Hepatocellular carcinoma (HCC) is the most common primary liver cancer with an increasing worldwide mortality rate. Cholangiocarcinoma (CCA) is the second most common primary liver cancer. In both types of cancers, early detection is very important. Biomarkers are a relevant part of diagnosis, enabling non-invasive detection and control of cancer recurrence, as well as in the application of screening tests in high-risk groups. Furthermore, some of these biomarkers are useful in controlling therapy and treatment selection. Detection of some markers presents higher sensitivity and specificity in combination with other markers when compared with a single detection. Some gene aberrations are also prognostic markers in the two types of cancers.
- biomarker
- genetic marker
- hepatocellular carcinoma
- cholangiocarcinoma
- screening
- diagnosis
- therapy
- treatment
1. Introduction
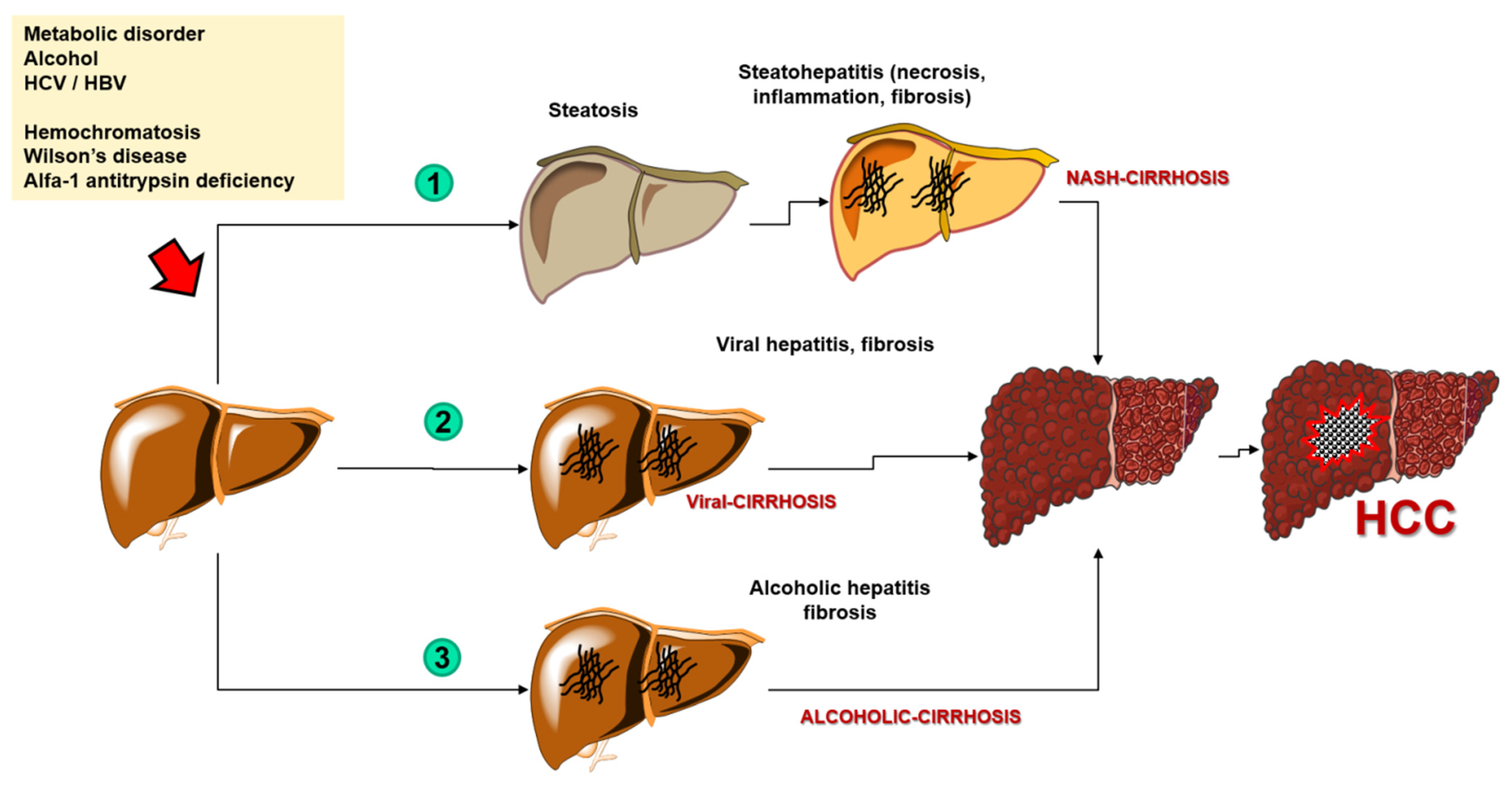
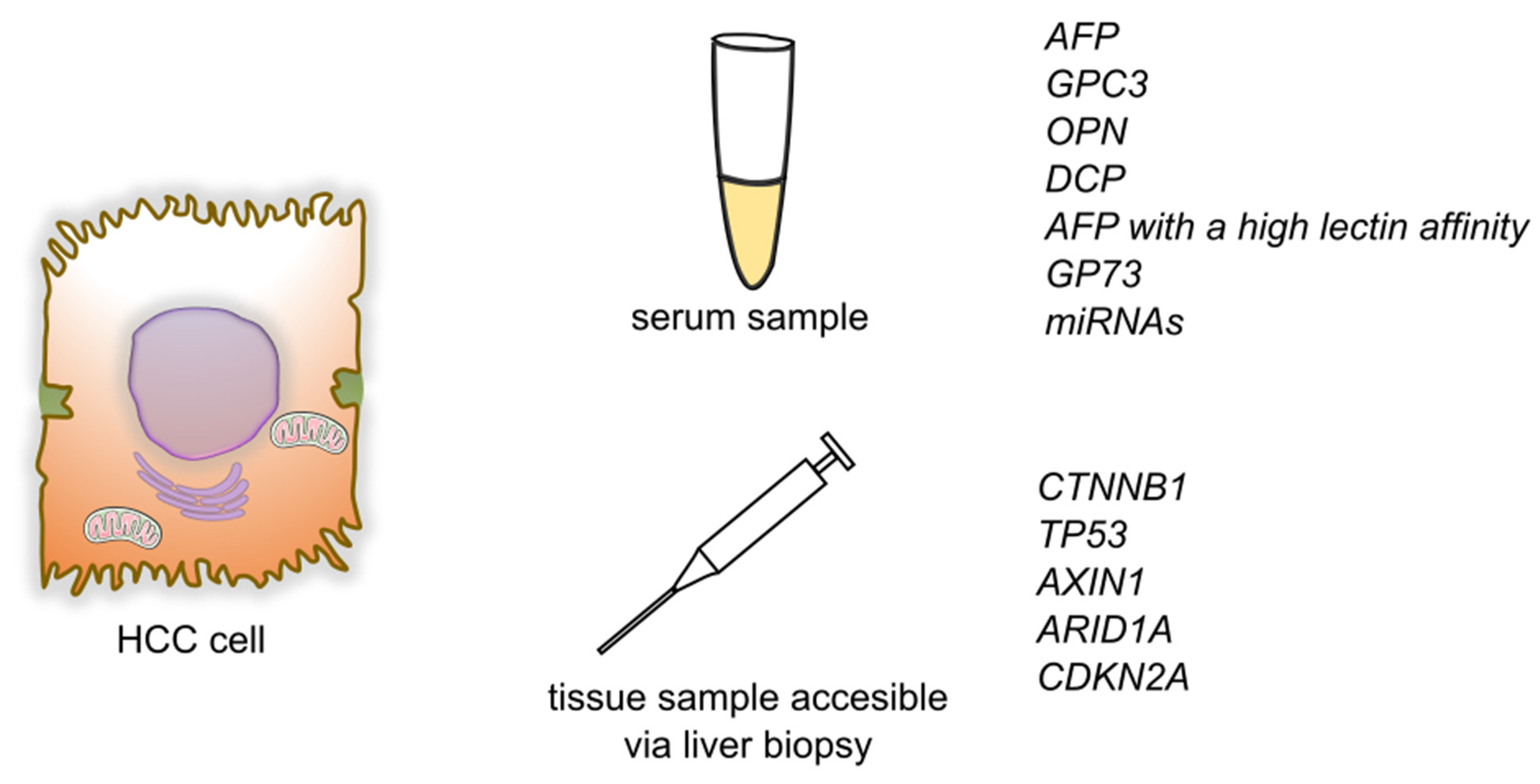
2. Genetic Markers for HCC
2.1. MicroRNAs
2.2. Genetic Markers
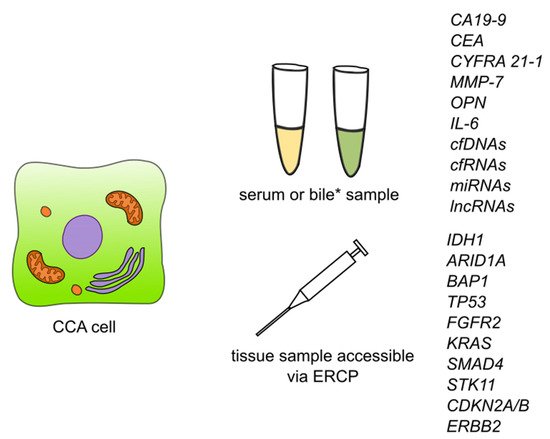
3. Genetic Markers of CCA
3.1. Circulating Nucleic Acids
3.1.1. Cell-Free DNA
3.1.2. Cell-Free RNA
3.1.3. Cell-Free Long Non-Coding RNA
3.1.4. Micro RNA
3.2. Genetic Markers
References
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261.
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309.
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.
- De Stefano, F.; Chacon, E.; Turcios, L.; Marti, F.; Gedaly, R. Novel biomarkers in hepatocellular carcinoma. Dig. Liver Dis. 2018, 50, 1115–1123.
- Khan, A.S.; Dageforde, L.A. Cholangiocarcinoma. Surg. Clin. N. Am. 2019, 99, 315–335.
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114.
- Bergquist, A.; Von Seth, E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 221–232.
- Intuyod, K.; Armartmuntree, N.; Jusakul, A.; Sakonsinsiri, C.; Thanan, R.; Pinlaor, S. Current omics-based biomarkers for cholangiocarcinoma. Expert Rev. Mol. Diagn. 2019, 19, 997–1005.
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39, 108–122.
- Shen, N.; Zhang, D.; Yin, L.; Qiu, Y.; Liu, J.; Yu, W.; Fu, X.; Zhu, B.; Xu, X.; Duan, A.; et al. Bile cell-free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol. Rep. 2019, 42, 549–560.
- Liu, C.H.; Huang, Q.; Jin, Z.Y.; Xie, F.; Zhu, C.L.; Liu, Z.; Wang, C. Circulating microRNA-21 as a prognostic, biological marker in cholangiocarcinoma. J. Cancer Res. Ther. 2018, 14, 220–225.
- Lixin, S.; Wei, S.; Haibin, S.; Qingfu, L.; Tiemin, P. miR-885-5p inhibits proliferation and metastasis by targeting IGF2BP1 and GALNT3 in human intrahepatic cholangiocarcinoma. Mol. Carcinog. 2020, 59, 1371–1381.
- Hann, H.W.; Wang, M.; Hafner, J.; Long, R.E.; Kim, S.H.; Ahn, M.; Park, S.; Comunale, M.A.; Block, T.M.; Mehta, A. Analysis of GP73 in patients with HCC as a function of anti-cancer treatment. Cancer Biomarkers 2010, 7, 269–273.
- Shen, S.; Lin, Y.; Yuan, X.; Shen, L.; Chen, J.; Chen, L.; Qin, L.; Shen, B. Biomarker MicroRNAs for Diagnosis, Prognosis and Treatment of Hepatocellular Carcinoma: A Functional Survey and Comparison. Sci. Rep. 2016, 6, 1–21.
- Cui, Y.; Xu, H.F.; Liu, M.Y.; Xu, Y.J.; He, J.C.; Zhou, Y.; Cang, S.D. Mechanism of exosomal microRNA-224 in development of hepatocellular carcinoma and its diagnostic and prognostic value. World J. Gastroenterol. 2019, 25, 1890–1898.
- Yang, C.; Ma, X.; Guan, G.; Liu, H.; Yang, Y.; Niu, Q.; Wu, Z.; Jiang, Y.; Bian, C.; Zang, Y.; et al. MicroRNA-766 promotes cancer progression by targeting NR3C2 in hepatocellular carcinoma. FASEB J. 2019, 33, 1456–1467.
- Liu, Y.; Tan, J.; Ou, S.; Chen, J.; Chen, L. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J. Physiol. Biochem. 2019, 75, 391–401.
- Lin, H.; Huang, Z.P.; Liu, J.; Qiu, Y.; Tao, Y.P.; Wang, M.C.; Yao, H.; Hou, K.Z.; Gu, F.M.; Xu, X.F. MiR-494-3p promotes PI3K/AKT pathway hyperactivation and human hepatocellular carcinoma progression by targeting PTEN. Sci. Rep. 2018, 8, 1–9.
- Jiang, L.; Cheng, Q.; Zhang, B.H.; Zhang, M.Z. Circulating micrornas as biomarkers in hepatocellular carcinoma screening a validation set from China. J. Med. 2015, 94, 1–10.
- Schulze, K.; Imbeaud, S.; Letouzé, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511.
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698.
- Wang, X.W.; Hussain, S.P.; Huo, T.I.; Wu, C.G.; Forgues, M.; Hofseth, L.J.; Brechot, C.; Harris, C.C. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology 2002, 181–182, 43–47.
- Long, J.; Wang, A.; Bai, Y.; Lin, J.; Yang, X.; Wang, D.; Yang, X.; Jiang, Y.; Zhao, H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine 2019, 42, 363–374.
- Ozen, C.; Yildiz, G.; Dagcan, A.T.; Cevik, D.; Ors, A.; Keles, U.; Topel, H.; Ozturk, M. Genetics and epigenetics of liver cancer. N. Biotechnol. 2013, 30, 381–384.
- Abitbol, S.; Dahmani, R.; Coulouarn, C.; Ragazzon, B.; Mlecnik, B.; Senni, N.; Savall, M.; Bossard, P.; Sohier, P.; Drouet, V.; et al. AXIN deficiency in human and mouse hepatocytes induces hepatocellular carcinoma in the absence of β-catenin activation. J. Hepatol. 2018, 68, 1203–1213.
- He, F.; Li, J.; Xu, J.; Zhang, S.; Xu, Y.; Zhao, W.; Yin, Z.; Wang, X. Decreased expression of ARID1A associates with poor prognosis and promotes metastases of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34.
- Yang, H.; Huo, J.; Li, X. Identification and validation of a five-gene prognostic signature for hepatocellular carcinoma. World J. Surg. Oncol. 2021, 19, 1–13.
- Nahon, P.; Nault, J.C. Constitutional and functional genetics of human alcohol-related hepatocellular carcinoma. Liver Int. 2017, 37, 1591–1601.
- Piao, Z.; Park, C.; Lee, J.S.; Yang, C.H.; Choi, K.Y.; Kim, H. Homozygous deletions of the CDKN2 gene and loss of heterozygosity of 9p in primary hepatocellular carcinoma. Cancer Lett. 1998, 122, 201–207.
- Rizzo, A.; Ricci, A.D.; Tavolari, S.; Brandi, G. Circulating tumor DNA in biliary tract cancer: Current evidence and future perspectives. Cancer Genom. Proteom. 2020, 17, 441–452.
- Wasenang, W.; Chaiyarit, P.; Proungvitaya, S.; Limpaiboon, T. Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin. Epigenet. 2019, 11, 39.
- Driescher, C.; Fuchs, K.; Haeberle, L.; Goering, W.; Frohn, L.; Opitz, F.V.; Haeussinger, D.; Knoefel, W.T.; Keitel, V.; Esposito, I. Bile-based cell-free DNA analysis is a reliable diagnostic tool in pancreatobiliary cancer. Cancers 2021, 13, 39.
- Wang, X.; Fu, X.H.; Qian, Z.L.; Zhao, T.; Duan, A.Q.; Ruan, X.; Zhu, B.; Yin, L.; Zhang, Y.J.; Yu, W.L. Non-invasive detection of biliary tract cancer by low-coverage whole genome sequencing from plasma cell-free DNA: A prospective cohort study. Transl. Oncol. 2021, 14, 100908.
- Gu, X.; Wang, C.; Deng, H.; Qing, C.; Liu, R.; Liu, S.; Xue, X. Exosomal piRNA profiling revealed unique circulating piRNA signatures of cholangiocarcinoma and gallbladder carcinoma. Acta Biochim. Biophys. Sin. 2021, 52, 475–484.
- Bai, J.G.; Tang, R.F.; Shang, J.F.; Qi, S.; Yu, G.D.; Sun, C. Upregulation of long non-coding RNA CCAT2 indicates a poor prognosis and promotes proliferation and metastasis in intrahepatic cholangiocarcinoma. Mol. Med. Rep. 2018, 17, 5328–5335.
- Angenard, G.; Merdrignac, A.; Louis, C.; Edeline, J.; Coulouarn, C. Expression of long non-coding RNA ANRIL predicts a poor prognosis in intrahepatic cholangiocarcinoma. Dig. Liver Dis. 2019, 51, 1337–1343.
- Yao, Y.; Jiao, D.; Liu, Z.; Chen, J.; Zhou, X.; Li, Z.; Li, J.; Han, X. Novel miRNA Predicts Survival and Prognosis of Cholangiocarcinoma Based on RNA-seq Data and in Vitro Experiments. Biomed Res. Int. 2020, 2020, 1–14.
- Asukai, K.; Kawamoto, K.; Eguchi, H.; Konno, M.; Asai, A.; Iwagami, Y.; Yamada, D.; Asaoka, T.; Noda, T.; Wada, H.; et al. Micro-RNA-130a-3p Regulates Gemcitabine Resistance via PPARG in Cholangiocarcinoma. Ann. Surg. Oncol. 2017, 24, 2344–2352.
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135.
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin. Cancer Res. 2018, 24, 4154–4161.
- Li, H.; Long, J.; Xie, F.; Kang, K.; Shi, Y.; Xu, W.; Wu, X.; Lin, J.; Xu, H.; Du, S.; et al. Transcriptomic analysis and identification of prognostic biomarkers in cholangiocarcinoma. Oncol. Rep. 2019, 42, 1833–1842.
