This work shows a global vision of the situation, placing the reader in a concise and orderly manner in perspective of the current state of treatment of metastatic uveal melanoma. Uveal melanoma (UM) is the most common primary intraocular malignancy in adults. The most frequent location is the choroid, representing 80% of the total, followed by the ciliary body, 12%, and the iris, 8%. The incidence of UM ranges from 5.3 to 10.9 cases per million inhabitants per year. Risk factors for developing UM include fair skin, congenital ocular melanocytosis, melanocytoma, and BAP1-tumor predisposition syndrome.
- uveal melanoma
- metastatic
- review
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Definition:
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults. The most frequent location is the choroid, representing 80% of the total, followed by the ciliary body, 12%, and the iris, 8%. The incidence of UM ranges from 5.3 to 10.9 cases per million inhabitants per year. Risk factors for developing UM include fair skin, congenital ocular melanocytosis, melanocytoma, and BAP1-tumor predisposition syndrome.
1.Introduction
More than 50% of patients with uveal melanoma end up developing metastases. Currently, there is no standard first-line treatment that facilitates proper management of the metastatic disease.
Therefore, improvements in primary tumor management have not translated into longer survival in patients with UM [1][2][15,16]. The 5-year survival rate among patients with the primary disease is approximately 60–70%; however, in advanced stages, with the presence of metastatic disease, the median overall survival falls to approximately 6–10 months, with only 8% of patients surviving to 2 years [3][4][13,17]. There has been considerable development in the field of treatment of metastatic UM over the past few decades, but new treatments do not appear to have demonstrated clear benefits [5][6][18,19].
2. Conventional Chemotherapy
Most of the systemic treatments in metastatic UM have been extrapolations from experience in cutaneous melanoma. With regard to conventional chemotherapy, the most commonly used drugs have been dacarbazine, fotemustine, and temozolomide, although studies have also been conducted with more modern agents, such as docosahexaenoic acid and paclitaxel and liposomal vincristine [7][20]. However, unlike its cutaneous counterpart, UM tends to be characterized by chemoresistance, as shown by the average survival rates provided by this review, which range from 4.6 to 17 months [7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][20–34].
In recent years, studies on temozolomide and dacarbazine with medians of OS between 5 and 13 months and progression-free interval (PFI) of up to 5.5 months have been the most consistent within this group [7][8][10][11][13][18][20,21,23,24,26,31]. The results with the combination of treosulfan and gemcitabine, on the other hand, are the most encouraging, even reaching medians of 14 months and annual survival rates of 80%, as in the Pföhler et al.’s trial [17][30].
Despite the large number of studies concerning conventional therapy, very few present exceptional data that deviate from the average. Among them, we found one patient alive at 5 years in the study by Leyvraz et al. [16][29], with fotemustine, a 57-month survival patient in the trial by Terheyden et al. [22][35], with gemcitabine and treosulfan, and another patient who survived 72 months in the study by Schinzari et al. [12][25], with a combination of cisplatin, dacarbazine, and vinblastine.
In terms of adverse effects, nausea and vomiting induced by chemotherapy represent the most frequent toxic effect, appearing in approximately 40–50% of the patients in the studies, and although they are often mild, they constitute one of the phenomena, which most deteriorate the quality of life of the oncological patient [23][36]. The vast majority of chemotherapy agents negatively affect the hematopoietic system [24][25][37,38], providing the most serious toxicities by affecting all cell series. Fotemustine [16][29] has the highest toxicity data, and dacarbazine or temozolomide [10][23] has the lowest (Figure 1).
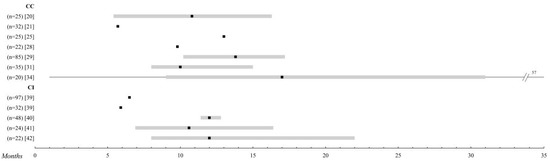
Figure 1.
Systemic chemotherapy. Comparison of the overall survival of the different treatments in metastatic uveal melanoma (UM). Those studies with n > 20 patients were selected. The black square indicates the median, and the 95% confidence intervals are represented in a grey bar. The range is established by the black lines. The overall survival is shown up to 35 months; when it is extended, the maximum time data (in months) is added. Abbreviations: CC, conventional chemotherapy; CI, chemoimmunotherapy
.
3. Chemoimmunotherapy
Different hypotheses maintain that the immune privilege enjoyed by the eye favors the growth and development of complex tissues, thanks to different mechanisms for the suppression of immune responses. Thus, to address the difficulty of metastatic treatment, different combinations of chemo-immune therapy have been proposed [26][39].
It is noteworthy that in this group [22][27][28][29][30][31][32][35,40–45], with the exception of the study of Pyrhönen et al. [30][43], all treatments are specifically studied as a first-line. The overall survival data range from 3, 7 to 12 months, although the range of PFI is much wider (1, 6–12 months). A multi-center study has analyzed the efficacy of BOLD (bleomycin, vincristine, lomustine, and dacarbazine) plus recombinant interferon α-2b, a form of TIQ, due to very promising pilot reports [33][34][46,47], but ultimately the expected results could not be confirmed. Adverse effects are practically superimposed on those of isolated chemotherapy, except for complications arising from the intra-arterial catheter used in the study by Becker et al. [28][41] and the liver toxicity of up to 80% found by Kivelä et al. [29][42] (Figure 1).
4. Immunotherapy
Immunotherapy has been shown to have a large survival benefit in the treatment of metastatic skin melanoma; however, it is unclear whether this advantage translates into UM, as it is a less immunogenic tumor [35][84].
For this reason, it has been precisely this immunological field on which studies in recent years have focused their attention in a very noticeable way, as can be seen in the articles included in this review [8][9][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][21,22,50,85–106].
Ipilimumab, a monoclonal antibody to cytotoxic T-lymphocyte antigen 4 (CTLA-4), blocks the effects of this regulator and increases T-cell responses against cancer cells, thus promoting increased immune system performance. Phase III trials have shown a clear survival benefit in metastatic skin melanoma [59][107]. However, in UM, survival is not increased with average values of approximately 10 months [8][38][43][50][21,86,91,98].
Anti-PD1 (anti-receptor of programmed death) therapy, such as pembrolizumab or nivolumab, is also being studied, but the results are very similar to those of ipilimumab in both OS and PFI, which is about 2–3 months long [7][41][42][45][46][49][52][21,89,90,93,94,97,100].
The best results of this group are obtained by combining several immunotherapies, as in Kirchberg et al. [44][92], (Ipilimumab + Pembrolizumab), and Pelster et al. [48][96], (Nivolumab + Ipilimumab), with median OS of 18.4 and 19.1 months, respectively.
In this treatment group, it is remarkable how a few exceptional events are observed in certain studies. Extreme survival can be appreciated in the four alive patients at 5 years in the study by Klemen et al. [37][85] or the 46 months reached by a patient in the study by Bol et al. [8][21] with ipilimumab.
The adverse effects are closely related to the hyperactivation of the immune system, with skin reactions and pseudo-flu symptoms being the most frequent in this group, which, in the vast majority of studies, are described as easily controllable. Among the most serious, hormonal alterations at the thyroid and pituitary levels and autoimmune colitis stand out, which, in the study by Rozeman et al. [38][86] with 10 mg of ipilimumab, affected 47% of the patients included (Figure 2).
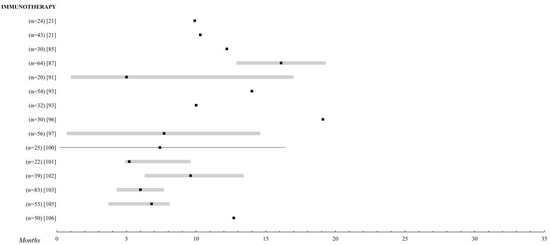
Figure 2.
Immunotherapy. Comparison of the overall survival of the different treatments for metastatic UM. Those studies with n > 20 patients were selected. The black square indicates the median, the 95% confidence intervals are the grey bar. The range is established by the black lines. The overall survival is shown up to 35 months; when it is extended, the maximum time data (in months) is added.
5. Targeted Therapy
Targeted therapy refers to drugs designed to interfere with a specific molecular pathway that is believed to play a critical role in tumor development or progression [59][108]. UM has a distinctive genetic profile that makes it an attractive candidate for the treatment with molecular target therapy. Unlike skin melanomas, BRAF mutations are extremely rare in uveal melanomas, where the vast majority show mutations in the genes GNAQ and GNA11 [60][61][109,110] that activate the mitogen-activated protein kinase (MAPK) pathway and, consequently, result in increased cell proliferation. These include drugs that can modify the pathways that regulate the cell cycle, inhibit the molecules involved in invasion and metastasis, and inhibit tumor angiogenesis [59][108] (Figure 3).
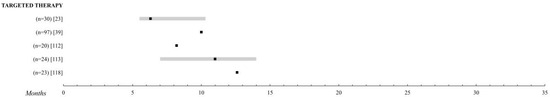
Figure 3.
Targeted therapy. Comparison of the overall survival of the different treatments for metastatic UM. Those studies with n > 20 patients were selected. The black square indicates the median, the 95% confidence intervals are the grey bar. The range is established by the black lines. The overall survival is shown up to 35 months; when it is extended, the maximum time data (in months) is added.
5.1. MAPK Inhibitors
The targeted therapies explored have been sunitinib [62][112], sorafenib [63][64][65][113–115], imatinib [66][67][116,117], cabozatinib [10][68][23,118], and selumetenib [27][40] alone or in combination with chemotherapy, reaching a median OS at 6, 3–12 months. The work of Niederkorn et al. [65][115] with sorafenib and fotemustine was unremarkable, providing an OS of 15.9 months and a 75% survival rate at one year, but this might be of little relevance due to the small sample size of the study—25 patients.
Promising results had initially been obtained with selumetinib, a phosphorylation inhibitor of MAPKs versus chemotherapy, but the recent SUMIT study led by Carvajal et al. [27][40], a phase II trial intended to confirm these results, finally found no difference between dacarbazine and dacarbazine with selumetinib with a median OS of about 10 months and a PFI of 2.8.
5.2. Heat Shock Protein 90 Protein Inhibitor
Hsp90 (heat shock protein) is a 90 kDa chaperone that promotes the folding of other proteins, allowing them to acquire their native three-dimensional conformation and thus perform their biological function [119]. It interacts with several client proteins, including signaling kinases (RAF and AKT), growth factor receptors (MET and KIT), and cell cycle regulators [69][70][120,121]. Several studies have found overexpression of Hsp90 in both solid and hematological malignancies, and data from cell line-based experiments suggest that this overexpression can also be seen in UM [71][122].
Ganetespib (STA-9090) is a synthetic small molecule that binds to Hsp90 and inactivates it [72][123]. Preclinical data have shown that in both in vitro and in vivo systems, ganetespib exhibits potent cytotoxicity and anti-tumor activity. Taking this as a reference, Shah et al. [73][111] conducted a prospective, controlled clinical trial with 17 patients in which different dosages of the drug were evaluated. Unfortunately, the median OS did not exceed 8.5 months.
6. Liver Thermotherapy
Other local ablative treatment techniques, such as CT-guided multi-probe stereotactic radiofrequency ablation (SRFA) or percutaneous magnetic resonance imaging (MRI)-guided laser-induced interstitial thermotherapy (LITT), have also been investigated for metastatic UM. The survival results are spectacular, reaching 38 months of the median OS with SRFA [73][140] and 33.6 months with LITT [74][141], making them an attractive alternative enhanced by minimal adverse effects. However, the scarcity of studies and their small sample size make it clear that new work is needed to confirm these theories.
