Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vicky Zhou and Version 1 by Vijay Bhooshan Kumar.
Carbon dots (CDs) are a novel type of carbon-based nanomaterial that has gained considerable attention for their unique optical properties, including tunable fluorescence, stability against photobleaching and photoblinking, and strong fluorescence, which is attributed to a large number of organic functional groups (amino groups, hydroxyl, ketonic, ester, and carboxyl groups, etc.). In addition, they also demonstrate high stability and electron mobility. The doping of CDs with organic and inorganic atoms and molecules leads to their functionalization to obtain desired physical and chemical properties for biomedical applications.
- CDs
- hybrid CDs
- doped CDs
- synthesis
1. Introduction
CDs are part of nanoscale carbon materials, including carbon nanotubes, graphene, fullerene, nano-diamonds, and nanofibers. Over the past few years, carbon and carbon-based nanomaterials have attracted significant scientific attention for their physical, chemical, and biological properties [1]. Carbon-based nanomaterials are highly biocompatible, have low toxicity, and have impressive thermal and mechanical properties, as well as the ability to be functionalized with a wide variety of organic and inorganic molecules [2]. Carbon nanoscale materials with strong fluorescence are commonly referred to as CDs because of their unique properties. CDs are also characterized by their high stability, reduced harmful activity, water solubility, and derivatization ability [3,4][3][4]. These properties enable them to be used in a number of disciplines, as presented in Figure 1. Doped or hybrid CDs consist of graphitic carbon and functional heteroatoms (nonmetal) attached with a carbonized core [5,6,7,8,9,10][5][6][7][8][9][10]. These are a relatively new type of nanomaterials and are considered to be promising for various applications [11]. Nanomaterials called CDs are also refer to polymer dots, carbon quantum nanodots, and graphene or graphene oxide quantum dots.
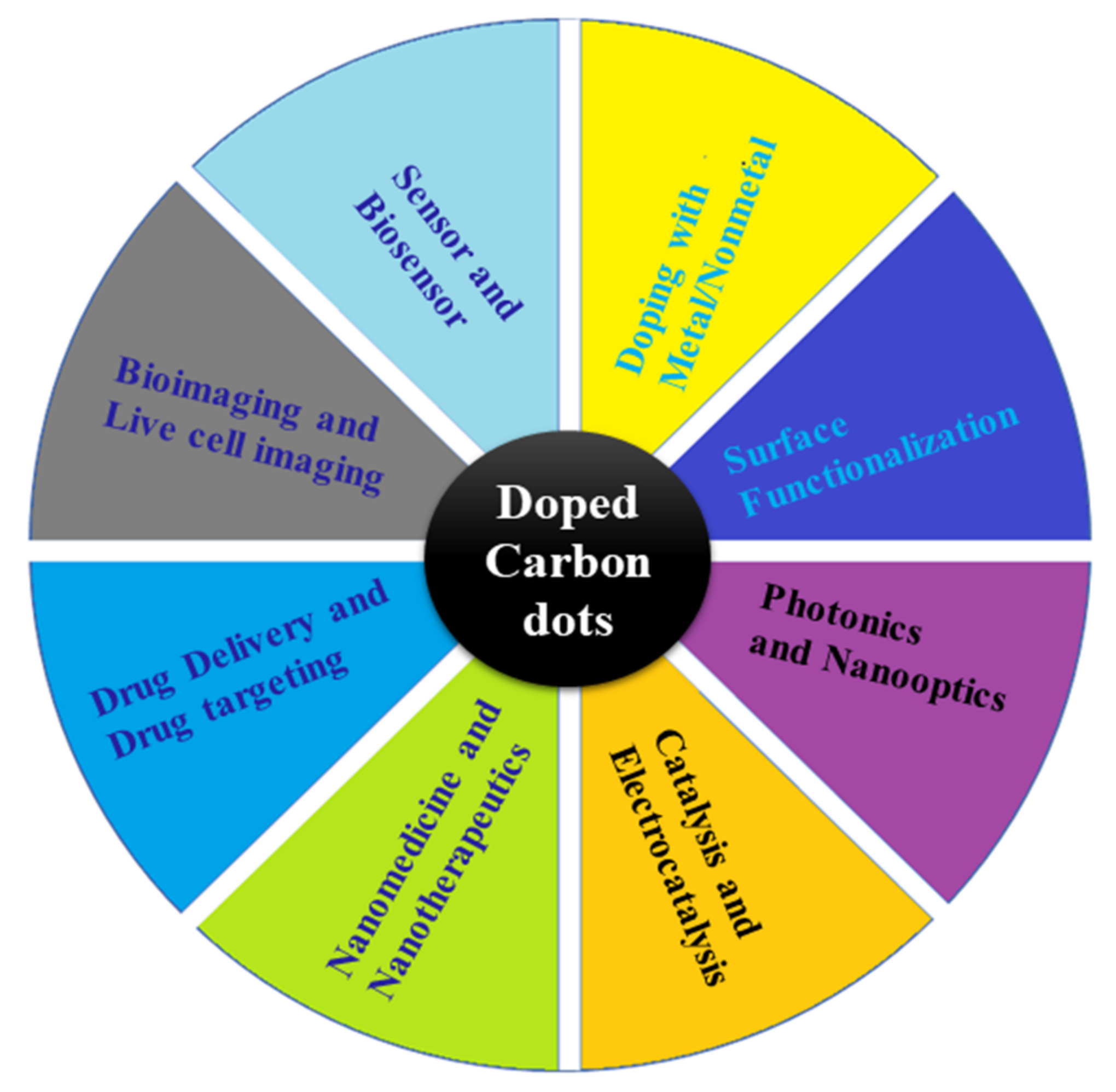
Figure 1. Overview of doped and hybrid CDs and showing the possible potential applications.
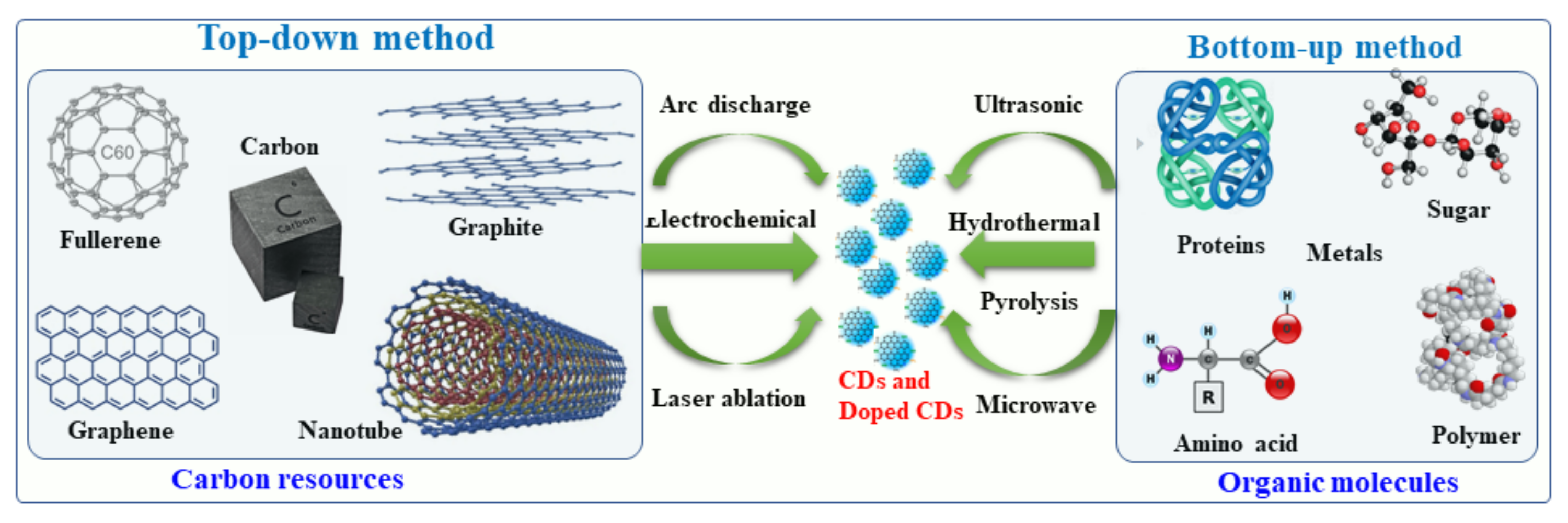
Figure 2. This diagram illustrates the top-down and bottom-up approaches to the synthesis of CDs.
2. Synthesis of Hybrid and Doped CDs
Recently, several synthetic approaches for the synthesis of CDs and hybrid/doped CDs have been explored and reported. These approaches were aimed at improving the physical-chemical characteristics of the hybrid/doped CDs, such as increasing the fluorescence quantum yield and transparency and narrowing the band gap. These approaches are described herein. CDs can be synthesized by several methods, for example arc-discharge, laser ablation, sonochemical, microwave, hydrothermal and electrochemical methods and strong acid oxidation [4,54,55,56][4][54][55][56]. The first synthetic procedure was developed for the formation of pristine CDs by an arc discharge procedure [57,58,59][57][58][59]. It involved a polymerization reaction of small organic molecules followed by carbonization to form CDs. Generally, metal- and nonmetal-doped CDs have been classified into two groups: (i) top-down methods and (ii) bottom-up methods. Under the top-down synthesis method, larger carbon structures are broken down using chemical oxidation, discharge, the electrochemical oxidation of carbon, and ultrasonic methods [58,59][58][59]. However, this top-down approach has a number of disadvantages, such as harsh reaction conditions, expensive materials, and long reaction times [60,61,62][60][61][62]. As an alternative, a bottom-up approach involves converting carbon-based small molecules and structures into CDs of the desired size. Bottom-up methods include sonochemical, microwave, and hydrothermal thermal decomposition of organic molecules, pyrolysis of carbon-based materials, and solvothermal reaction synthesis [63]. In this rHeport, werein will discuss primarily the bottom-up approach to the synthesis of metal- and non-metal doped CDs using organic molecules and a variety of metal or non-metal precursors.2.1. Synthesis of Metal-Doped CDs
Several bottom-up approaches have been extensively adopted for the creation of metal-doped CDs because of their low cost and better efficiency. CDs have been synthesized by solvothermal reactions of carbon-based materials and metallic salts to contain ions of silver [64], manganese [65], copper [49], magnesium [66,67][66][67], cobalt [68], zinc [69], gadolinium [28,70][28][70] and chromium [71]. As an example, aqueous GQDs (graphene quantum dots, which is the same as CDs) (0.1 mg/mL) were prepared by using an electrochemical cyclic voltammetry instrument [66]. Following this, a series of AgNO3 fresh aqueous solutions (0.1 mol/L) with measured volumes (5–60 mL) were added to CD (GQD) solutions (2 mL), respectively, and the above mixtures were stirred for 1 h to form a homogeneous solution of Ag-GQDs [64]. The resulting mixtures were then illuminated by UV light at the wavelength of 365 nm (18 W) from a distance of 20 mm for 3 h. When the photochemical reaction was completed, the color of the solutions transformed from yellow to brownish red (depending on the volume ratio of GQDs to Ag ions), indicating that Ag NPs had been formed [64]. The silver-doped CD (Ag-CD) nanoparticles on silica were engineered (Figure 3a) to form highly active surfaces enhanced by Raman scattering [64]. A number of researchers have also synthesized Zn-doped CDs for biomedical applications [69,72,73,74,75,76][69][72][73][74][75][76]. Dehydration, coordination, and carbonization are the steps required to synthesize Zn2+ doped CDs (Figure 3b) according to Cheng et al. [69]. In detail, Zn2+-doped CDs were prepared by dissolving citric acid, urea, and ZnCl2 in toluene. The resultant mixture was transferred to an autoclave hydrothermal reactor and heated at 200 °C for 12 h. It was then allowed to cool to room temperature. The brown solution obtained after the hydrothermal reaction contained the Zn ion-doped CDs. Evaporating the solvents led to the formation of solid samples, and the Zn2+ doped CDs showed excellent solubility in many organic solvents, as well as in water. In addition, wthe group applied the as-synthesized Zn2+ doped CDs with intense bright yellow PL to a variety of applications. Additionally, Figure 3ca study shows different metal-doped CDs (M = Ga, Sn, Zn, Bi, Au, and Ag) and carbon dot-decorated substrates (Figure 3c) [4][4]. CDs were doped with low melting point metals by sonicating the molten metal overlayered with polyethylene glycol 400 (PEG-400) (PEG-400) [14,77,78][14][77][78]. The formation and doping of the CDs occurred simultaneously, and the effects of several experimental parameters, such as reaction temperature (4–150 °C), sonication duration (3 min–3 h) and sonication amplitude (40–70%), [37] were studied [79]. Doping the CDs with metallic Ga, In, Sn, Pb, Zn, Au or Ag was performed by ultrasound irradiation without the use of any catalyst or post-acid/base treatment [12]. Ga-doped CDs were also decorated with Ga nanoparticles and given the terminology Ga@CDs@Ga nanoparticles. Unlike the other reported synthetic routes for Metal@CDs the sonochemical synthesis. Sonications is the only direct technique for the preparation of Metal@CDs. Likewise, Sn-doped CDs [80] was synthesized by sonochemical reaction of polyethylene glycol-400 and metallic Sn as a precursor [78,79][78][79]. It yielded both Sn@CDs@Sn nanoparticles and Sn@CDs as depicted in Figure 3d. In a recent study, Xu et al., synthesized manganese-doped CDs (Mn-CDs) that demonstrated ultrahigh quantum yield of 54% for metal-doped CDs (Figure 3e) [76][76]. The Mn-CDs nanomaterials were prepared by a facile hydrothermal synthesis route using sodium citrate and manganese carbonate as the precursors and citric acid as an auxiliary agent. The materials displayed bright blue fluorescence and the capability to switch their luminescence on or off in the presence of compatible ions [76]. Taking advantage of the superior reversible fluorescence properties of the Mn-CD, Xu et al., developed a reusable universal flourescence particles for the nanomolar detection of a mercury ion in simulated polluted water samples [76]. Moreover, Duan et al., described a facile solid-phase synthesis method for the Cu-doped CDs nanomaterials using citric acid as the carbon source and Cu(NO3)2·3H2O as the dopant materials, respectively [49]. These Cu-CDs show superior peroxidase-like activity to horseradish peroxidase and were stable under a wide range of pH and temperatures [49].
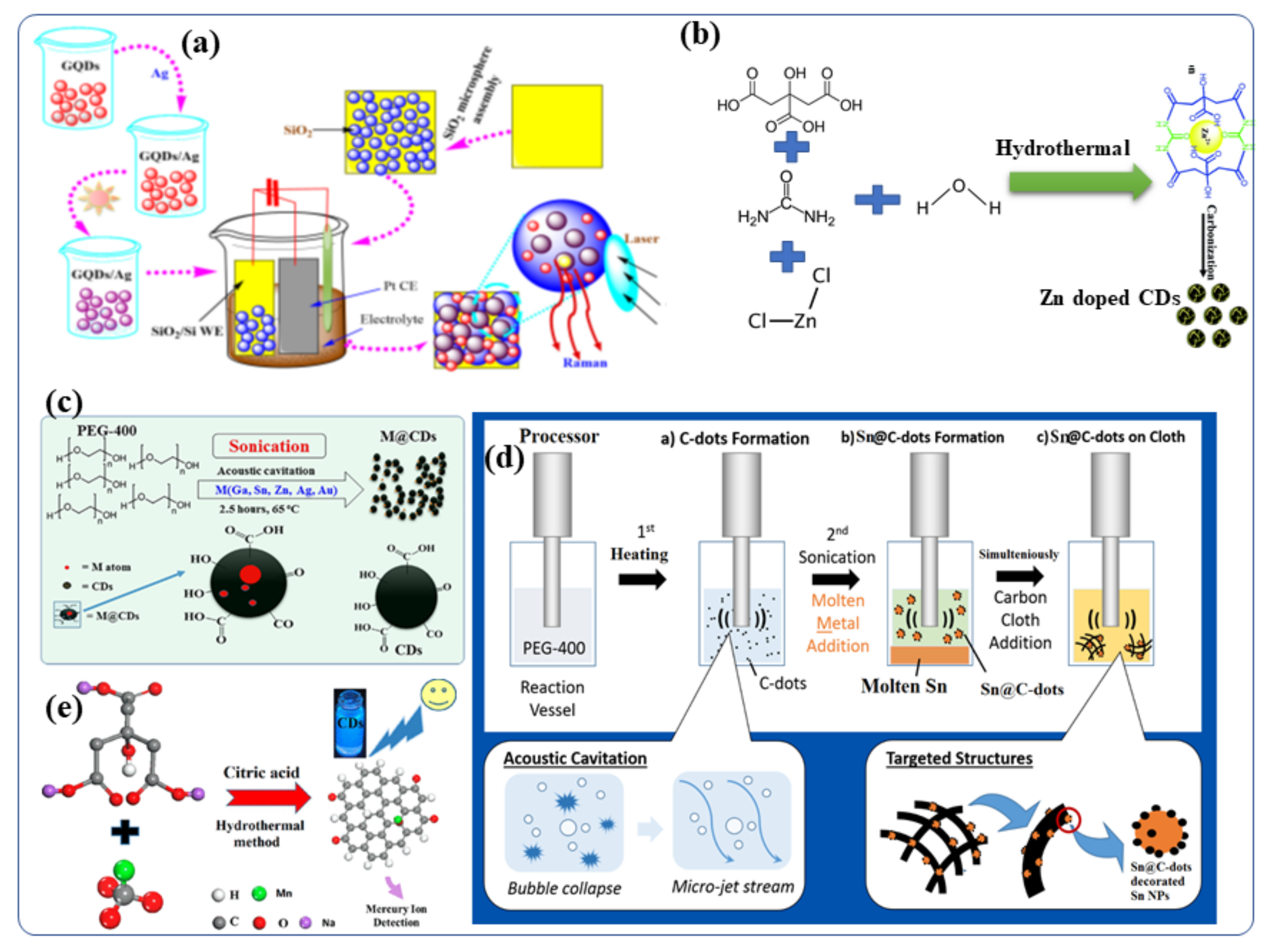

Figure 3. Schematic presentation of the synthetic procedures for Metal-doped CDs: (a) silver-doped CDs [5,64] (b) Synthesis of Zn-doped CDs, including the dehydration, coordination, and carboncarbonation steps. It also illustrates applications of the Zn doped CDs in fluorescent hybrid materials for inkjet printing [69]. (c) Synthesis of CDs and metal-doped CDs protocol [12]. (d) Sonochemical synthesis route of Sn@C-dots and Sn@C-dots@Sn nanoparticles [80]. (e) Synthetic process for the Mn-doped CDs [76]. Reproduced with copyright permission.
2.2. Synthesis of Nonmetal-Doped CDs
The Doping of nonmetals in CDS can affect the overall electrical distribution and the relevant levels of electronic energy in the nonmetal-doped CDs [81]. CDs can be tuned to exhibit their desired fluorescent and other properties by doping foreign atoms [81]. In order to improve the properties of CDs for further applications, various nonmetals such as boron [82], nitrogen [83], phosphorous [84], sulfur [85], silicon [86], and halogens (F, Cl) [87] have been doped into the CDs, using either the “top-down” or “bottom-up” approaches. B, N, and P- doped CDs were recently synthesized by Kumar et al., using hydrothermal and sonochemical methods [45]. They synthesized N-doped carbon quantum dots (NCDs) from bovine serum albumin protein in water via a hydrothermal chemical reaction [15]. The involved the steps of fragmentation, aromatization, polymerization, and carbonization (The synthesis procedure are depicted in Figure 4a) [88][88]. During the hydrothermal reaction (>175 °C) carbonization of the BSA polymer occurs leading to the formation of a final NCDs material with a complicated surface structure [88]. The same hydrothermal method was used to synthesize boron-doped and phosphorus-doped CDs [45]. A temperature-dependent mechanism for the synthesis of N-doped CDs has been proposed. These NCDs were used for doping/functionalization of hydroxyapatite (NCDs-HA) nanomaterials in water by stirring of hydroxyapatite (HA) with NCDs at room temperature [89]. A Figure 4bstudy presents a schematic illustration of doping NCDs with the HA particles. NCDs were used as the stabilizer in three different compositions of HA particles accommodate 1% to 5% NCDs [89]. Adding P and N did not improve the fluorescence properties of N/P-co-doped CDs, although their aqueous dispersibility increases significantly [90]. The purpose of S-doping in CDs is typically to enhance metal ion (Fe3+ ions, other heavy metal ion) detection sensitivity by 0–500 µM [91]. However, if the S atom is fixed to the ring of polythiophene, strong red-emitting light can be observed when the excitation wavelength is 543 nm [92]. Further, the B/ N/S-co-doped CDs demonstrate high-efficiency red emission at wavelength near 600 nm [92]. Even though a variety of nonmetals, such as N, P, S, and B, have been reported, there is still a strong demand for the development of highly efficient CDs in term of real application for ourthe society [92]. Therefore, further exploration of other nonmetal doping systems is highly desirable. For example, although fluorine is not present in biological systems, the incorporation of F-containing grafts can increase the therapeutic efficacy of many drugs, improve the chemical stability of proteins, and enhance phase separation in both polar and nonpolar conditions [93]. Thus, by modifying CDs with the F, it may be possible to significantly tune their fluorescence properties including wavelengths and behavior in biological systems or biomedical applications [93]. Zuo et al., studied the F-doping strategy to dramatically lengthen the emission wavelength of CDs [94]. Using 1,2-diamino-4,5-difluorobenzene as the fluorine source and tartaric acid to improve aqueous solubility, they synthesized a kind of F-doped CD (F-CD) in a one-pot solvothermal process [94]. When compared with undoped CDs, the emission wavelength of F-CDs is redshifted by 50 nm [63,94,95,96][63][94][95][96]. Using an excitation wavelength of 480 nm, the F-CD emits intense yellow fluorescence, while the emitted red light is found upon excitation at 540 nm [94]. Additionally, the F-CDs provide highly sensitive detection of intracellular Ag+ as well as high imaging sensitivity of red blood cells in various cell systems [94]. The hydrothermal method was by Kalaiyarasan to synthesize phosphorus-doped CQDs (PCQDs) from phosphoric acid and Trisodium citrate (TSC) (Figure 4d) [97][97]. They investigated the effect of the reaction temperature, durationand precursor concentrations on the formation of PCQDs and the doping effect of phosphorus in CDs. and found that fluorometric probes for iron detection. Moreover, the P-CDs with high fluorescence QY have been examined for changes in the fluorescence intensities induced by the addition of Fe3+. These changes are a consequence of the formation of a stable fluorescent inactive complex (P-CDs-Fe3+), which may be useful for biomedical applications (Such as live cell imaging, Fe3+ detection in blood, urine) [97]. In addition, a highly sensitive fluorescent probe based on sulfur-doped carbon dots (S-CDs) was synthesized by Kamali et al., using a microwave irradiation method (Figure 4e) [36][36]. Using this probe, a strong blue emission was observed with 36% QY [36]. This class of nonmetal-doped CDs (B, N, P, S, F, etc) or co-doping two/three nonmetals was utilized as an effective fluorescent materials for live cell imaging owing to their excellent QY, good water dispersibility, tunable strong fluorescence, better biocompatibility and low cytotoxicity.
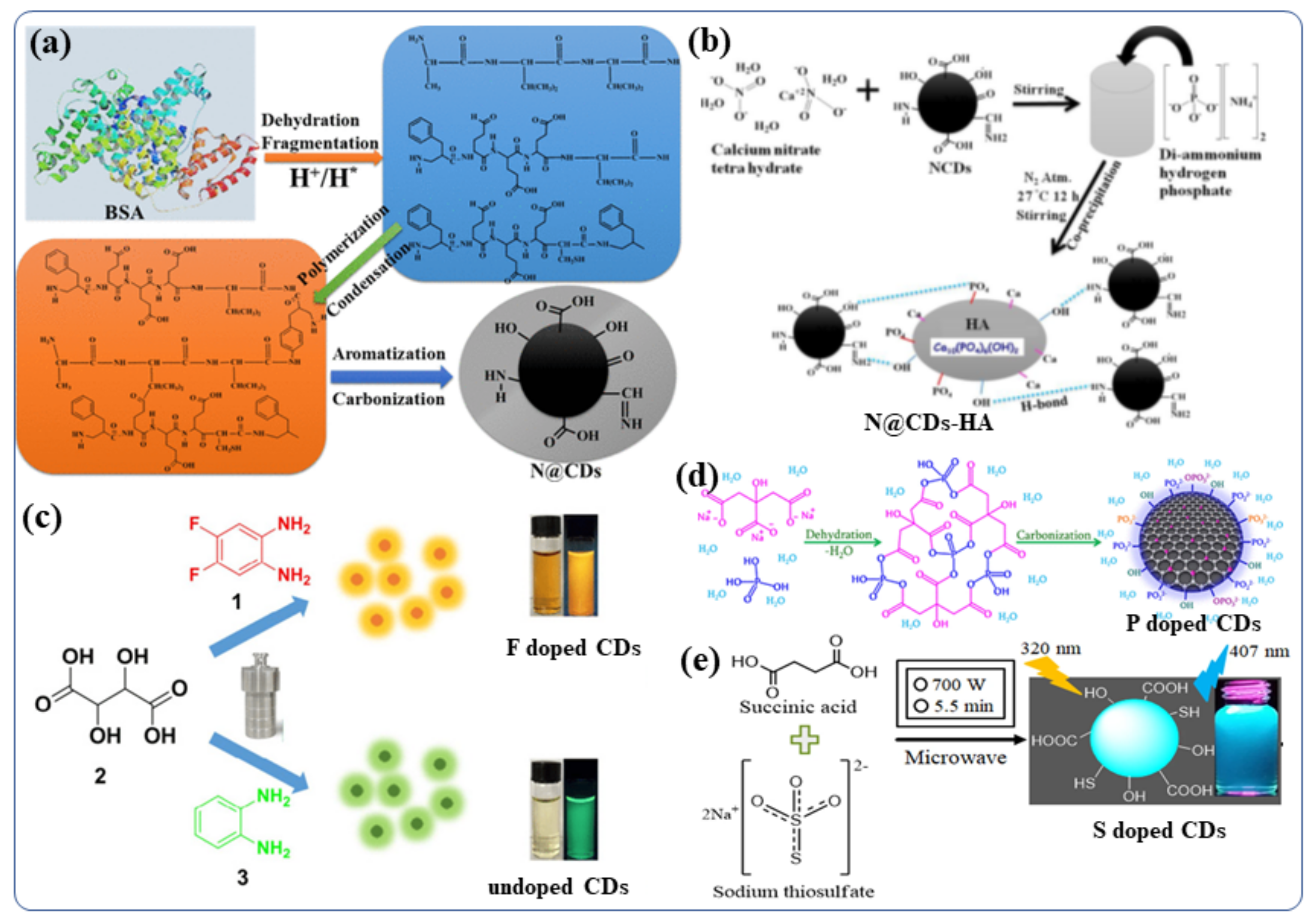 Figure 4. Synthetic procedures for non-metal doped CDs (a) NCDs (N@CDs) [88]. (b) NCDs-HA nanoparticles and their possible binding mechanism with HA particles [89]. (c) F-CDs and undoped CDs [94]. (d) P-doped CDs [97]. (e) S-CDs nanomaterials [36]. Reproduced with copyright permission.
Figure 4. Synthetic procedures for non-metal doped CDs (a) NCDs (N@CDs) [88]. (b) NCDs-HA nanoparticles and their possible binding mechanism with HA particles [89]. (c) F-CDs and undoped CDs [94]. (d) P-doped CDs [97]. (e) S-CDs nanomaterials [36]. Reproduced with copyright permission.
 Figure 4. Synthetic procedures for non-metal doped CDs (a) NCDs (N@CDs) [88]. (b) NCDs-HA nanoparticles and their possible binding mechanism with HA particles [89]. (c) F-CDs and undoped CDs [94]. (d) P-doped CDs [97]. (e) S-CDs nanomaterials [36]. Reproduced with copyright permission.
Figure 4. Synthetic procedures for non-metal doped CDs (a) NCDs (N@CDs) [88]. (b) NCDs-HA nanoparticles and their possible binding mechanism with HA particles [89]. (c) F-CDs and undoped CDs [94]. (d) P-doped CDs [97]. (e) S-CDs nanomaterials [36]. Reproduced with copyright permission.2.3. Nanohybrids of CDs with Metals and Metal Oxides
CDs-metal nanohybrids are prepared mainly by mixing CDs with dispersions of metallic nanoparticles followed by sonochemical reaction. Kumar et al., synthesized TiO2/Sn@CDs hybrid nanomaterials by adding TiO2 nanoparticles in the powder form into an aqueous solution of Sn@CDs and sonicating for 20 min, followed by drying at 80 °C for 12 h in a vacuum chamber [17]. Recently, Li et al., prepared Cu2O/CDs hybrid nanomaterials using ultrasonic reaction [98]. Kumar et al., also developed hybrid nanostructured CDs decorated with Fe3O4 for neuronal growth enhancement [13]. These Fe3O4 functionalized CDs nanospheres were dispersed in an aqueous solution by ultrasonication for 30 min for further study the physical, chemical and biological properties. Tang et al., prepared CDs-BiVO4 nanosphere by refluxing BiVO4 powder in an aqueous solution of synthesized CDs at 90 °C for three hours. Jiang et al., prepared gold-doped CDs (Au@CDs) or gold decorated CDs nanomaterials by using anisotropic gold nanomaterials via microwave assisted method [99]. Tiny gold clusters were found scattered within the skeletons of CDs or on the surfaces of CDs. Au@CDs effectively combine the properties of CDs and gold nanoclusters (AuNCs). In order to obtain the nanoprobes, antibodies were attached to the surface of Au@CDs for further biomedical applications, especially intracellular imaging of cancer-derived exosomes) [99,100][99][100].
3. Conclusions and Summary
The goal was to provide an overview of recent advances in metal and nonmetal doping of CDs, including synthesis approaches, physical-chemical properties, and biomedical applications. Furthermore, metal ion/nonmetal ion doping has been demonstrated to be a promising synthetic route to enhance the chemical, optical, electrical, and magnetic properties of doped CDs by modifying the electronic and atomic arrangement of the CD structure. Thus, doped CD-based nanomaterials have demonstrated remarkable performance in nano probing, sensing, imaging, drug delivery, and tissue engineering. Researchers have significantly improved the biomedical performance of nonmetal/metal-doped CDs by using innovative synthesis techniques. Due to their unique fluorescent properties, excellent biocompatibility, and high aqueous stability, doped C-dots can be a versatile material for biomedical and sensing applications.
