Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Bruce Ren and Version 4 by Bruce Ren.
Food supply disruption and shortage verified during the current pandemic events are a scenario that many anticipate for the near future. The impact of climate changes on food production, the continuous decrease in arable land, and the exponential growth of the human population are important drivers for this problem.
- bioactive compounds
- fruit waste
- vegetable waste
- health
- disease
- peels
- seeds
1. Introduction
According to UN estimates, the world’s population will reach 9.8 billion by 2050, nearly 20% higher than today [1]. This exponential increase combined with continuous climate changes, water scarcity, and decreasing of agricultural areas constitutes societal and global problems that challenge food production for the next generations. With current global trends in diets, the exponentially growing population and considering the several millions of tons of food that are lost and wasted every year in different steps of the food chain, including production, post-harvesting, processing, and distribution, in 2050 50–60% more food will be needed than today to feed everyone. These overwhelming numbers clearly show that food challenges and planet sustainability are intrinsically bound and must determine urgent measures to mitigate them. It will be important to develop strategies to produce more food and better food with less waste. In addition, the implementation of sustainable food production systems through the optimization of food processes will be essential to achieve a better environmental footprint, lower production costs, and improve the quality and nutritional value of food (Figure 1).
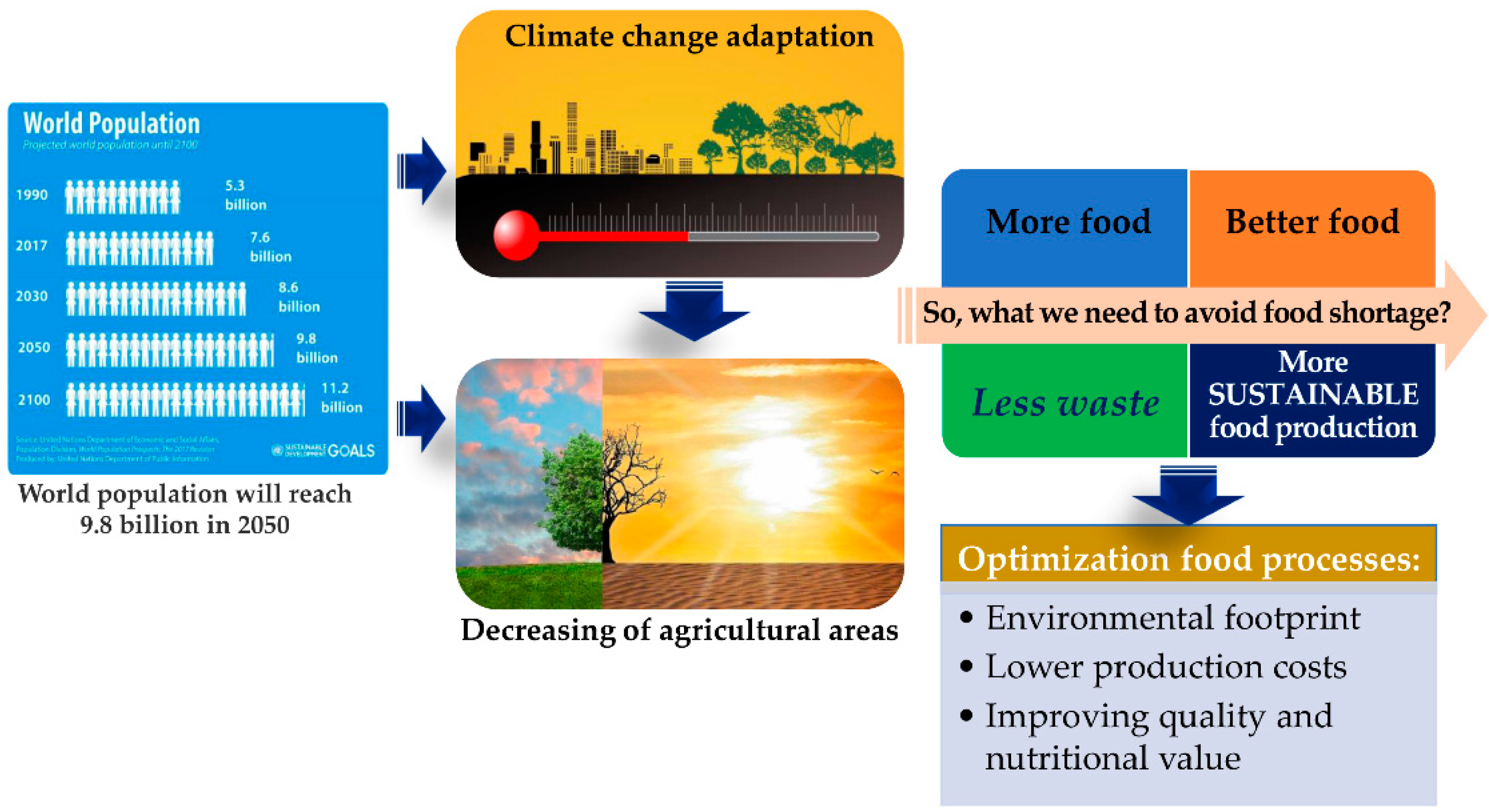
Figure 1. Global and societal challenges in the production of foods for the next generations.
Given these facts, the European Commission established food waste as one of the priority areas of the Action Plan for the European Circular Economy Strategy [2]. This includes a zero-waste strategy envisaging agri-food waste to reduce environmental pollution. This strategy is based on the extraction of compounds from food waste that have a high demand or innovative applications with potentially high economic return in different industrial sectors, such as nutraceutical, cosmetic, and pharmaceutical industries [3][4]. In this context, the extraction of phytochemicals from fruit and vegetable waste able to substitute the use of synthetic preservatives in food products and confer additional health protection effects to the researchers diet is of utmost importance [3][4][5][6]. However, these strategies mostly target the food industries and food chain stakeholders and not the final consumer. Remarkably, almost half of the losses and waste in fruits and vegetables are caused by human eating habits, being the second cause of greenhouse gas emission. Statistically, this is equivalent to 1.3 billion tons of wasted foods causing the emission of around 4.4 gigatons of greenhouse gas [1]. This means that every one of us, as final consumers, has important responsibilities to share when a great part of food waste occurs on the supermarket shelves and in the researchers kitchens and fridges. This includes the fruits and vegetables that are rejected because of their non-standardized measures or defects, commonly known as ugly foods, but also the peels, seeds, rinds, or cores, which despite being edible and rich in nutrients and bioactive compounds, are discarded to the food waste bin.
2. Nutritional and Bioactive Potential of Fresh Fruit and Vegetable Waste
According to the most recent data available, approximately half of fruits and vegetables produced worldwide are wasted. This represents around 1.3 billion tons of wasted foods and constitutes the second highest cause of greenhouse gas emission, representing the emission of around 4.4 gigatons of greenhouse gas [7]. However, beyond the obvious environmental impact, this means that millions of tons of nutrients present in this food waste are being lost. Additionally, this is happening on the same planet where currently more than 500 million people do not have access to enough food to live, and the problem will be exacerbated as the world population continues to grow exponentially [7]. It is, therefore, imperative that each consumer contributes to wasting less food and part of that objective can be easily achieved just by integrating into the researchers diet fruits and vegetables that the researchers wrongly considered as inedible. The list of fruits and vegetables used in the human diet is potentially endless and so here is focused on those with higher production and impact in terms of waste being discarded that can and should be further used in the researchers diet. Their nutritional and bioactive potential is reviewed below in detail and summarized in Table 1. It should be noted, however, that this behavioral change should be accompanied by due diligence regarding food safety. Fruit and vegetable peels are often loaded with microorganisms and agrochemicals that should be thoroughly removed to maintain safety.
Table 1. Main fruits and vegetables consumed worldwide with a rich bioactive waste composition.
| Fruit or Vegetable | Edible Part Composition: Nutritional and Bioactive Value | Discarded Part Composition: Nutritional and Bioactive Value | Possible Dietary Uses | Preparation | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit | |||||||||||
| Apple | flesh: flavan-3-ols, phenolic acids, flavonols, dihydrochalcones, anthocyanins, and ascorbic acid |
|
|
| [8][9][10] | ||||||
| Banana | flesh: fiber, carbohydrates, phenolics, biogenic amines, phytosterols, minerals; low-fat content |
|
|
| [11][12][13][14] | ||||||
|
|
| [15] | ||||||||
| Citrus | flesh: carbohydrates, amino acids, polyphenols, flavonoids, vitamin C, minerals |
|
|
| [14][16][17][18] | ||||||
| Grape | flesh: carbohydrates, organic acids, terpenoids, vitamins, minerals, polyphenols |
|
|
| [19][20][21][22][23][24][25][26] | ||||||
|
| ||||||||||
| Kiwi fruit | flesh: dietary fiber, phenolic compounds, vitamins, minerals |
|
|
| [27][28][29] | ||||||
| Mango | flesh: rich in dietary fiber, carbohydrates, proteins, fats, and phenolic compounds |
|
|
| [14][30][31][32][33][34] | ||||||
|
| ||||||||||
| Passion fruit | flesh: vitamin A and C, minerals, dietary fiber, protein, phenolic compounds |
|
|
| [35][36][37][38][39][40][41][42][43] | ||||||
|
| ||||||||||
| Pear | flesh: sugars, vitamins, organic and fatty acids, amino acids, volatiles, polyphenols, minerals |
|
|
| [44][45] | ||||||
| Peach | flesh: dietary fiber, minerals, sugars, organic acids, phenolic compounds, carotenoids, volatiles |
|
|
| [46][47][48] | ||||||
| Pineapple | flesh: carbohydrates, dietary fiber, sugars, organic acids, vitamins, minerals |
|
|
| [49][50][51][52] | ||||||
|
|
| |||||||||
| Plum | flesh: phenolic compounds (chlorogenic and gallic acids, resorcinol, and rutin) and ascorbic acid. |
|
|
| [53][54][55] | ||||||
|
|
| [56][57][58][59][60][61][62][63] | ||||||||
| Watermelon | flesh: glycosides, carotenoids, flavonoids, alkaloids, carbohydrates, fatty acids, essential oils. |
|
| [64][65][66][67][68] | |||||||
|
| ||||||||||
| Vegetables | |||||||||||
| Broccoli | florets: minerals, vitamins, phenolic, flavonoid compounds |
|
|
| [69][70][71] | ||||||
| Cauliflower | florets: fiber, minerals, phenolics, ascorbic acid, carotenoids, glucosinolates |
|
|
| [72][73][74] | ||||||
| Potato | flesh: carbohydrates, polyphenols, vitamins, and minerals |
|
|
| [75][76][77][78][79] | ||||||
| Pumpkin | flesh: dietary fiber, carotenoids, phenolic acids, flavonols, minerals, vitamins |
|
|
| [80][81] |
2.1. Fresh Fruits
2.1.1. Apple
Apples are one of the most consumed fruits in Europe, and they are a great source of several bioactive compounds, especially phenolic compounds such as flavan-3-ols, phenolic acids, flavonols, dihydrochalcones, and anthocyanins. Furthermore, apples present a high content of ascorbic acid [8]. Apple waste is mainly composed of peels that are rich in bioactive compounds, in concentrations even higher than in the flesh. Apple peels are an important source of polyphenols, being the most abundant chlorogenic acid, procyanidin B2, and epicatechin [9].
2.1.2. Banana
The edible banana fruit (Musa spp.) is soft and sweet, and usually eaten raw. In contrast, plantain bananas (Musa × paradisiaca) have a firm and starchy pulp suitable for cooking as a vegetable. Both are seedless fruits, rich in carbohydrates, dietary fibers, certain vitamins, minerals (such as phosphorus, sodium, potassium, calcium, magnesium, iron, copper, zinc, and manganese), and several health-promoting bioactive phytochemicals [11]. Different studies have identified many of these bioactive compounds, such as carotenoids (β-carotene), flavonoids, phenolics, biogenic amines, vitamins A, B, C, and E, and phytosterols [11][12][82][83]. Extracts from different parts of banana fruit and plants were shown to elicit effective protection against oxidative damage to cells and different human diseases triggered by these deleterious events, notably different forms of cancer (reviewed in [84]). These effects are possibly associated with the existence of specific phytoconstituents in banana plants and fruit, including ferulic acid, protocatechualdehyde, 2-pentanone, 4-epicyclomusalenone, cycloeucalenol acetate, and chlorogenic acid, which have been shown to exhibit therapeutic cancer-preventing and anticancer abilities (reviewed in [84]). These bioactive compounds have been widely shown to have many positive effects on human health and banana has an effective antioxidant capacity higher than many berries [85]. This phytochemical composition certainly explains the efficiency of the traditional uses of bananas against degenerative diseases triggered by oxidative stresses [12]. Among the flavonoids found in bananas are quercetin, myricetin, kaempferol, and cyanidin [11][86]. Banana has been shown to contain a good amount of phytosterols, such as stigmasterol, β-sitosterol, campesterol, 24-methylene cycloartenol, cycloeucalenol, and cycloartenol, as well as a group of biogenic amines, the catecholamines [11]. In particular, the composition of the biogenic amines L-dopa and dopamine in banana makes this fruit very relevant as a supplement in the treatment of Parkinson’s disease [82].
In contrast, and with exception of some producing countries in Asia and Latin America, banana peel and other parts of the fruit and plant are traditionally discarded in soil and used as compost to provide nutrients for the next harvests. However, banana peel is also rich in anthocyanins (delphinidin) and cyanidins [11], as well as in β-carotene, gallocatechin, and vitamins A, C, and E [87][88]. Additionally, it has a high content of dietary fibers, including hemicellulose and pectin polysaccharides [87]. Like the pulp, banana peels are also rich in phytosterols and catecholamines (biogenic amines). Borges, et al. [89] reported that banana peel showed superior phenolic compound and mineral levels compared to pulp. This composition of banana peels certainly explains the bioactive effects reported in traditional uses to promote wound healing, mainly from burns, and to help overcome or prevent several illnesses, such as depression [82].
The banana rhizome is very rich in phenolic compounds with important antioxidant activities [90]. The main phenolics found include ferulic, sinapic, salicylic, gallic, p-hydroxybenzoic, vanillic, syringic, gentisic, and p-coumaric acids, besides catechin, epicatechin, tannins, and anthocyanins [11]. Banana flowers or blossoms are also treated as agricultural waste when they are extirpated from the plant during the growing process, as they compete for the nutrients necessary for the growing fruit [91]. However, high amounts of different bioactive compounds, such as umbelliferone and lupeol, have been reported in ethanol extracts of banana flowers [92]. Part of these bioactive compounds has potential against cardiovascular diseases and diabetes. Evidence of such effects was provided by Wistar rats fed with an experimental diet incorporating banana blossom, which revealed modulation of hypocholesterolemic and hypoglycemic responses [84]. Recently, Sheng, et al. [93] also provided evidence that banana flower phytosterols, notably β-sitosterol, have the potential to prevent diseases associated with abnormal blood sugar and AGE levels, such as diabetes. Finally, extracts from the bracts of the banana flower were shown to contain anthocyanins (delphinidin, pelargonidin, peonidin, and malvidin), alkaloids, glucosides, flavonoid and phenolic compounds, saponins, terpenoids, coumarins, and steroids [91], as well as minerals (K, Cu, Ca, Fe, and P) and vitamins (A, C, and E) [15].
2.1.3. Citrus Fruit
Citrus fruits, mainly orange and lemon, are very popular worldwide. These fruits are rich in carotenoids, flavonoids, terpenes, limonoids, and many other bioactive compounds of nutritional and nutraceutical value, including polymethoxylated flavones (PMFs). These flavones were shown to elicit several bioactive effects against metabolic disorders, atherosclerosis, inflammation, neuroinflammation, cancer, and oxidation (reviewed in [94]). In addition, citrus essential oils are rich in limonoids and terpenes, which are widely appreciated for their antioxidant, antimicrobial, and flavoring properties with broad industrial applications [94]. Citrus peels are the main residue originating from citrus fruit, being very rich in dietary fibers (cellulose, hemicellulose, and pectin), bioactive compounds (phenolic compounds, flavonoids, and carotenoids), and vitamin C [18][95][96]. A myriad of bioactive compounds has been reported in citrus peels. A study recently conducted by Yaqoob, et al. [97], for instance, revealed that kinnow peel contains six flavones, eight favon-3-ols, five flavanones, four favan-3-ols, one anthocyanin, phenolic acids, and limonoids. Other reports point to citrus peels as promising sources of naringin, β-carotene, hesperidin, neohesperidin, and pectin [98][99]. Tangerines grown in Madeira, for instance, have a very intense and unique aroma which is mainly due to the presence of high amounts of volatile bioactive compounds in their peels, including thymol and dimethyl anthranilate [100]. Overall, citrus peels gained considerable attention due to their content in essential oils, mainly constituted by a mixture of volatile compounds such as terpenes (limonene) and oxygenated derivates such as aldehydes (citral), alcohols, and esters [17][99].
2.1.4. Grape
Grape is a fruit crop with a wide range of products, from fresh fruit and juice to other highly processed products, such as jelly and wine. Interest in these products is greatly related to the high content of phytonutrients belonging to numerous families, such as carbohydrates, organic acids, terpenoids, vitamins, and minerals, but most importantly polyphenols [22]. Studies have proved that these health-promoting compounds are highly accumulated in the skin, seeds, stems, and leaves of grapes [22][101]. Several studies have identified various phenolic compounds in grape skins, including phenolic acids, stilbenes, flavanols, flavonols, and anthocyanins [19][23][26], namely malvidin-3,5-di-O-glucoside and the flavonol quercetin-β-D-glucoside [102]. Regarding the nutritional value of grape pomace, it can be observed that red grape varieties are rich in dietary fibers, consisting mainly of lignin, cellulose, and hemicellulose [21]. Grape seeds’ most relevant component is oil, which is rich in unsaturated fatty acids, such as linoleic and oleic acids, vitamin E, and sterols. Seeds also present significant amounts of essential minerals, predominantly K, Fe, and Zn [24].
2.1.5. Kiwi Fruit
Kiwi fruit is one of the most commercialized fruits in the international markets and is loaded with nutrients such as vitamins, minerals, and a myriad of bioactive compounds [28][29]. Its peel is a source of macronutrients, namely carbohydrates, dietary fiber, lipids, proteins, and vitamins C and E [27]. It also presents high biological activity due to the content in phenolic compounds, such as flavonoids, organic acids (quinic, malic, and malic), epicatechin, and anthocyanins [29]. Kiwi fruit skin has been reported to exert higher antioxidant, antibacterial, and anticancer activities than the pulp [103].
2.1.6. Mango
Mangoes are one of the most popular fruits worldwide, and their production causes high amounts of waste. Mango peels and seeds are the main byproducts produced, having a considerable concentration of bioactive compounds [32]. Mango peel is rich in vitamins C and E, and in several minerals (Ca, K, Mg, Na, Fe, Mn, Zn, and Cu) [33], and contains pectins and anthocyanins [31] and a higher polyphenol content than its pulp [33]. Among the phytochemicals in mango peel reported in the literature, the researchers can find mangiferin, benzophenone derivates, xanthones, phenolic acids, fatty acids, flavonoids, procyanidins, penta-O-galloyl-glucoside, methyl gallate, tetra-O-galloyl-glucoside, maclurin di-O-galloyl-glucoside, and isoquercitrin [34]. Mango seeds also have a very interesting bioactive composition which includes high concentrations of the major flavonoids and phenolic acids, gallic acid, catechin, chlorogenic acid, caffeic acid, ellagic acid, rutin, quercitrin, quercetin, and kaempferol [34]. Additionally, these seeds are a source of fats, being particularly rich in palmitic, stearic, oleic, and linoleic acids [30].
2.1.7. Passion Fruit
Passion fruit is a tropical fruit with worldwide popularity attributed to its appealing sensorial properties, and its processing for juice results in great quantities of byproducts [104]. Passion fruit peel comprehends about 52% of the total fruit weight and has been shown to have a high content of fiber [36], pectin [40], and phenolic compounds, predominantly isoorientin, and lower concentrations of other flavonoids, such as vicenin, isovitexin, vitexin, orientin, and schaftoside [41][43]. The peels have also been indicated as an excellent source of essential minerals, such as K and Ca [37]. The use of orange passion fruit peel flour in foods (bread, cakes, biscuits, and cereal bars) has likewise been reported to increase their nutritional qualities, contributing to the enrichment of the total dietary fiber, minerals, and bioactive compounds [42]. Besides the peels, passion fruit seeds are also a byproduct with great nutritional value, mainly due to their high lipid content, especially oleic and linoleic acids [37].
2.1.8. Peach
Peach is a fruit highly appreciated for its aromatic characteristics and its richness in dietary fiber, minerals, sugars, organic acids, phenolic compounds, carotenoids, and volatiles [47]. The peach peel contains plenty of polyphenols, such as flavanols, hydroxybenzoic and hydroxycinnamic acids, and flavonols [48]. Furthermore, high carotenoid content was observed in peach peel extracts [46].
2.1.9. Pear
Pear fruit is widely consumed worldwide and can be found in several processed products. It is famous for its many medical functions, such as antidiabetic, anticarcinogenic, and anti-inflammatory properties [45], and various nutrients and bioactive compounds, including sugars, vitamins, organic and fatty acids, amino acids, volatiles, polyphenols, and minerals are attributed to pear fruit [44]. A variety of bioactive compounds has been identified in pear peel, including arbutin, chlorogenic acid, catechin, quercetin, kaempferol, hydroxycinnamoylmalic acids and their ethyl esters, and procyanidins and triterpenes. These compounds were found in pear peel in concentrations approximately 6–20 times higher than those in the flesh of the pear [44].
2.1.10. Pineapple
Pineapple is another highly appreciated tropical fruit in the world due to its nutritional and organoleptic properties. In recent years, the pineapple market has grown significantly, generating huge volumes of byproducts (mainly the core and peel), which have negative economic and environmental impacts [50]. Pineapple core is a rich source of fibers, bromelain [52], and vitamin C [51]. The peel of pineapple contains carbohydrates, proteins, pectin, bromelain, and phenolic compounds, namely gallic acid, catechin, epicatechin, and ferulic acid [52].
2.1.11. Plum
Plum (Spondias spp.) has been widely used for medicinal and therapeutic purposes since it is rich in phenolic compounds, namely chlorogenic and gallic acids, resorcinol, and rutin [55]. Moreover, it is a source of ascorbic acid [54]. Plum peel is proved to have high amounts of polyphenols, including flavonoids and anthocyanins, and ascorbic acid [53].
2.1.12. Watermelon
Watermelon is the second-largest fruit crop worldwide and causes a great amount of biowaste, mainly the rind and seeds, that represent an environmental hazard [105]. Watermelon rind is an interesting natural source of citrulline, a non-protein α-amino acid reported to have antioxidant and vasodilatation activity [106], and pectin, a polysaccharide widely used as an additive in the food industry [105]. Additionally, higher levels of phenolic compounds are found in watermelon rind than in its flesh [106]. In turn, watermelon seeds are an excellent source of dietary oils [64], with high levels of linoleic acid and lower concentrations of oleic, palmitic, and stearic acids, as well as minerals (P, K, Na, and Mg) [65]. Enemor, Oguazu, Odiakosa and Okafor [66] also reported a vast vitaminic repertoire in watermelon seeds, namely vitamins A and C and in minor concentrations B1, B2, B3, B6, B9, B12, D, E, and K. A myriad of other bioactive compounds has been also reported in these seeds, mainly sinapic acid, and lower amounts of other phenols, flavonoids, saponin, tannins, cardiac and cyanogenic glycosides, terpenoids, phytosterol, steroids, and phytates [67][107].
2.2. Vegetables
2.2.1. Broccoli
Broccoli is a Brassica vegetable widely consumed worldwide, owing to its health-promoting properties [72][108]. About 60–75% of world broccoli production is wasted during harvesting [70], creating a large number of byproducts, most of which are edible [69]. Broccoli stalks, for instance, constitute an abundant source of pectin, also being rich in phenolic compounds, fructose, glucose, mannitol, polysaccharides, free amino acids, and glucosinolates [69][70].
2.2.2. Cauliflower
Cauliflower is another Brassica vegetable rich in natural antioxidants. It is known to have anticancerous properties due to its abundance in glucosinolates [72][108]. Cauliflower stems and leaves present carbohydrates, protein, fat, and glucosinolates [73]. Further, cauliflower leaves are very rich in dietary fiber and minerals [74].
2.2.3. Potato
Potato is the fourth main crop consumed worldwide and it is an important constituent in the human diet [77].
Potato peels represent about 10% of the total potato waste and are an inexpensive source of valuable bioactive compounds, such as secondary metabolites and cell wall materials, which can be used to functionalize foods or replace synthetic additives with natural ingredients [109][110]. Studies have shown that potato peels contain glycoalkaloids, such as α-solanine and α-chaconin, polysaccharides, the phenolic compounds quercetin and rutin, and ferulic, gallic, p-coumaric, caffeic, and chlorogenic acids [110][111], which are important precursors for steroid hormones and natural antioxidants, respectively [78]. Acylated anthocyanins were identified in the peels of red and purple potato varieties, with pelargonidin, peonidin, and malvidin being the most prominent aglycones [79].
2.2.4. Pumpkin
The pumpkin (Cucurbita spp.) is a well-known vegetable all over the world and is unquestionably a source of valuable nutrients, such as carotenoids [58]. Pumpkin seeds are rich in oil and several health-promoting compounds [57][61]. The oil extracted from pumpkin seed kernels is rich in protein and fat, including palmitic, stearic, oleic, and linoleic acids. However, the level of accumulation of these fatty acids differs greatly among pumpkin varieties [63]. The main proteins previously found in pumpkin seed consist of storage salt-soluble globulins (cucurbitin), albumins, glutelins, and prolamins [56]. According to the literature, pumpkin seed kernels also accumulate high levels of minerals such as Fe, Zn, K, Ca, Mg, Mn, Cu, and Na, as well as tocopherol [62][63]. Pumpkin skins have broad variations in terms of composition and texture, but overall, they are edible and very rich in many bioactive compounds, notably high amounts of carotenoids and polyphenols with broad uses in food, pharmaceutical, and cosmetic industries. Bioactive colorant pigments and skin regeneration formulations are examples of promising applications for pumpkin peel extracts [80].
2.3. Dietary Uses of Fruit and Vegetable Waste
The inclusion in diet of fruit and vegetable spare parts, often seeds and peels that are discarded and here generically considered as waste, is not new. Banana peels and blossoms, for instance, have been widely consumed for centuries as a curry, boiled, fried, etc. in Asia (Sri Lanka and India) and some countries in Latin (Brazil) and Central America (Caribbean islands) [84]. In Ethiopia, the Indian wild banana Ensete superbum (Cliff banana) is the main staple food because this variety is very resistant to severe drought. Moreover, its seeds have been used for generations to treat several health problems such as diabetes, leukorrhea, kidney stones, and dysuria [112]. Banana flowers, pseudostem, and unripe fruits are also consumed as vegetables by many indigenous communities [113], including rural peoples in India [114]. Despite the popularity of the fruit, banana peels are not used in the human diet in most developing countries. Recently, however, the growing concerns of consumers about the sustainability of the planet placed cooking banana peels under social media attention. Hopefully, Nigella Lawson’s banana peel curry [115] will trigger a change in the way the researchers consider fruit and vegetable waste in diet. Similarly, banana blossoms seem to be a very versatile food to substitute meat proteins, taking into consideration the great number of different potential recipes such as curries, fish and chips, linguini, paella, salads, soups, fish pies, and crab cakes. Beyond the historic use of banana waste in the human diet, which would be the strongest argument that it is a safe procedure, evidence from different studies demonstrates the nutritional and bioactive value of such waste. Boiling bananas, often plantain or cooking banana varieties, increases the phenolic compound content of banana [89] because heat softens the vegetable structures, therefore releasing the bioactive compounds into the medium. In this case, banana peel prevents the loss of those bioactives in the water. Similar studies reported an increase in phenolic content during thermal processing of banana peel [114][116].
For most people, the hairy skin of kiwi fruit is not very appellative to eat. However, not only are the peels edible, eating skin-on kiwi greatly increases nutrient intake, namely of fibers, vitamins C and E, and a great number of bioactive compounds [29][117]. Consumption of whole SunGold kiwifruit (including the skin), for instance, was shown to increase the fiber, vitamin E and folate contents by 50, 32, and 34%, respectively [118]. To facilitate the ingestion of kiwi skin, the hairs can be easily removed by rubbing the fruit, scrubbing it with a vegetable brush, or scraping it lightly with a spoon.
Tannins are widely present in many fruits, particularly in their wasted parts, such as citrus and banana peels. This abundance is the main reason why consumers reject those parts because tannins confer high astringency and unpleasant flavors. When present in lower amounts, however, tannins are more easily tolerated by consumers, as occurs with the proanthocyanidins present in grape skins. Different strategies, however, can be used to mitigate the high abundance of tannins, such as sweetening to obtain marmalades and jams, or fermentation to obtain beverages, such as tepache [49]. This is a traditional Mexican beverage that is obtained by fermentation of different fruits and fruit waste, according to the region where it is produced. Pineapple rinds and tamarind, banana peels, corn husks, crushed corn, persimmon, apple, jujube, pomegranate, plum, cranberry, sour cherry, soursop, mango, orange, and papaya are among the tepache varieties in traditional Mexican food folklore [49]. Although there are not many studies in the literature about these traditional fermented beverages, it is known that the use of fruit during the fermentation process of beverages such as beer enhances the content of bioactive compounds, in particular polyphenols and carotenoids [14]. Acetic fermentation to obtain vinegar is also a popular process to give additional uses to many fruit wastes [10]. Traditionally associated with wine, vinegar can be produced from many fruit wastes such as the peels and cores from apple, kiwi fruit, pineapple, pear, mango, melon, watermelon, etc. In simple terms, any fruit and its waste can be fermented to obtain vinegar enriched with different cocktails of nutrients and bioactive compounds. In this respect, Sandor Katz is carrying out very relevant work unveiling ancient techniques behind fermentations that everyone can use at home [119].
Cucurbita moschata Duchesne (squash and pumpkin varieties) is a plant food highly appreciated for the content of nutrients and bioactive compounds, including polyphenols and carotenoids. Its peel (including the rind layers immediately below the peel) are particularly rich in pectins which can be used in various food processing operations due to their gelling properties. Pectins are also used as thickening agents and stabilizers in jams, jellies, etc. [81]. Pumpkin peel therefore has many industrial applications, but it can also be very useful for consumers even with minimal cooking procedures. Although ripe pumpkin peel is very hard to eat, it can be easily softened by different cooking procedures, such as boiling, roasting, frying, or baking, for use in pies and cakes. Table 2 presents selected examples from the literature showing cooking procedures to incorporate fruit wastes in diet.
Table 2. Examples of possible uses and cooking procedures for several fruit and vegetable wastes.
| Fruit or Vegetable Waste | Suggested Cooking Procedure | Ref. |
|---|---|---|
| Apple peel | Cookies: peels were blanched in hot water for 30 s, washed and dried (48 h, 60 °C), ground, and used to make cookies. | [120] |
| Citrus peel | Jam: washed and crushed peels were mixed with water to form a puree, and then sugar, pectin, and citric acid were added to obtain a jam-like texture. | [121] |
| Passion fruit peel | Cookies: peels were immersed in water (100 °C, 15 min) and cooled on ice, epicarp was separated with a spoon, dried (60 °C), ground, and used to make cookies. | [122] |
| Pineapple core | Pineapple dried core: core was cut into small pieces and dried in a microwave oven. | [123] |
| Pineapple core fiber was extracted and incorporated into dough and steamed bean. | [124] | |
| Pineapple peel | Beef marination: the peel was washed, cut, mashed with a blender, and filtered; the juice was then used to marinate beef. | [125] |
| Gluten-free muffins: peels were sanitized with 100 ppm chlorinated solution for 15 min, dried for 8 h at 60 °C, and milled to obtain flour. | [126] | |
| Plum peel | Marmalade: peels were mixed with water and filtered to make a puree, and then sugar syrup, pectin, and citric acid were added to obtain marmalade. | [127] |
| Jam: washed and crushed peels were mixed with water to form a puree, and then sugar, pectin, and citric acid were added to obtain a jam-like texture. | [128] | |
| Potato peel | Potato peel chips: peels were dried, milled, and sieved to obtain flour, then mixed with wheat flour to make deep-fried chips. | [129] |
| Potato peel dough: peels were dried at 50 °C in an oven for 48 h, ground in a mixer grinder to make flour, mixed with wheat flour, and incorporated into dough. | [130] | |
| Gluten-free bread: peels were sanitized with sodium hypochlorite solution, dried at 70 °C for 24 h in a forced-air oven, and ground using a blender; the flour obtained was used to produce gluten-free bread. | [131] | |
| Pumpkin rind and seeds | Bread: rinds and seeds were dried in an oven at 60 °C for 24 h and ground using a grinder to obtain a fine particle size, then the flour obtained was incorporated into bread. | [132] |
| Watermelon rind | Marmalade: rind was mixed with water and filtered to make a puree, and then sugar syrup, pectin, and citric acid were added to obtain marmalade. | [133] |
| Watermelon rind flour: rind was cut into small chips, dried at 60 °C for 48 h, and milled into powdered flour to use in cakes. | [134] | |
| Watermelon seeds | Watermelon snacks: seeds were ground in a mixer grinder into a fine powder and sieved to obtain uniform size distribution of the grains; the flour obtained was mixed with rice and corn flour and used to make snacks. | [135] |
Many more examples of home food applications and recipes for fruit and vegetable waste could be included. This would be, however, a very long list that obviously cannot be included here. Instead, the selected examples constitute a flavor of the bioactive potential that is being neglected in the fruit and vegetable waste that is discarded in kitchens (Figure 2).
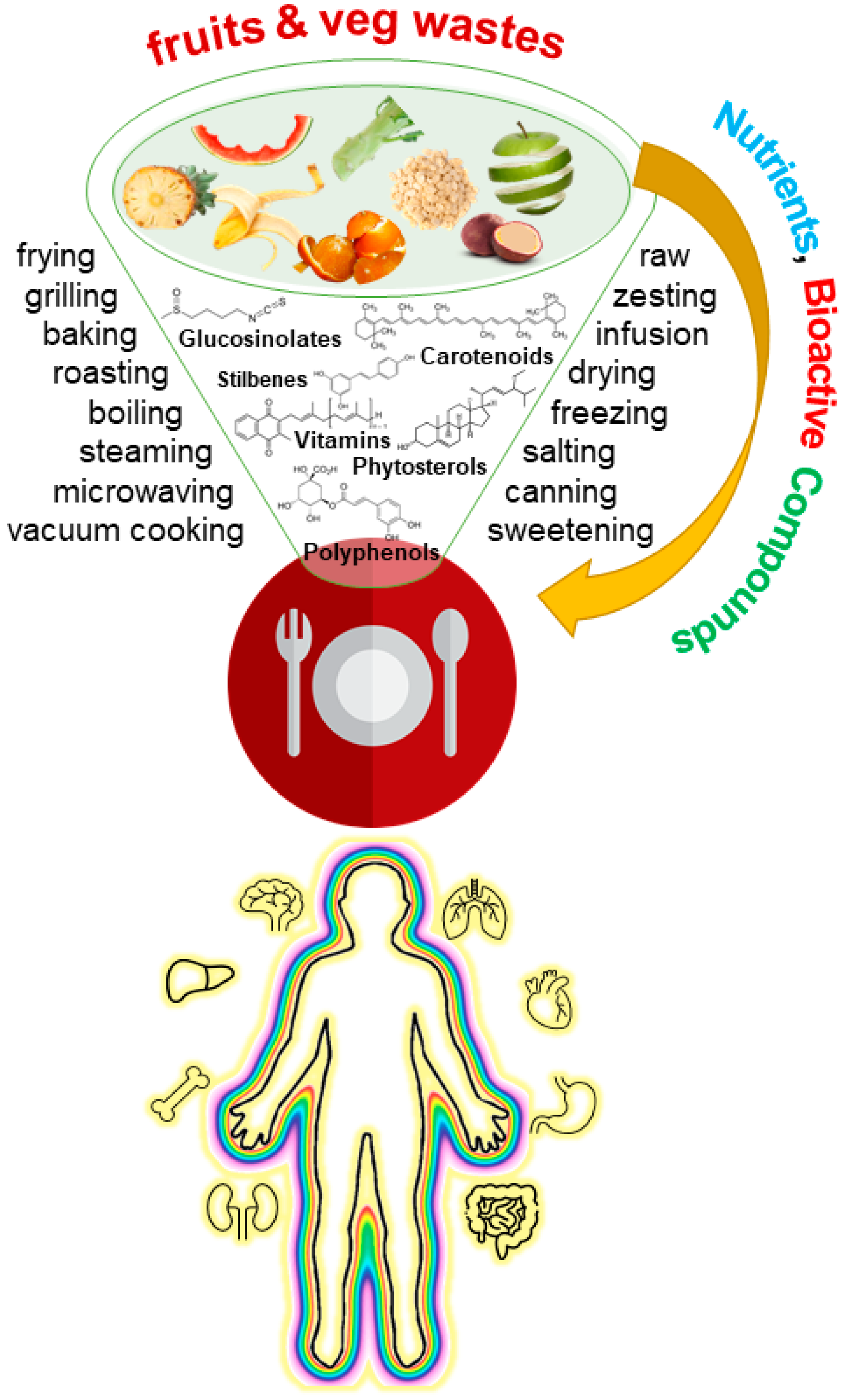

Figure 2. Integrative overview of the rationale behind fruit and vegetable waste cooking to include important molecules with reported bioactive effects in diet.
References
- United Nations Department of Economic and Social Affairs. World Population Prospects 2019: Highlights; United Nations Department of Public Information: New York, NY, USA, 2019.
- European Commission, Directorate-General for Research and Innovation. A sustainable bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment: Updated Bioeconomy Strategy, Publications Office. 2018. Available online: https://data.europa.eu/doi/10.2777/478385 (accessed on 10 February 2022).
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26.
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2061–2108.
- Genkinger, J.M.; Platz, E.A.; Hoffman, S.C.; Comstock, G.W.; Helzlsouer, K.J. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am. J. Epidemiol. 2004, 160, 1223–1233.
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C.C. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int. J. Cancer 2008, 122, 2330–2336.
- FAO. FAO Statistical Yearbook 2021—World Food and Agriculture; FAO: Rome, Italy, 2021.
- Almeida, D.P.F.; Gião, M.S.; Pintado, M.; Gomes, M.H. Bioactive phytochemicals in apple cultivars from the Portuguese protected geographical indication “Maçã de Alcobaça:” Basis for market segmentation. Int. J. Food Prop. 2017, 20, 2206–2214.
- Loncaric, A.; Matanovic, K.; Ferrer, P.; Kovac, T.; Sarkanj, B.; Skendrovic Babojelic, M.; Lores, M. Peel of Traditional Apple Varieties as a Great Source of Bioactive Compounds: Extraction by Micro-Matrix Solid-Phase Dispersion. Foods 2020, 9, 80.
- Viroli, S.L.M.; Viroli, S.G.; Carvalho, N.P.; Bernardi, D.P.d.S.; Coelho, R.G. Production and characterization of acetic fermentation with different fruit peels. Res. Soc. Dev. 2021, 10, e84101421878.
- Sidhu, J.S.; Zafar, T.A. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018, 2, 183–188.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11.
- Oyeyinka, B.O.; Afolayan, A.J. Comparative Evaluation of the Nutritive, Mineral, and Antinutritive Composition of Musa sinensis L. (Banana) and Musa paradisiaca L. (Plantain) Fruit Compartments. Plants 2019, 8, 598.
- Kesa, A.L.; Pop, C.R.; Mudura, E.; Salanta, L.C.; Pasqualone, A.; Darab, C.; Burja-Udrea, C.; Zhao, H.; Coldea, T.E. Strategies to Improve the Potential Functionality of Fruit-Based Fermented Beverages. Plants 2021, 10, 2263.
- Muhammad Suffi, N.S.; Mohamed, E.; Camalxaman, S.N.; Rambely, A.S.; Haron, N. The medicinal benefits, phytochemical constituents and antioxidant properties of banana blossom: A mini review. Healthscope Off. Res. Book Fac. Health Sci. UiTM 2021, 4, 113–118.
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Int. 2017, 33, 587–619.
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895.
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219.
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC–DAD–ESI-MSn. Food Chem. 2012, 135, 94–104.
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.D.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69.
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594.
- Pintac, D.; Majkic, T.; Torovic, L.; Orcic, D.; Beara, I.; Simin, N.; Mimica-Dukic, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390.
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138.
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627.
- Monteiro, G.C.; Minatel, I.O.; Pimentel, A.; Gomez-Gomez, H.A.; de Camargo, J.P.C.; Diamante, M.S.; Basilio, L.S.P.; Tecchio, M.A.; Lima, G.P.P. Bioactive compounds and antioxidant capacity of grape pomace flours. Lwt-Food Sci. Technol. 2021, 135, 110053.
- Perra, M.; Lozano-Sanchez, J.; Leyva-Jimenez, F.J.; Segura-Carretero, A.; Pedraz, J.L.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Manca, M.L.; Manconi, M. Extraction of the antioxidant phytocomplex from wine-making by-products and sustainable loading in phospholipid vesicles specifically tailored for skin protection. Biomed. Pharmacother. 2021, 142, 111959.
- Dias, M.; Caleja, C.; Pereira, C.; Calhelha, R.C.; Kostic, M.; Sokovic, M.; Tavares, D.; Baraldi, I.J.; Barros, L.; Ferreira, I. Chemical composition and bioactive properties of byproducts from two different kiwi varieties. Food Res. Int. 2020, 127, 108753.
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588.
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315.
- Jahurul, M.H.; Zaidul, I.S.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180.
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydr. Polym. 2019, 219, 29–38.
- Hazarudin, N.H.; Razali, N.F. A Review on Extraction of Antioxidants from Mangifera indica L. (Mango) Peel and Seed with Highest Extraction Yield. Prog. Eng. Appl. Technol. 2021, 2, 699–710.
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public Health 2021, 18, 741.
- Quintana, S.E.; Salas, S.; Garcia-Zapateiro, L.A. Bioactive compounds of mango (Mangifera indica): A review of extraction technologies and chemical constituents. J. Sci. Food Agric. 2021, 101, 6186–6192.
- Vigano, J.; Brumer, I.Z.; Braga, P.A.D.; da Silva, J.K.; Marostica, M.R.; Reyes, F.G.R.; Martinez, J. Pressurized liquids extraction as an alternative process to readily obtain bioactive compounds from passion fruit rinds. Food Bioprod. Process. 2016, 100, 382–390.
- Coelho, E.M.; Gomes, R.G.; Machado, B.A.S.; Oliveira, R.S.; Lima, M.d.S.; de Azêvedo, L.C.; Guez, M.A.U. Passion fruit peel flour—Technological properties and application in food products. Food Hydrocoll. 2017, 62, 158–164.
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318.
- Mandal, G.; Thokchom, R. Production Preference and Importance of Passion Fruit (Passiflora Edulis): A Review. J. Agric. Eng. Food Technol. 2017, 4, 27–30.
- Coelho, E.M.; de Azevedo, L.C.; Viana, A.C.; Ramos, I.G.; Gomes, R.G.; Lima, M.D.S.; Umsza-Guez, M.A. Physico-chemical properties, rheology and degree of esterification of passion fruit (Passiflora edulis f. flavicarpa) peel flour. J. Sci. Food Agric. 2018, 98, 166–173.
- Moia, T.A.; Pimentel, T.C.; Barão, C.E.; Feihrmann, A.C.; Favareto, R.; Reis, A.V.; Cardozo-Filho, L. Bioactive compounds and pectin from residues of the passion fruit processing: Extraction using Green Technology and Characterization. Chem. Eng. Trans. 2019, 75, 157–162.
- Dominguez-Rodriguez, G.; Garcia, M.C.; Plaza, M.; Marina, M.L. Revalorization of Passiflora species peels as a sustainable source of antioxidant phenolic compounds. Sci. Total Environ. 2019, 696, 134030.
- Reis, L.C.R.D.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; Rios, A.d.O. Characterization of Orange Passion Fruit Peel Flour and Its Use as an Ingredient in Bakery Products. J. Culin. Sci. Technol. 2018, 18, 214–230.
- Pereira, D.T.V.; Zabot, G.L.; Reyes, F.G.; Iglesias, A.H.; Martinez, J. Integration of pressurized liquids and ultrasound in the extraction of bioactive compounds from passion fruit rinds: Impact on phenolic yield, extraction kinetics and technical-economic evaluation. Innov. Food Sci. Emerg. Technol. 2021, 67.
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538.
- Hong, S.Y.; Lansky, E.; Kang, S.S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. 2021, 21, 219.
- Loizzo, M.R.; Pacetti, D.; Lucci, P.; Nunez, O.; Menichini, F.; Frega, N.G.; Tundis, R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive Compounds and Antioxidant Activity of Pulp, Peel and Seed Ethanolic Extracts. Plant Foods Hum. Nutr. 2015, 70, 331–337.
- Machado, M.I.R.; Machado, A.R.; Zambiazi, R.C. Pêssego: Características Físico-Químicas e Conteúdo de Compostos Bioativos. Res. Soc. Dev. 2020, 9, e216973103.
- Kan, J.A.; Chen, C.C.; Huo, T.B.; Xie, W.J.; Hui, Y.Y.; Liu, J.; Jin, C.H. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. J. Funct. Foods 2020, 72, 104082.
- Ojeda-Linares, C.; Alvarez-Rios, G.D.; Figueredo-Urbina, C.J.; Islas, L.A.; Lappe-Oliveras, P.; Nabhan, G.P.; Torres-Garcia, I.; Vallejo, M.; Casas, A. Traditional Fermented Beverages of Mexico: A Biocultural Unseen Foodscape. Foods 2021, 10, 2390.
- Valdes Garcia, A.; Domingo Martinez, M.I.; Ponce Landete, M.; Prats Moya, M.S.; Beltran Sanahuja, A. Potential of Industrial Pineapple (Ananas comosus (L.) Merrill) By-Products as Aromatic and Antioxidant Sources. Antioxid 2021, 10, 1767.
- Hikal, W.M.; Mahmoud, A.A.; Said-Al Ahl, H.A.H.; Bratovcic, A.; Tkachenko, K.G.; Kačániová, M.; Rodriguez, R.M. Pineapple (Ananas comosus L. Merr.), Waste Streams, Characterisation and Valorisation: An Overview. Open J. Ecol. 2021, 11, 610–634.
- Vieira, I.M.M.; Santos, B.L.P.; Santos, C.V.M.; Ruzene, D.S.; Silva, D.P. Valorization of Pineapple Waste: A Review on How the Fruit’s Potential Can Reduce Residue Generation. BioEnergy Res. 2021.
- Medina-Meza, I.G.; Barbosa-Canovas, G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. J. Food Eng. 2015, 166, 268–275.
- Sarmento, J.D.A.; de Morais, P.L.D.; de Souza, F.I.; da Costa, L.R.; Melo, N.J.D. Bioactive compounds and antioxidant activity of Ximenia americana coming from different collection sites. Arch. Latinoam. Nutr. 2015, 65, 263–270.
- Hernandez-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chavez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.J.; Del-Toro-Sanchez, C.L.; Marquez-Rios, E.; Lopez-Mata, M.A.; Rodriguez-Felix, F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200.
- Rodriguez-Miranda, J.; Hernandez-Santos, B.; Herman-Lara, E.; Vivar-Vera, M.A.; Carmona-Garcia, R.; Gomez-Aldapa, C.A.; Martinez-Sanchez, C.E. Physicochemical and functional properties of whole and defatted meals from Mexican (Cucurbita pepo) pumpkin seeds. Int. J. Food Sci. Technol. 2012, 47, 2297–2303.
- Montesano, D.; Blasi, F.; Simonetti, M.S.; Santini, A.; Cossignani, L. Chemical and Nutritional Characterization of Seed Oil from Cucurbita maxima L. (var. Berrettina) Pumpkin. Foods 2018, 7, 30.
- Kulczynski, B.; Gramza-Michalowska, A. The Profile of Secondary Metabolites and Other Bioactive Compounds in Cucurbita pepo L. and Cucurbita moschata Pumpkin Cultivars. Molecules 2019, 24, 2945.
- Ahmad, G.; Khan, A.A. Pumpkin: Horticultural Importance and Its Roles in Various Forms; a Review. Int. J. Hortic. Agric. 2019, 4, 1–6.
- WHO, World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; WHO: Geneva, Switzerland, 2003.
- Ferreira, D.F.; Barin, J.S.; Binello, A.; Veselou, V.V.; Cravotto, G. Highly efficient pumpkin-seed extraction with the simultaneous recovery of lipophilic and hydrophilic compounds. Food Bioprod. Process. 2019, 117, 224–230.
- Syed, Q.A. Nutritional and Therapeutic Importance of the Pumpkin Seeds. Biomed. J. Sci. Tech. Res. 2019, 21.
- Koltsov, V.A.; Danilin, S.I. Chemical composition of a seed kernel of a pumpkin produced in the Central Chernozem region of Russia. IOP Conf. Ser. Earth Environ. Sci. 2021, 845, 012004.
- Wani, A.A.; Sogi, D.S.; Singh, P.; Shivhare, U.S. Characterization and Functional Properties of Watermelon (Citrullus lanatus) Seed Protein Isolates and Salt Assisted Protein Concentrates. Food Sci. Biotechnol. 2011, 20, 877–887.
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Msaada, K.; Hamdi, S. Chemical composition and bioactive compounds of Cucurbitaceae seeds: Potential sources for new trends of plant oils. Process Saf. Environ. Prot. 2019, 127, 73–81.
- Enemor, V.H.A.; Oguazu, C.E.; Odiakosa, A.U.; Okafor, S.C. Evaluation of the Medicinal Properties and Possible Nutrient Composition of Citrullus lanatus (Watermelon) Seeds. Res. J. Med. Plants 2019, 13, 129–135.
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aadil, R.M. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 275–291.
- Sorokina, M.; McCaffrey, K.S.; Deaton, E.E.; Ma, G.; Ordovas, J.M.; Perkins-Veazie, P.M.; Steinbeck, C.; Levi, A.; Parnell, L.D. A Catalog of Natural Products Occurring in Watermelon-Citrullus lanatus. Front. Nutr. 2021, 8, 729822.
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393.
- Petkowicz, C.L.O.; Williams, P.A. Pectins from food waste: Characterization and functional properties of a pectin extracted from broccoli stalk. Food Hydrocoll. 2020, 107, 105930.
- Xu, Y.Y.; Xiao, Y.D.; Lagnika, C.; Song, J.F.; Li, D.J.; Liu, C.Q.; Jiang, N.; Zhang, M.; Duan, X. A comparative study of drying methods on physical characteristics, nutritional properties and antioxidant capacity of broccoli. Dry. Technol. 2020, 38, 1378–1388.
- Vanlalneihi, B.; Saha, P.; Kalia, P.; Jaiswal, S.; Kundu, A.; Saha, N.D.; Sirowa, S.S.; Singh, N. Chemometric approach based characterization and selection of mid-early cauliflower for bioactive compounds and antioxidant activity. J. Food Sci. Technol. 2020, 57, 293–300.
- Drabinska, N.; Jez, M.; Nogueira, M. Variation in the Accumulation of Phytochemicals and Their Bioactive Properties among the Aerial Parts of Cauliflower. Antioxid 2021, 10, 1597.
- Mythili, S.; Rajeswari, N.; Bosco, S.J.D.; Rajalechumi, A.K.A. Impact of blanching treatments on the chemical composition, total dietary fiber, physicochemical, functional, and structural properties of underutilized cauliflower leaves (Brassica oleracea var. botrytis). J. Food Process. Preserv. 2021, 45, e15910.
- Zaheer, K.; Akhtar, M.H. Potato Production, Usage, and Nutrition--A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721.
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835.
- Pathak, P.D.; Mandavgane, S.A.; Puranik, N.M.; Jambhulkar, S.J.; Kulkarni, B.D. Valorization of potato peel: A biorefinery approach. Crit. Rev. Biotechnol. 2018, 38, 218–230.
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods 2020, 9, 1598.
- Sampaio, S.L.; Petropoulos, S.A.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Fernandes, A.; Leme, C.M.M.; Alexopoulos, A.; Santos-Buelga, C.; Ferreira, I.; et al. Phenolic composition and cell-based biological activities of ten coloured potato peels (Solanum tuberosum L.). Food Chem. 2021, 363, 130360.
- Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787.
- Lalnunthari, C.; Devi, L.M.; Badwaik, L.S. Extraction of protein and pectin from pumpkin industry by-products and their utilization for developing edible film. J. Food Sci. Technol. 2020, 57, 1807–1816.
- Pereira, A.; Maraschin, M. Banana (Musa spp.) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015, 160, 149–163.
- Borges, C.V.; Amorim, E.P.; Leonel, M.; Gomez, H.A.G.; dos Santos, T.P.R.; Ledo, C.A.D.; Belin, M.A.F.; de Almeida, S.L.; Minatel, I.O.; Lima, G.P.P. Post-harvest physicochemical profile and bioactive compounds of 19 bananas and plantains genotypes. Bragantia 2019, 78, 284–296.
- Mondal, A.; Banerjee, S.; Bose, S.; Das, P.P.; Sandberg, E.N.; Atanasov, A.G.; Bishayee, A. Cancer Preventive and Therapeutic Potential of Banana and Its Bioactive Constituents: A Systematic, Comprehensive, and Mechanistic Review. Front. Oncol. 2021, 11, 697143.
- Ling, S.S.; Chang, S.K.; Sia, W.C.; Yim, H.S. Antioxidant effcacy of unripe banana (Musa acuminata Colla) peel extracts in sunflower oil during accelerated storage. Acta Sci. Pol. Technol. Aliment. 2015, 14, 343–356.
- Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J.O.; Dommes, J.; Pincemail, J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Agric. Food. Chem. 2007, 55, 8596–8603.
- Mostafa, H.S. Banana plant as a source of valuable antimicrobial compounds and its current applications in the food sector. J. Food Sci. 2021, 86, 3778–3797.
- Toh, P.Y.; Leong, F.S.; Chang, S.K.; Khoo, H.E.; Yim, H.S. Optimization of extraction parameters on the antioxidant properties of banana waste. Acta Sci. Pol. Technol. Aliment. 2016, 15, 65–78.
- Borges, C.V.; Maraschin, M.; Coelho, D.S.; Leonel, M.; Gomez, H.A.G.; Belin, M.A.F.; Diamante, M.S.; Amorim, E.P.; Gianeti, T.; Castro, G.R.; et al. Nutritional value and antioxidant compounds during the ripening and after domestic cooking of bananas and plantains. Food Res. Int. 2020, 132, 109061.
- Kandasamy, S.; Aradhya, S.M. Polyphenolic profile and antioxidant properties of rhizome of commercial banana cultivars grown in India. Food Biosci. 2014, 8, 22–32.
- Chiang, S.H.; Yang, K.M.; Lai, Y.C.; Chen, C.W. Evaluation of the in vitro biological activities of Banana flower and bract extracts and their bioactive compounds. Int. J. Food Prop. 2021, 24, 1–16.
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Ranganatha, L.V.; Nagendra Prasad, M.N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S. Afr. J. Bot. 2014, 95, 54–63.
- Sheng, Z.; Dai, H.; Pan, S.; Ai, B.; Zheng, L.; Zheng, X.; Prinyawiwatkul, W.; Xu, Z. Phytosterols in banana (Musa spp.) flower inhibit α-glucosidase and α-amylase hydrolysations and glycation reaction. Int. J. Food Sci. Tech. 2017, 52, 171–179.
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxid 2022, 11, 239.
- Jeong, D.; Park, H.; Jang, B.K.; Ju, Y.; Shin, M.H.; Oh, E.J.; Lee, E.J.; Kim, S.R. Recent advances in the biological valorization of citrus peel waste into fuels and chemicals. Bioresour. Technol. 2021, 323, 124603.
- Yadav, V.; Sarker, A.; Yadav, A.; Miftah, A.O.; Bilal, M.; Iqbal, H.M.N. Integrated biorefinery approach to valorize citrus waste: A sustainable solution for resource recovery and environmental management. Chemosphere 2022, 293, 133459.
- Yaqoob, M.; Aggarwal, P.; Babbar, N. Extraction and screening of kinnow (Citrus reticulata L.) peel phytochemicals, grown in Punjab, India. Biomass Convers. Biorefin. 2022.
- Tsitsagi, M.; Ebralidze, K.; Chkhaidze, M.; Rubashvili, I.; Tsitsishvili, V. Sequential extraction of bioactive compounds from tangerine (Citrus Unshiu) peel. Ann. Agrar. Sci. 2018, 16, 236–241.
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167.
- Figueira, J.A.; Porto-Figueira, P.; Pereira, J.A.M.; Camara, J.S. Tangerines Cultivated on Madeira Island-A High Throughput Natural Source of Bioactive Compounds. Foods 2020, 9, 1470.
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404.
- de Andrade, R.B.; Machado, B.A.S.; Barreto, G.A.; Nascimento, R.Q.; Correa, L.C.; Leal, I.L.; Tavares, P.; Ferreira, E.S.; Umsza-Guez, M.A. Syrah Grape Skin Residues Has Potential as Source of Antioxidant and Anti-Microbial Bioactive Compounds. Biology 2021, 10, 1262.
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Zhai, X.; Pang, Y.; Yang, X. Antioxidant, antimicrobial, and antiproliferative activity-based comparative study of peel and flesh polyphenols from Actinidia chinensis. Food Nutr. Res. 2019, 63, 1577.
- de Toledo, N.; de Camargo, A.; Ramos, P.; Button, D.; Granato, D.; Canniatti-Brazaca, S. Potentials and Pitfalls on the Use of Passion Fruit By-Products in Drinkable Yogurt: Physicochemical, Technological, Microbiological, and Sensory Aspects. Beverages 2018, 4, 47.
- Marinelli, V.; Lucera, A.; Incoronato, A.L.; Morcavallo, L.; Del Nobile, M.A.; Conte, A. Strategies for fortified sustainable food: The case of watermelon-based candy. J. Food Sci. Technol. 2021, 58, 894–901.
- Tarazona-Diaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J. Sci. Food Agric. 2011, 91, 805–812.
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793.
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal 2018, 12, 91–101.
- Martinez-Fernandez, J.S.; Gu, X.Y.; Chen, S.L. Techno-economic assessment of bioactive compound recovery from potato peels with sequential hydrothermal extraction. J. Clean. Prod. 2021, 282, 124356.
- Rodriguez-Martinez, B.; Gullon, B.; Yanez, R. Identification and Recovery of Valuable Bioactive Compounds from Potato Peels: A Comprehensive Review. Antioxid 2021, 10, 1630.
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C.; et al. Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads. J. Agric. Food Chem. 2018, 66, 7942–7947.
- Yesodharan, K.; Sujana, K.A. Ethnomedicinal knowledge among Malamalasar tribe of Parambikulam wildlife sanctuary, Kerala. Indian J. Tradit. Knowl. 2007, 6, 481–485.
- Monica, K.; Agarwal, S.; Sanjay, P. Characterization of a chromaffin derivative isolated from the seeds of Ensete superbum Cheesm. Phcog. Mag. 2008, 4, 114–117.
- Sasipriya, G.; Maria, C.L.; Siddhuraju, P. Influence of pressure cooking on antioxidant activity of wild (Ensete superbum) and commercial banana (Musa paradisiaca var. Monthan) unripe fruit and flower. J. Food Sci. Technol. 2014, 51, 2517–2525.
- Lawson, N. Cook, Eat, Repeat: Ingredients, Recipes and Stories.; ECCO: New York, NY, USA, 2021.
- Gonzalez-Montelongo, R.; Lobo, M.G.; Gonzalez, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039.
- Newgent, J. The Big Green Cookbook: Hundreds of Planet-Pleasing Recipes and Tips for a Luscious, Low-Carbon Lifestyle; Houghton Mifflin Harcourtv: San Francisco, SF, USA, 2009.
- Sivakumaran, S.; Huffman, L.; Sivakumaran, S.; Drummond, L. The nutritional composition of Zespri(R) SunGold Kiwifruit and Zespri(R) Sweet Green Kiwifruit. Food Chem. 2018, 238, 195–202.
- Katz, S. Sandor Katz’s Fermentation Journeys; Chelsea Green Publishing: Hartford, VT, USA, 2021.
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Jukic, M.; Komlenic, D.K.; Lukinac, J. Influence of apple peel powder addition on the physico-chemical characteristics and nutritional quality of bread wheat cookies. Food Sci. Technol. Int. 2020, 26, 574–582.
- Teixeira, F.; Santos, B.A.D.; Nunes, G.; Soares, J.M.; Amaral, L.A.D.; Souza, G.H.O.; Resende, J.T.V.; Menegassi, B.; Rafacho, B.P.M.; Schwarz, K.; et al. Addition of Orange Peel in Orange Jam: Evaluation of Sensory, Physicochemical, and Nutritional Characteristics. Molecules 2020, 25, 1670.
- Ning, X.; Wu, J.; Luo, Z.; Chen, Y.; Mo, Z.; Luo, R.; Bai, C.; Du, W.; Wang, L. Cookies fortified with purple passion fruit epicarp flour: Impact on physical properties, nutrition, in vitro starch digestibility, and antioxidant activity. Cereal Chem. 2020, 98, 328–336.
- Zaki, N.A.M.; Rahman, N.A.; Zamanhuri, N.A.; Hashib, S.A. Ascorbic acid content and proteolytic enzyme activity of microwave-dried pineapple stem and core. Chem. Eng. Trans. 2017, 56, 1369–1374.
- Shiau, S.Y.; Wu, M.Y.; Liu, Y.L. The effect of pineapple core fiber on dough rheology and the quality of mantou. J. Food Drug Anal. 2015, 23, 493–500.
- Hafid, H.; Patriani, P.; Sepriadi, S.; Ananda, S.H. Organoleptic properties of pineapple peel juice marinated beef (Ananas comosus L. Merr). E3S Web Conf. 2021, 332, 03005.
- Brigagao, T.C.S.; Fortes, R.R.; Lourenco, C.O.; Carvalho, E.E.N.; Cirillo, M.A.; Nachtigall, A.M.; Boas, B.M.V. Optimization of gluten-free muffins made with pineapple peel, banana peel, and pumpkin seed flours. J. Food Process. Preserv. 2021, 45, e16037.
- Mohammadi-Moghaddam, T.; Firoozzare, A. Investigating the effect of sensory properties of black plum peel marmalade on consumers acceptance by Discriminant Analysis. Food Chem. X 2021, 11, 100126.
- Moghaddam, T.N.; Elhamirad, A.H.; Asl, M.R.S.; Noghabi, M.S. Pulsed electric field-assisted extraction of phenolic antioxidants from tropical almond red leaves. Chem. Pap. 2020, 74, 3957–3961.
- Durmaz, A.; Yuksel, F. Deep fried wheat chips added with potato peel flour—Effect on quality parameters. Qual. Assur. Saf. Crops Foods 2021, 13, 115–124.
- Ben Jeddou, K.; Bouaziz, F.; Zouari-Ellouzi, S.; Chaari, F.; Ellouz-Chaabouni, S.; Ellouz-Ghorbel, R.; Nouri-Ellouz, O. Improvement of texture and sensory properties of cakes by addition of potato peel powder with high level of dietary fiber and protein. Food Chem. 2017, 217, 668–677.
- Jacinto, G.; Stieven, A.; Maciel, M.J.; Souza, C.F.V.d. Effect of potato peel, pumpkin seed, and quinoa flours on sensory and chemical characteristics of gluten-free breads. Braz. J. Food Technol. 2020, 23, 2020.
- Kl, N.; Lau, M.; Cp, T. Fibre from Pumpkin (C. pepo L.) Seeds and Rinds: Application as Bakery Product Ingredients Fibre from Pumpkin (Cucurbita pepo L.) Seeds and Rinds: Physico-chemical Properties, Antioxidant Capacity and Application as Bakery Product Ingredients. Mal. J. Nutr. 2013, 19, 99–109.
- Zabala, V.B.; Goles, C.E. Watermelon Rind-Ponkan Marmalade: A Physico-chemical Analysis. J. Phys. Conf. Ser. 2021, 1835, 012114.
- Adegunwa, M.O.; Oloyede, I.O.; Adebanjo, L.A.; Alamu, E.O. Quality attribute of plantain (Musa paradisiaca) sponge-cake supplemented with watermelon (Citrullus lanatus) rind flour. Cogent Food Agric. 2019, 5, 1631582.
- Raja, M.P.; Praveen Raja, M.; Karthiayani, A.; Selvan, P.; Nithyalakshmi, V. Production of extruded snacks by utilization of watermelon (Citrullus vulgaris) seed flour. J. Postharvest Technol. 2019, 2019, 56–67.
More
