Aging is a broad process that occurs as a time-dependent functional decline and tissue degeneration in living organisms. On a smaller scale, aging also exists within organs, tissues, and cells. As the smallest functional unit in living organisms, cells “age” by reaching senescence where proliferation stops. Such cellular senescence is achieved through replicative stress, telomere erosion and stem cell exhaustion. It has been shown that cellular senescence is key to tissue degradation and cell death in aging-related diseases (ARD). However, senescent cells constitute only a small percentage of total cells in the body, and they are resistant to death during aging. This suggests that ARD may involve interaction of senescent cells with non-senescent cells, resulting in senescence-triggered death of non-senescent somatic cells and tissue degeneration in aging organs.
- cell senescence
- SACTAI
- SASP
1. Introduction
2. Cell Senescence Is Cell-Autonomous: Telomere Shortening, Replicative Stress and SASP Manifestation
Almost 60 years ago, Leonard Hayflick and Paul Moorhead first reported that primary human cells derived from embryonic tissues exhibited limitations while dividing [5]. These fibroblasts could only divide for 40–60 times in cell culture before entering senescence. Hayflick defined the different stages of cell culture into three phases (Figure 1). Phase I is the original primary culture, where cells divide to cover the surface at a relatively slow rate. Phase II represents the period when robust cell division occurs. Continuous subcultures of the same batch of cells leads to Phase III, when cells not only stopped growth but also died in a short period of time. Hayflick postulated that cell alterations must have occurred during cell growth in Phase II, which led to cell growth arrest and death in Phase III. He called this phenomenon cell senescence and further postulated that it could be a mechanism to counter unlimited cell proliferation in cancer. This phenomenon of cell growth arrest is later defined as Hayflick Limit due to replicative senescence (Figure 1A). In a discovery that led to a Nobel prize on telomerase, it was found that the primary cause for Hayflick Limit, or replicative senescence, is telomere shortening [7,8][7][8].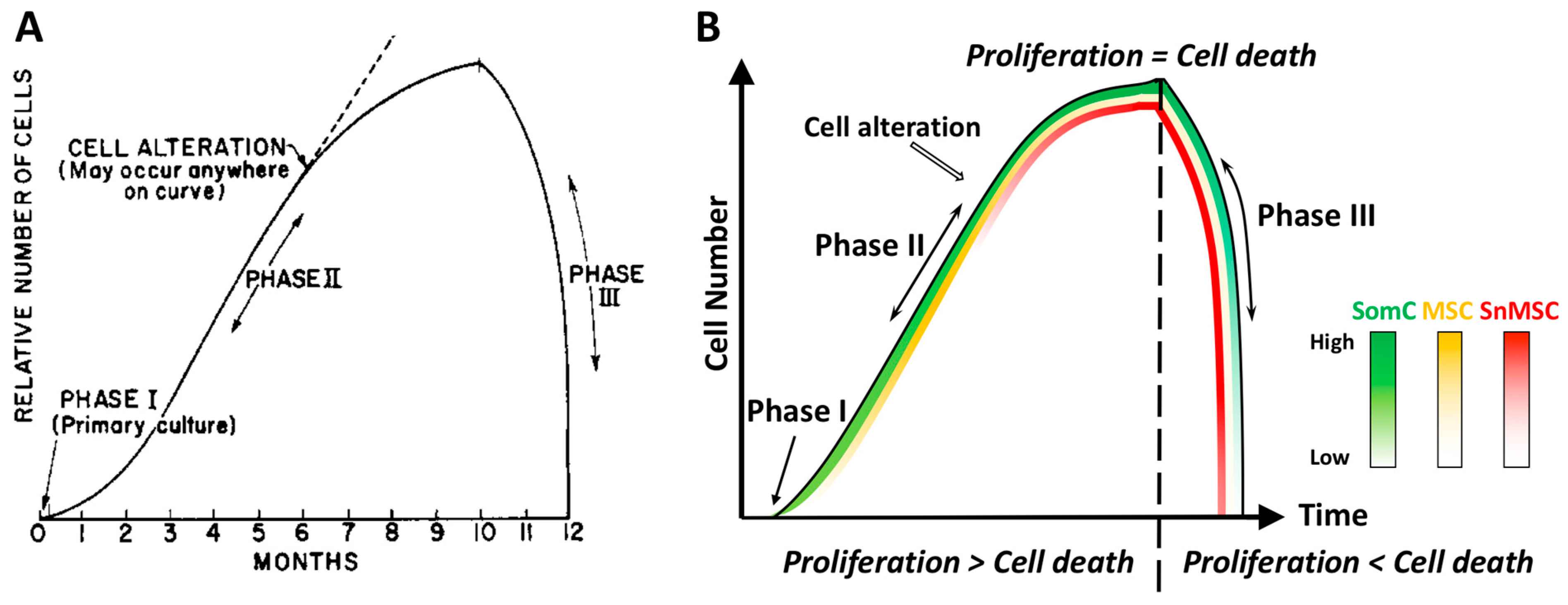
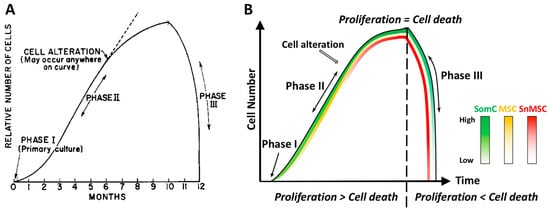 Figure 1. Diagram representations of the Hayflick phenomenon. (A) Classic Hayflick model [5]. Phase I is the primary culture. Phase II is defined as the multiple cell passage period characterized by robust cell proliferation and increase of cell number. After subcultures of 40–60 passages, cells reach Phase III with halting proliferation followed by rapid cell death. Such an event is termed Hayflick Limit, or replicative senescence. (B) The SACTAI model. Phase I contains SomCs. During Phase II, cell passaging-induced cell proliferation triggers cell alteration. Some SomCs are converted into MSC and SnMSC due to replicative senescence. Cell proliferation is more than cell death in Phase II. The emergence of SnMSC results in its communication with SomC via SASP, which triggers catabolism and death of SomC. At the end of Phase II, cell proliferation equals death resulting in a cell number plateau. During Phase III, SASP triggered SomC death results in a rapid decline of total cell number. The loss of SomCs depletes the cell pool for proliferation. As a result, SnMSC triggered SomC cell death outpaces its proliferation, resulting in tissue degeneration. Thus, different cell types (SomC, MSC, and SnMSC) contribute to the cell transition and interaction during various phases. They account for aging-associated cellular changes. SomC: somatic cell; MSC: mesenchymal stromal cell; SnMSC: senescent mesenchymal stromal cell.
Figure 1. Diagram representations of the Hayflick phenomenon. (A) Classic Hayflick model [5]. Phase I is the primary culture. Phase II is defined as the multiple cell passage period characterized by robust cell proliferation and increase of cell number. After subcultures of 40–60 passages, cells reach Phase III with halting proliferation followed by rapid cell death. Such an event is termed Hayflick Limit, or replicative senescence. (B) The SACTAI model. Phase I contains SomCs. During Phase II, cell passaging-induced cell proliferation triggers cell alteration. Some SomCs are converted into MSC and SnMSC due to replicative senescence. Cell proliferation is more than cell death in Phase II. The emergence of SnMSC results in its communication with SomC via SASP, which triggers catabolism and death of SomC. At the end of Phase II, cell proliferation equals death resulting in a cell number plateau. During Phase III, SASP triggered SomC death results in a rapid decline of total cell number. The loss of SomCs depletes the cell pool for proliferation. As a result, SnMSC triggered SomC cell death outpaces its proliferation, resulting in tissue degeneration. Thus, different cell types (SomC, MSC, and SnMSC) contribute to the cell transition and interaction during various phases. They account for aging-associated cellular changes. SomC: somatic cell; MSC: mesenchymal stromal cell; SnMSC: senescent mesenchymal stromal cell.3. Cell Senescence-Associated Apoptosis and Degeneration Is Senescent Cell Non-Autonomous: The SACTAI Mechanism for Aging
4. Two-Way Communications between Senescent and Non-Senescent Cells: Altered Intercellular Signaling Mechanisms during Aging
An important consequence of senescence-associated cell transition is enabling heterotypic cell interaction between SnMSCs and non-senescent SomC. SASPs appear to be major signaling molecules mediating the SACTAI, as shown by recent publications [29,31][27][28]. The interaction between senescent and non-senescent cells is a two-way communication. The first is signaling from senescent cells to non-senescent cells (Figure 32). snMSC acquire a pro-inflammatory phenotype and secrete cytokine IL-1β due to cell transition and senescence. On the other hand, chondrocytes are the main recipient cells of inflammatory signaling by expressing IL-1R [20]. In addition to cytokine signaling, a recent study also identified Sonic Hedgehog (SHH) to mediate senescence signaling from snMSC to SomC [29][27]. SHH is known to play an important role during skeletal development and homeostasis [32,33][29][30]. SHH is expressed by NCSCs and increased in OA-MSCs during aging [29][27].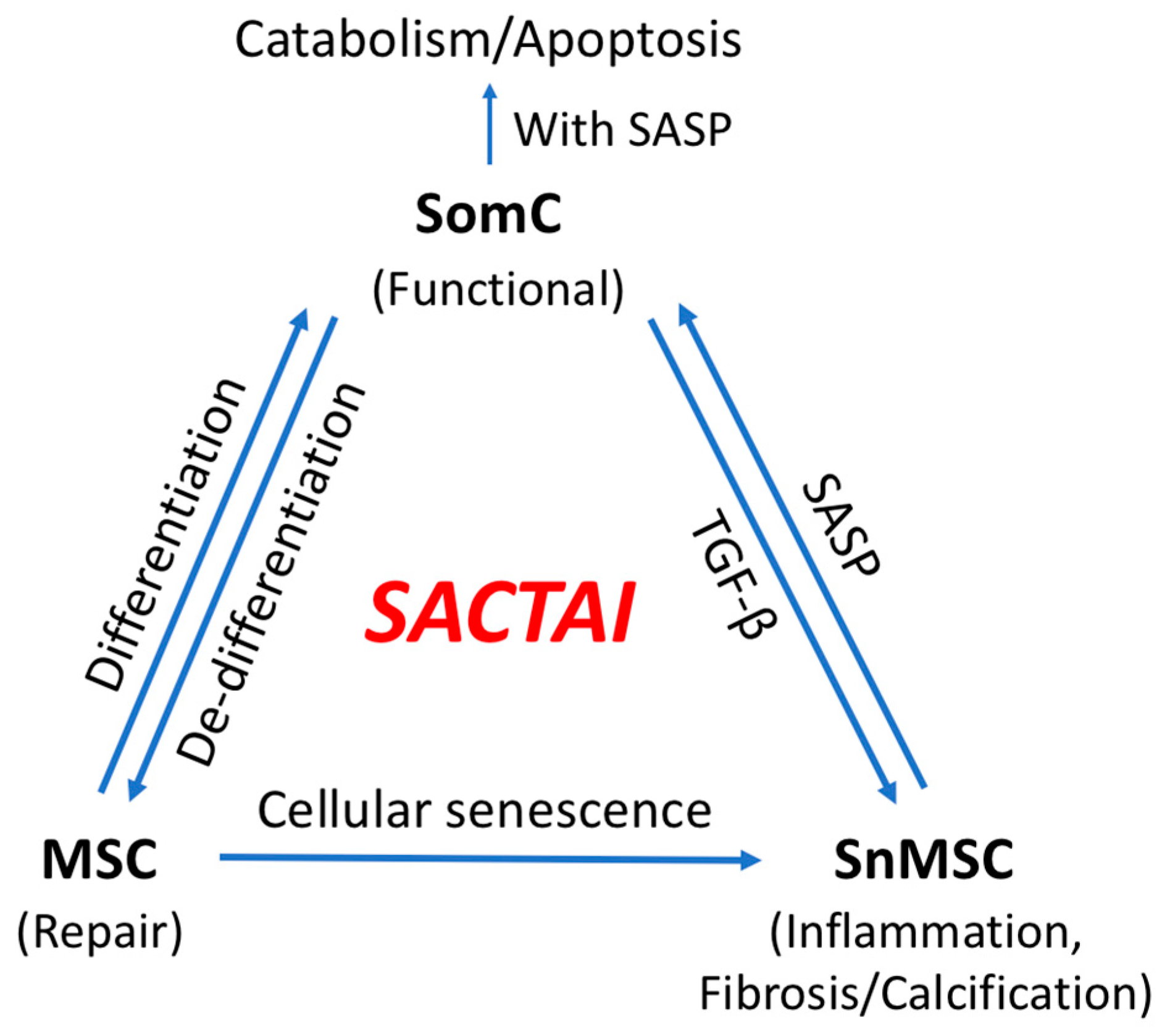
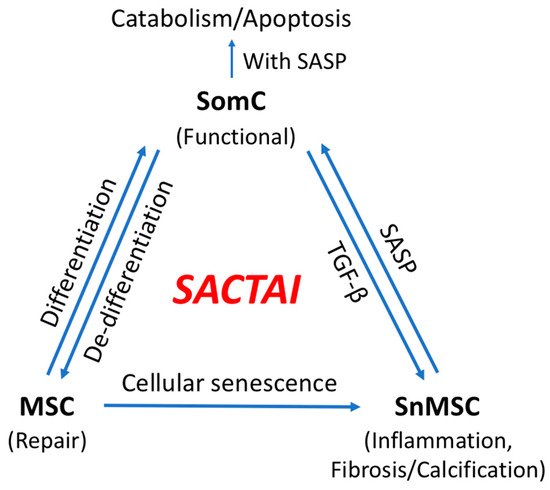 Figure 2. Senescence-associated cell transition and interaction (SACTAI) showing the relationship among SomC, MSC, and SnMSC. Due to aging-induced stress, MSCs proliferate and differentiate while SomCs de-differentiate and result in MSC-like cells for tissue repair. Repeated activation of MSC’s replication induces replicative senescence, resulting in SnMSCs. SnMSCs synthesize pro-inflammatory SASPs, which in turns act on SomCs to trigger catabolism and SomC death. Feedback of SomC with TGF-b reinforces the SnMSC properties including inflammation, fibrosis and abnormal calcification.
Figure 2. Senescence-associated cell transition and interaction (SACTAI) showing the relationship among SomC, MSC, and SnMSC. Due to aging-induced stress, MSCs proliferate and differentiate while SomCs de-differentiate and result in MSC-like cells for tissue repair. Repeated activation of MSC’s replication induces replicative senescence, resulting in SnMSCs. SnMSCs synthesize pro-inflammatory SASPs, which in turns act on SomCs to trigger catabolism and SomC death. Feedback of SomC with TGF-b reinforces the SnMSC properties including inflammation, fibrosis and abnormal calcification.