.32 |
19.08 |
* SD: standard deviation; ** FW: fresh weight
3.3. Phenolic Composition of Bud-derivatives
In this study, the health-promoting compounds were grouped into four different polyphenolic classes in order to assess the contribution of each class to the phytocomplex composition of buds belonging to the eight different species: cinnamic acids (as sum of caffeic acid, chlorogenic acid, coumaric acid, ferulic acid), flavonols (as sum of hyperoside, isoquercitrin, quercetin, quercitrin and rutin), benzoic acids (ellagic and gallic acids) and catechins ((+)catechin and (-)epicatechin).
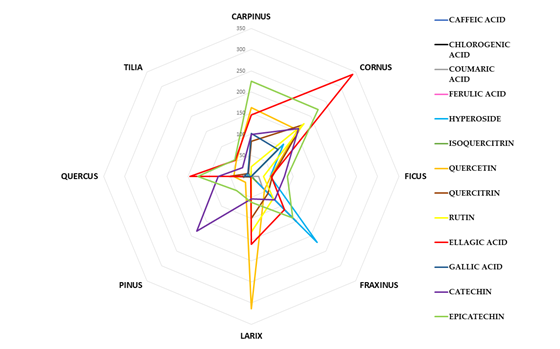
The identification and quantification of each single bioactive compound is shown in Figure 5 which shows the radar plot, made considering for each botanical species the mean values obtained from the 4 different extracts (M_A, M_B, US_A, US_B) for each marker compound quantified.
Figure 5.
The mean content of each phenolic marker (caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, hyperoside, isoquercitrin, quercetin, quercitrin and rutin, ellagic acid, gallic acid, (+)catechin and (-)epicatechin) for the eight botanical species investigated.
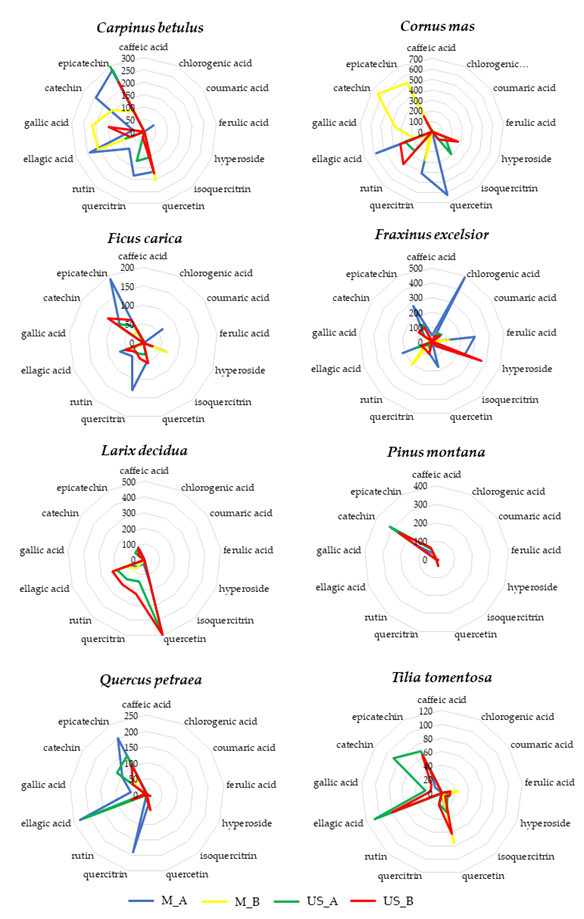
All BDs analyzed showed a good content of phenolics although there was a high variability both between the different vegetal species and between the extracts obtained by the different manufacturing method and experimental conditions starting from the same botanical species. Figure 6 showed the radar plots of each botanical species in order to better highlight the phenolic composition of the 4 different extracts (M_A, M_B, US_A, US_B).
Figure 6.
For each botanical species
the phenolic composition (caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, hyperoside, isoquercitrin, quercetin, quercitrin and rutin, ellagic acid, gallic acid, (+)catechin and (-)epicatechin) of the 4 different extracts (M_A: blue line, M_B: yellow line, US_A: green line, US_B: red line) was reported.
Detailed information are reported in the original article available online
https://www.mdpi.com/2304-8158/9/10/1343/htm
.
As showed in Figure 6, the manufacturing methods (conventional maceration or sonication) and the experimental conditions used for the preparation of BDs (i.e., extraction solvent, extraction time, solid/ solvent ratio, extraction time) strongly influenced the phenolic extraction yield despite having removed the variability of the raw material (same batch of buds for each vegetal species).
Due to the lack of a single regulation and an unique preparation protocol for these botanicals, very different products can be found on the market in terms of their polyphenolic fraction depending on both the raw materials (i.e., taking into account their specific agro-environmental and biological traits) and on the experimental conditions of manufacturing (method of preparation, extraction solvent, solid/solvent ratio, extraction time).
4. Conclusions
Although BDs have been widely used in traditional medicine because of the peculiar content of buds in phenolic compounds, nowadays they are a category of botanicals still poorly studied. The lack of detailed scientific information and a clear and unique regulation, it makes these products high risk and vulnerable for accidental mistakes in the attribution of the botanical species, but also frauds and adulterations. Moreover, the polyphenols content of BDs is strongly influenced by the manufacturing processes whose parameters are often not strictly defined (e.g., solvent ratios in the extraction mixtures, raw material/extraction mixture ratios, extraction time) and thus they affect their final compositions.
This research, within the Finnover project, aims to answer to the growing demand for efficient quality control in the BDs field to guarantee the proper attribution of the botanical source and their content. Moreover, a manufacturing process specification should be advisable to monitor the bioactive contents.
UV-Visible spectroscopy and HPLC-DAD analysis have been employed to obtain an untargeted and a targeted phytochemical fingerprint of BDs, respectively. UV-Visible coupled with an appropriate chemometric data processing is a simple, rapid and low-cost technique proved to be very useful to identify the botanical source regardless the manufacturing method and the experimental conditions used. Moreover, the targeted phytochemical fingerprint by HPLC-DAD allowed to obtain a detailed screening of the BDs polyphenolic profile which highlighted an high variability due to the different vegetal species and to the manufacturing method and protocol. The ultrasonic extraction of buds compared to conventional maceration proved less sensitive to the different extraction protocols.
The proposed strategy offers to those operating in this industrial sector an untargeted method for the identification of the bud’s botanical species and a green extraction strategy (PUAE) which is more robust with respect to the different extractive protocols that can be used. The same approach, described for BDs, could be analogously applied to other botanical productions.
The article has been published on https://doi.org/10.3390/foods9101343.
References
- Czepielewska, E.; Makarewicz-Wujec, M.; Różewski, F.; Wojtasik, E. Kozłowska-Wojciechowska, M. Drug adulteration of food supplements: A threat to public health in the European Union? Toxicol. Pharmacol. 2018, 97, 98–102, doi:10.1016/j.yrtph.2018.06.014.
- Italian Ministry of Health. Available online: http://www.salute.gov.it/portale/temi/p2_5.jsp?lingua=italiano&area=Alimentiparticolarieintegratori&menu=integratori (accessed on 20 March 2020).
- European Commission, Food Supplements. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/supplements_en (accessed on 21 May 2020).
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in Functional Foods and Food Supplements: Tradition, Efficacy and Regulatory Aspects. Sci. 2020, 10, 2387, doi:10.3390/app10072387.
- FINNOVER Interreg Alcotra Project 2017–2020. Available online: http://www.interreg-finnover.com/ (accessed on 20 May 2020).
- Pharmacopée Française. Codex Medicamentarius Gallicus, Codex Français: Monographie, Préparations Homéopathiques; Ordre National des Pharmaciens: Paris, France, 1965. Available online: http://ansm.sante.fr/Mediatheque/Publications/Pharmacopee-francaise-Plan-Preambule-index (accessed on 21 May 2020).
- Turrini, F.; Donno, D.; Boggia, R.; Beccaro, G.L.; Zunin, P.; Leardi, R.; Pittaluga, A.M. An innovative green extraction and re-use strategy to valorize food supplement by-products: Castanea sativa bud preparations as case study. Food Res. Int. 2019, 115, 276–282, doi:1016/j.foodres.2018.12.018.
- Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project. Foods 2019, 8, 466, doi:10.3390/foods8100466.
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud Extracts as New Phytochemical Source for Herbal Preparations-Quality Control and Standardization by Analytical Fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health, 1st ed.; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; pp. 187–218, doi:10.5772/59759.
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus Buds. Pharmaceuticals 2016, 9, 7, doi:10.3390/ph9010007.
- Sanzini, E.; Badea, M.; Dos Santos, A.; Restani, P.; Sievers, H. Quality control of plant food supplements. Food Funct. 2011, 2, 740–746, doi:10.1039/C1FO10112A.
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. Food Sci. Technol. 2016, 53, 1071–1083, doi:10.1007/s13197-015- 2115-6.
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. Available online: https://eur-lex.europa.eu/eli/dir/2002/46/2017-07-26 (accessed on 5 September 2020).
- Decreto Legislativo 21 Maggio 2004, n.169, Attuazione Della Direttiva 2002/46/CE Relativa Agli Integratori Alimentari. Available online: https://www.gazzettaufficiale.it/eli/id/2004/07/15/004G0201/sg (accessed on 5 September 2020).
- European Federation of Associations of Health Product Manufacturers (EHPM). Available online: https://www.ehpm.org/attachments/article/117/EHPM%20Quality%20Guide%20101214.pdf (accessed on 5 September 2020).
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Metab. Cardiovasc. Dis. 2014, 24, 639–647, doi:10.1016/j.numecd.2013.12.014.
- Kwok, C.S.; Boekholdt, S.M.; Lentjes, M.A.H.; Loke, Y.K.; Luben, R.N.; Yeong, J.K.; Wareham, N.J.; Myint, P.K.; Khaw, K.T. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart 2015, 101, 1279–1287, doi:10.1136/heartjnl-2014-307050.
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. Diabetes Investig. 2016, 7, 56–69, doi:10.1111/jdi.12376.
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. Nutr. Biochem. 2014, 25, 1–18, doi:10.1016/j.jnutbio.2013.09.001.
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233, doi:10.1016/j.tifs.2018.06.007.
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273, doi:10.1155/2012/914273.
- Liu, X.; Du, X.; Han, G.; Gao, W. Association between tea consumption and risk of cognitive disorders: A dose-response meta-analysis of observational studies. Oncotarget 2017, 8, 43306–43321, doi:10.18632/oncotarget.17429.
- Allio, A.; Calorio, C.; Franchino, C.; Gavello, D.; Carbone, E.; Marcantoni, A. Bud extracts from Tilia tomentosa Moench inhibit hippocampal neuronal firing through GABAA and benzodiazepine receptors activation. Ethnopharmacol. 2015, 172, 288–296, doi:10.1016/j.jep.2015.06.016.
- Calorio, C.; Donno, D.; Franchino, C.; Carabelli, V.; Marcantoni, A. Bud extracts from Salix caprea L. inhibit voltage gated calcium channels and catecholamines secretion in mouse chromaffin cells. Phytomedicine 2017, 36, 168–175, doi:10.1016/j.phymed.2017.09.006.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901, doi:10.3390/molecules21070901.
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Bonvegna, L.; Bounous, G. Castanea spp. buds as a phytochemical source for herbal preparations: Botanical fingerprint for nutraceutical identification and functional food standardization. Sci. Food Agric. 2014, 94, 2863–2873.doi:10.1002/jsfa.6627.
- Boggia, R.; Turrini, F.; Anselmo, M.; Zunin, P.; Donno, D.; Beccaro, G.L. Feasibility of UV-VIS-Fluorescence Spectroscopy combined with pattern recognition techniques to authenticate a new category of plant food supplements. Food Sci. Technol. 2017, 54, 2422–2432, doi:10.1007/s13197-017-2684-7.
- Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Andriamaniraka, H.; Gamba, G.; Beccaro, G.L. Traditional and Unconventional Dried Fruit Snacks as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 396, doi:3390/antiox8090396.
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenol. Nutrients 2014, 6, 6020–6047, doi:10.3390/nu6126020.
- Mok, D.K.W.; Chau, F.T. Chemical information of Chinese medicines: A challenge to chemist. Intell. Lab. Syst. 2006, 82, 210–217, doi:10.1016/j.chemolab.2005.05.006.
- Italian Chemical Society. Division of Analytical Chemistry-Group of Chemometrics. CAT Chemometric Agile Tool. Available online: http://www.gruppochemiometria.it/index.php/software (accessed on 25 May 2020).
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Intell. Lab. Syst. 1987, 2, 37–52, doi:10.1016/0169-7439(87)80084-9.
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer Series in Statistics; Springer: New York, NY, USA, 2002.
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Spectrosc. 1989, 43, 772–777, doi:10.1366/0003702894202201.
- Oliveri, P. Class-modelling in food analytical chemistry: Development, sampling, optimisation an validation issues—A tutorial. Chim. Acta 2017, 982, 9–19. doi:10.1016/j.aca.2017.05.013.
- Wold, S.; Johansson, E.; Cocchi, M. 3D QSAR in Drug Design: Theory, Methods and Applications; Hugo, K., Ed.; ESCOM Science Publishers: Leiden, The Netherlands, 1993; p. 523.
- Chemat, F.; Ashokkumar, M. Preface: Ultrasound in the processing of liquid foods, beverages and alcoholic drinks. Sonochem. 2017, 38, 753, doi:10.1016/j.ultsonch.2017.01.041.
- Paniwnyk, L. Applications of ultrasound in processing of liquid foods: A review. Sonochem. 2017, 38, 794–806, doi:10.1016/j.ultsonch.2016.12.025.
- Falcão, L.; Araújo, M.E.M. Vegetable Tannins Used in the Manufacture of Historic Leathers. Molecules 2018, 23, 1081, doi:10.3390/molecules23051081.
- Okuda, T.; Yoshida, T.; Hatano, T.; Iwasaki, M.; Kubo, M.; Orime, T.; Yoshizaki, M.; Naruhashi, N. Hydrolysable tannins as chemotaxonomic markers in the rosaceae. Phytochemistry 1992, 31, 3091–3096, doi:1016/0031-9422(92)83451-4.
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural features and biological properties of ellagitannins in some plant families of the order Myrtales. J. Mol. Sci. 2010, 11, 79–106, doi:10.3390/ijms11010079.
- Moilanen, J.; Koskinen, P.; Salminen, J.P. Distribution and content of ellagitannins in Finnish plant species. Phytochemistry 2015, 116, 188–197, doi:10.1016/j.phytochem.2015.03.002.
- Rocha, L.D.; Monteiro, M.C.; Teodoro, A.J. Anticancer properties of hydroxycinnamic acids—A Review. Cancer Clin. Oncol. 2012, 1, 109–121, doi:10.5539/cco.v1n2p109.
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949, doi:10.1111/liv.13435.