Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Bruce Ren and Version 2 by Bruce Ren.
Severe cardiac arrhythmias developing in the course of seizures increase the risk of SUDEP (sudden unexpected death in epilepsy). Hence, epilepsy patients with pre-existing arrhythmias should receive appropriate pharmacotherapy. Concomitant treatment with antiarrhythmic and antiseizure medications creates, however, the possibility of drug–drug interactions. This is due, among other reasons, to a similar mechanism of action. Both groups of drugs inhibit the conduction of electrical impulses in excitable tissues.
- antiarrhythmic drugs
- antiepileptic drugs
- interactions
- maximal electroshock
1. Introduction
According to WHO, epilepsy is the second most common neurological disorder globally. Around 65 million people worldwide suffer from this disease. Since Western populations are aging fast and the incidence of epilepsy increases with age, the prevalence of this condition is rising constantly. The first written document about epilepsy was found in Mesopotamia. Back in antiquity and the Middle Ages, seizures were thought to result from possession and contributed to the condemnation of innocent people for witchcraft. Nowadays, nobody associates epilepsy with the action of demons. However, many myths have arisen around the disease and numerous patients still feel stigmatized and discriminated. Furthermore, patients with drug-resistant epilepsy cannot live fully fledged lives, as they are unable to fulfill their professional and social roles. The cumulative effects of recurring seizures lead to an increased rate of marital and family breakdown, unemployment, impaired career progress, and consequent financial difficulties [1]. Moreover, drug-resistant patients have an increased risk of SUDEP (sudden unexpected death in epilepsy) development. The exact pathophysiology of SUDEP is currently unknown, although it is believed to be related to frequent incidence of generalized tonic–clonic convulsions and the resulting cardiac, respiratory, and brainstem disorders in the mechanism affecting the function of the central autonomic control centers [2][3]. This was one of the reasons why the maximal electroshock (MES) test was selected. It is the most commonly used animal model reflecting generalized tonic–clonic seizures in humans.
In up to even 42% of cases, epilepsy coexists with arrhythmias, the most frequent one being atrial fibrillation, sudden cardiac arrest, bundle branch block, and ventricular tachycardia [4]. This co-occurrence may result from the common genetic background of the two disorders, e.g., mutation of genes encoding Na+, K+, and Ca2+ ion channels [5][6]. Patients with long-lasting epilepsy often present some interictal cardiac changes, including QT prolongation, decreased heart rate variability, subtle signs of ischemia (ST-segment depression), and ventricular late potentials [7]. Importantly, significant prolongation of QT, probably due to the altered function of sodium channels, was observed in patients who experienced SUDEP [5][8]. Experimental research provided evidence that seizures can disturb autonomic regulation of the heart and lead to fatal arrhythmias [9]. In turn, clinical studies showed that seizures may be preceded by tachycardia or atrial/ventricular ectopy [8].
Epilepsy patients with serious arrhythmias should be treated with antiseizure and antiarrhythmic drugs simultaneously. Either classical or newer antiepileptic drugs are also used in the treatment of disorders other than epilepsy, including bipolar disorders (valproate, carbamazepine, oxcarbazepine, lamotrigine), migraine (topiramate, lamotrigine), neuropathic pain (valproate, carbamazepine, lamotrigine, oxcarbazepine, pregabalin), and fibromyalgia (pregabalin) [10]. Such a wide range of applications increases the likelihood of polypragmasy with antiseizure and antiarrhythmic drugs, which considerably rises the risk of interactions between them. The two groups of medications present similar mechanisms of action, modulating the function of ion channels. There are also similarities between cardiac and neural action potentials. This creates the theoretical basis for antiarythmogenic properties of antiepileptic drugs and anticonvulsant effects of antiarrhythmic medications [4][11][12]. Interestingly, phenytoin is classified as both an antiseizure and antiarrhythmic drug. On the other hand, lidocaine has been effective in certain cases of drug-resistant seizures. It should be underlined, however, that “the dose makes the poison”. Antiepileptics in overdose can be arrhythmogenic, On the other hand, antiarrhytmic drugs (particularly sodium channel blockers) applied at supratherapeutic doses may, though rarely, generate seizures [4]. Furthermore, some antiepileptic drugs induce the hepatic microsomal system, which decreases the effectiveness of the concomitant antiarrhythmic treatment [13].
According to Vaughan Williams, antiarrhythmic drugs are classified into four groups [4]. Class I includes moderate (IA), weak (IB), or marked (IC) sodium channel blockers, which reduce action potential phase 0 slope and overshoot while increasing, decreasing or preserving the action potential duration and effective refractory period, respectively. The resulting reduction in the excitability of cardiomyocytes suppresses abnormal rhythms. Since sinoatrial and atrioventricular nodes use calcium ions to depolarize, class I antiarrhythmic have a negligible effect on the pacemaker cells. Hence, sodium channel blockers can be used in re-entry tachyarrhythmias, in which a blockade of the atrioventricular node is unfavorable. A representative of class IA is procainamide. Class IB comprises lidocaine, mexiletine, and phenytoin, while propafenone and flecainide belong to class IC. Class II contains β-adrenergic blockers that reduce action potential phase 4 slope, decrease sinoatrial node pacing rates, and slow atrioventricular node conduction. B-blockers differ from each other in terms of β1/β2 receptor selectivity, intrinsic sympathomimetic activity, and membrane-stabilizing (local anesthetic) activity. Examples of class II antiarrhythmics are propranolol, metoprolol, atenolol, esmolol, and timolol. Class III, comprising potassium channel blockers, inhibit action potential phase 3 repolarization (potassium efflux) and lengthen the effective refractory period. Representatives of this class are: amiodarone, bretylium, dofetilide, ibutilide, and sotalol. Sotalol, in addition to class III properties, is also a nonselective β-blocker without intrinsic sympathomimetic or membrane-stabilizing activity. Finally, class IV drugs, containing calcium channel blockers, reduce action potential phase 0 in the sinoatrial and atrioventricular nodes, decreasing heart rate and conduction. Calcium blockers also inhibit action potential phase 2 in cardiomyocytes and suppress contraction. The main representatives of class IV antiarrhythmics are verapamil and diltiazem [4][14][15] (Figure 1).
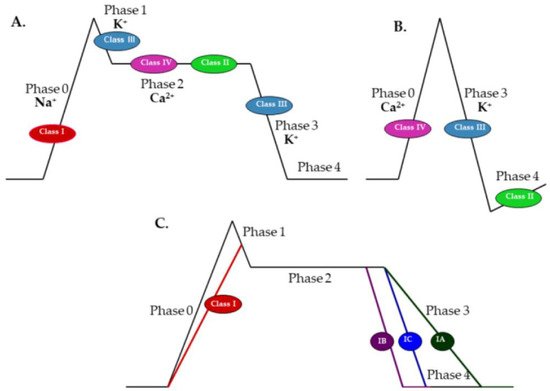
Figure 1. Effects of class I–IV antiarrhythmic drugs on phases of the action potential in: (A) cardiomyocytes; (B) sinoatrial and atrioventricular nodes; and (C) effects of subclasses IA, IB, and IC on phases of the action potential in cardiomyocytes (adapted and modified from https://www.ezmedlearning.com/blog/antiarrhythmics (accessed on 1 February 2022)).
Recently, several other classes have been added to this classification. Class ID relates to actions on late sodium current (INaL) components important in long QT syndrome type 3. Class II takes into account advances in the understanding of autonomic, often G protein-mediated, signaling. Class III covers numerous subsequently discovered potassium channels regulating action potential and refractory period durations. Class IV comprises not only calcium channel blockers, but also drugs that modify intracellular calcium homeostasis. Further new classes have been added, such as class 0, taking into account cardiac automaticity, class V, including drugs acting on mechanically sensitive channels, class VI, gathering factors affecting electrotonic coupling between cells, and class VII, containing substances modifying structural remodeling (class VII) [15]. The only representative of class 0 is ivabradine, which slows the heart rate by inhibiting If/Ih currents conducted through hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Other considered mechanisms of action include deactivation of outward delayed rectifier potassium current and activation of inward currents, such as sodium-dependent background currents (IbNa), T- and L-type calcium currents (ICaL and ICaT), and sustained inward currents (Ist) [15]. If (“funny current”) occurs in the sinoatrial node, while corresponding Ih currents are observed in neurons. Both of them are described as voltage-activated Na+/K+ hyperpolarization-activated depolarizing currents facilitated by cAMP. To date, four isoforms of HCN have been identified. In the heart, If is responsible for initiation and regulation of the heart beat (“pacemaker current”). Roles attributed to Ih in the nervous tissue include control of rhythmic activity in neuronal circuits (e.g., in the thalamus), regulation of excitability, determination of resting membrane potential, dendritic integration, and synaptic transmission. Therefore, dysfunctions of HCN channels may be involved in some forms of epileptic activity [10][16][17][18], and ivabradine has a theoretical background in interacting with antiseizure drugs.
2. Antiarrhythmic Drugs Have Different Effects on Tonic–Clonic Convulsions
The MES parameters were as follows: 25 mA, 50 Hz, and 0.2 s. In remaining cases, MES details and their results were described together. Results were considered significant when p was lower than 0.05.
2.1. Class 0 Antiarrhythmic Drugs
Ivabradine administered at doses of 5 and 10 mg/kg did not alter the electroconvulsive threshold in mice; however, at higher doses of 15 and 20 mg/kg, it significantly elevated this parameter. Ivabradine (10 mg/kg) significantly enhanced the antielectroshock activity of valproate and reduced the action of phenytoin and lamotrigine in the MES test in mice. The effectiveness of carbamazepine, phenobarbital, lacosamide, pregabalin, and topiramate remained unchanged. Furthermore, ivabradine significantly diminished the brain concentration of phenytoin and had no effect on the brain levels of remaining antiepileptic drugs used. This suggests that the interaction between ivabradine and phenytoin is at least partially due to pharmacokinetic events, while the interaction between ivabradine and valproate seems to be pharmacodynamic [19][20][21].
2.2. Class I Antiarrhythmic Drugs
Propafenone at the dose range of 60–90 mg/kg significantly elevated the electroconvulsive threshold (ECT), being ineffective at lower doses. Applied at its subthreshold dosages, propafenone potentiated the antielectroshock action of seven antiepileptic drugs: carbamazepine, valproate, phenytoin, phenobarbital, oxcarbazepine, topiramate, and pregabalin. The action of lamotrigine remained unaffected. Interactions between propafenone and valproate or pregabalin may be in part due to pharmacokinetic events, since propafenone significantly elevated the brain levels of the two antiepileptics. On the other hand, propafenone potentiated the action of carbamazepine despite its lowered concentration in the brain. It may suggest actual synergism between propafenone and carbamazepine, which is, however, masked by pharmacokinetic interactions [22][23].
Mexiletine exhibited properties of an antiseizure drug, being active not only in the ECT but also in the MES test. This enabled isobolographic analysis of interactions between mexiletine and antiepileptic drugs. Regarding classical antiepileptics, antagonistic interaction was revealed between mexiletine and valproate for two fixed-ratio combinations of 1:1 and 3:1. Additivity was observed between mexiletine and valproate applied in the proportion of 1:3. Moreover, mexiletine interacted additively with carbamazepine, phenytoin, and phenobarbital in all three fixed ratios. Since mexiletine did not significantly alter brain concentrations of carbamazepine, phenobarbital, and phenytoin, the observed interactions seem to be pharmacodynamic in nature. In contrast, the antiarrhythmic drug decreased the brain level of valproate. This could be, at least in part, the reason for antagonistic interaction between the two drugs [24].
In relation to new-generation antiepileptic drugs, the mixture of mexiletine and pregabalin at the fixed ratios of 1:1 and 3:1 led to synergistic interaction, while the combination in the proportion of 1:3 was additive. Synergism was also demonstrated for the combination of mexiletine with topiramate in all three proportions. Combinations of this antiarrhythmic drug with lamotrigine or oxcarbazepine were found to be additive. Interestingly, synergism between mexiletine and topiramate at the dose ratio of 1:1 existed despite the mexiletine-induced decrease in the brain concentration of topiramate. This may indicate strong pharmacodynamic interaction between the two drugs. A similar situation occurred with the combination of mexiletine and oxcarbazepine (1:1) as well as pregabalin (1:3), where additivity was enough to overcome decreased brain levels of the two antiepileptics. It may suggest that in this case, pharmacokinetic interactions mask the actual synergism between above-mentioned drugs. It is worth underlining that pharmacokinetic events may vary depending on the proportion of combined drugs. Mexiletine decreased the brain concentration of pregabalin at the dose ratio of 1:3, increased this level at 1:1, and did not alter it in the proportion of 3:1 [25].
2.3. Class II Antiarrhythmic Drugs
In one of the first studies on this topic, propranolol applied at doses of 5, 10, and 20 mg/kg increased the ECT in mice in a dose-dependent manner. According to the authors, the anticonvulsant action of this β-blocker is related to its membrane-stabilizing properties [26]. Moreover, propranolol (40 mg/kg) showed antielectroshock activity comparable to that of phenytoin (30 mg/kg). Parameters of the MES were: 60-Hz current of 50 mA intensity for 0.2 s through ear-clip electrodes [27]. In the study conducted by Fischer and Müller [28], some β-blockers with local anesthetic properties (propranolol, alprenolol, and pindolol, all at 10 mg/kg) increased the antielectroshock action of phenobarbital. The MES was provided with following parameters: 35-Hz current of 50 mA intensity for 0.4 s through ear-clip electrodes. In another study, (+/−) propranolol (1–50 mg/kg), (+) propranolol (50 mg/kg), and pindolol (10–50 mg/kg) exhibited significant protective effects against the MES (30 mA, 0.2 s, no data about current frequency), whereas timolol (1 mg/kg), and propranolol analog UM-272 (1 and 10 mg/kg) were ineffective in this respect [29].
In another study, propranolol, acebutolol, metoprolol, and atenolol potentiated the antiseizure action of certain antiepileptic drugs against the MES test in mice. Propranolol and metoprolol increased the effectiveness of valproate and diazepam. Acebutolol enhanced the action of valproate but not that of diazepam. In contrast, atenolol, which does not penetrate the blood–brain barrier, had no effect on the two antiepileptic drugs. None of the β-blockers changed the protective activity of carbamazepine and phenytoin against the MES. Revealed interactions do not seem to be pharmacokinetic, since β-blockers did not change the brain levels of valproate or diazepam. Propranolol and metoprolol are highly lipophilic agents, easily penetrating to the brain, whereas acebutolol crosses the blood–brain barrier to a moderate degree. Hence, it may be suggested that the action of separate beta-blockers on the action of antiepileptic drugs depends largely on their brain levels [30]. Some β-receptor blockers with local anesthetic properties (e.g., propranolol 5–10 mg/kg, alprenolol 10 mg/kg, pindolol 10 mg/kg, all at the dose of 10 mg/kg) were able to enhance the protective effect of phenobarbital in higher concentrations [28].
Finally, it was shown that nebivolol (0.5–15 mg/kg) did not raise the ECT but, at the dose of 15 mg/kg, it reduced the antielectroshock properties of carbamazepine. The effect of valproate, phenytoin, and phenobarbital remained unchanged by this β-blocker. Nebivolol significantly decreased the brain concentration of valproate but did not affect concentrations of remaining antiepileptic drugs [31].
2.4. Class III Antiarrhythmic Drugs
Although amiodarone (25–75 mg/kg) did not change the ECT, when applied at the dose of 75 mg/kg, it significantly enhanced the antielectroshock activity of carbamazepine, oxcarbazepine, and pregabalin in mice. The action of valproate, phenytoin, phenobarbital, lamotrigine, and topiramate remained unaffected. Brain concentrations of antiepileptic drugs were not affected by amiodarone. Therefore, the interaction between amiodarone and carbamazepine also seems to be pharmacodynamic in nature [32][33].
Dronedarone, another multichannel blocker, administered alone (in doses of 50, 75, and 100 mg/kg), increased the ECT in mice. Surprisingly, this amiodarone derivative (50 mg/kg) significantly reduced the anticonvulsant action of phenytoin in the MES test. No effect on the action of carbamazepine, phenobarbital, or valproate was observed [34]. Dronedarone (50 mg/kg) significantly enhanced the anticonvulsant potency of lamotrigine but did not affect the anticonvulsant properties of lacosamide, pregabalin, or topiramate in the MES test in mice. The measurement of total brain concentrations of phenytoin and lamotrigine revealed that dronedarone did not significantly alter total brain concentrations of lamotrigine in experimental animals, so interactions observed between dronedarone and the two antiepileptics seem to be pharmacodynamic in nature [35].
Sotalol at doses up to 100 mg/kg did not affect the ECT. This antiarrhytmic drug applied at the dose range of 60–100 mg/kg potentiated the antielectroshock action of valproate, whilst it potentiated that of phenytoin at doses of 80–100 mg/kg. Sotalol did not, however, affect the action of carbamazepine, phenobarbital, oxcarbazepine, lamotrigine, pregabalin, or topiramate in the MES test. Furthermore, sotalol significantly decreased the brain concentration of lamotrigine, increased those of oxcarbazepine and topiramate, and did not change the levels of remaining antiepileptic drugs. This indicates that interactions between sotalol and valproate as well as sotalol and phenytoin are most likely pharmacodynamic [36][37].
2.5. Class IV Antiarrhythmic Drugs
In the study conducted by Czuczwar et al. [38], diltiazem elevated the ECT at doses of 2.5 and 5 mg/kg, but at doses of 1.25 and 10 mg/kg, it did not affect this parameter. This calcium blocker at the dose of 1.25 mg/kg markedly potentiated the protective action of carbamazepine and diphenylhydantoin against the MES-induced seizures in mice. When applied at the higher dose of 2.5 mg/kg, it also enhanced the action of phenobarbital and valproate [38].
In newer reports, diltiazem (up to 10 mg/kg, ip) and verapamil (up to 20 mg/kg, ip) did not significantly affect the ECT in mice. Diltiazem (5 and 10 mg/kg) markedly potentiated the antielectroshock activity of topiramate, but not that of lamotrigine. In contrast, verapamil (5, 10, and 20 mg/kg) had no effect on the antiseizure action of topiramate. Pharmacokinetic verification revealed that diltiazem did not affect the brain concentration of topiramate or lamotrigine; thus, the observed interactions were considered as pharmacodynamic in nature [39][40].
All data outlines can be found in Table 1, Table 2 and Table 3.
Table 1. Effects of antiarrhythmic drugs on the antielectroshock action of antiepileptic drugs.
Table 3. Isobolographic interactions between mexiletine and antiepileptic drugs in the MES test [24][25].
| MXL/AED Ratio | VPA | CBZ | PHT | PB | OXC | TPM | PGB | LTG | |
|---|---|---|---|---|---|---|---|---|---|
| 1:3 | Add | Add | Add | Add | Add ph↓ | S | Add ph↓ | Add | |
| Propafenone | [22][23] | ↑ ** | ↑VPA ***, ↑CBZ ***, ↑PB **, ↑OXC **, ↑TPM *, ↑PGB **, ↔LTG | ||||||
| 1:1 | Ant ph↓ | Add | Add | ↑VPA ***, ↑CBZ ***, ↑PGB * | |||||
| Add | Add | S | ph↓ | S | ph↑ | Add | Amiodarone | [32][33] | ↔ |
| 3:1 | ↑CBZ **, ↑OXC *, ↑PGB ***, ↔VPA, ↔PHT, ↔PB, ↔TPM, ↔LTG | not found for any AEDs | |||||||
| Ant | Add | Add | Add | Add | Dronedarone | [34][35] | ↑ *** | ↑LTG *, ↓PHT *, ↔VPA, ↔CBZ, ↔PB, ↔TPM, ↔PGB, ↔LSM | not found for VPA, CBZ, PHT, PB, LTG, not tested for remaining AEDs |
| Sotalol | [36][37] | ↔ | ↑VPA **, ↑PHT **, ↔CBZ, ↔PB, ↔OXC, ↔TPM, ↔PGB, ↔LTG | ↑OXC ***, ↑TPM ***, ↓LTG **, not found for remaining AEDs | |||||
| Propranolol | |||||||||
| ] | |||||||||
| [ | |||||||||
| 40 | |||||||||
| ] | |||||||||
| ↔ | |||||||||
| ↑CBZ **, ↑PHT ***, ↑TPM **, ↔VPA, ↔PB, ↔LTG | not found for CBZ, PHT, TPM, LTG, not tested for remaining AEDs |
Table 2. Effects of antiarrhythmic drugs on the antielectroshock action of individual antiepileptic drugs.
| Antiepileptic Drugs | Antiarrhythmic Drugs Affecting the Antielectroshock Action of a Given Antiepileptic Drug | |||||
|---|---|---|---|---|---|---|
| Valproate | ↑propafenone, sotalol, propranolol, acebutolol, metoprolol, ivabradine | |||||
| Carbamazepine | ↑propafenone, amiodarone, diltiazem, ↓nebivolol | |||||
| Phenytoin | ↑propafenone, sotalol, diltiazem, ↓dronedarone, ivabradine | |||||
| S | S | ph↓ | Add | Phenobarbital | ↑propafenone, propranolol, alprenolol, pindolol | |
| Oxcarbazepine | ↑propafenone, amiodarone | [26][28][29][30] | ↑ a | ↑VPA ***, ↑PB *, ↑DZP *, ↔CBZ, ↔PHT | not found for VPA, DZP, not tested for remaining AEDs | |
| Topiramate | ↑propafenone, diltiazem | Acebutolol | [30] | ↔ | ↑VPA **, ↔CBZ, ↔PHT, ↔DZP | not found for VPA, DZP, not tested for remaining AEDs |
| Pregabalin | ↑propafenone, amiodarone | Metoprolol | [30] | ↔ | ↑VPA ***, ↑DZP **, ↔CBZ, ↔PHT | not found for VPA and DZP, not tested for remaining AEDs |
| Lamotrigine | ↑dronedarone, ↓ivabradine | Atenolol | [30] | ↔ | ↔VPA, ↔CBZ, ↔OXC, ↔DZP | not tested |
| Pindolol | [28] | ↑ a | ↑PB * | not tested | ||
| Alprenolol | [28] | ↑PB * | not tested | |||
| Timolol | [29] | ↔ | not tested | |||
| Nebivolol | [31] | ↔ | ↓CBZ **, ↔VPA, ↔PHT, ↔PB | ↓VPA, not found for other AEDs | ||
| Verapamil | [38][39][40] | ↔ | ↔VPA, ↔CBZ, ↔PB, ↔PHT, ↔TPM, ↔LTG | not found | ||
| Diltiazem | [38][ | |||||
| 39 |
3.6. Clinical Data
Clinical knowledge of the influence of antiarrhythmic drugs on seizures is very scarce. Lidocaine and propranolol were effective in patients with chronically unstable generalized epilepsy [41][42]. In 3 of 11 patients, propranolol decreased, by at least 50%, the frequency of startle-induced epileptic seizures [43]. Mexiletine and lidocaine attenuated seizures in symptomatic partial epilepsy and Lennox–Gastaut syndrome and controlled neonatal seizures resistant to phenobarbital, lamotrigine, and midazolam [44][45][46]. On the other hand, verapamil limited convulsions in recurrent status epilepticus [47][48].
References
- Janson, M.T.; Bainbridge, J.L. Continuing burden of refractory epilepsy. Ann. Pharmacother. 2021, 55, 406–408.
- Maguire, M.J.; Jackson, C.F.; Marson, A.G.; Nevitt, S.J. Treatments for the prevention of sudden unexpected death in epilepsy (SUDEP). Cochrane Database Syst. Rev. 2020, 4, CD011792.
- Serdyuk, S.; Davtyan, K.; Burd, S.; Drapkina, O.; Boytsov, S.; Gusev, E.; Topchyan, A. Cardiac arrhythmias and sudden unexpected death in epilepsy: Results of long-term monitoring. Heart Rhythm 2021, 18, 221–228.
- Borowicz, K.K.; Banach, M. Antiarrhythmic drugs and epilepsy. Pharmacol. Rep. 2014, 66, 545–551.
- Bouza, A.A.; Isom, L.L. Voltage-Gated Sodium Channel β Subunits and Their Related Diseases. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2018; Volume 246, pp. 423–450.
- Li, M.C.H.; O’Brien, T.J.; Todaro, M.; Powell, K.L. Acquired cardiac channelopathies in epilepsy: Evidence, mechanisms, and clinical significance. Epilepsia 2019, 60, 1753–1767.
- Velagapudi, P.; Turagam, M.; Laurence, T.; Kocheril, A. Cardiac arrhythmias and sudden unexpected death in epilepsy (SUDEP). Pacing Clin. Electrophysiol. 2012, 35, 363–370.
- Biet, M.; Morin, N.; Lessard-Beaudoin, M.; Graham, R.K.; Duss, S.; Gagné, J.; Sanon, N.T.; Carmant, L.; Dumaine, R. Prolongation of action potential duration and QT interval during epilepsy linked to increased contribution of neuronal sodium channels to cardiac late Na+ current: Potential mechanism for sudden death in epilepsy. Circ. Arrhythm. Electrophysiol. 2015, 8, 912–920.
- Davis, A.M.; Natelson, B.H. Brain-heart interactions. The neurocardiology of arrhythmia and sudden cardiac death. Tex. Heart Inst. J. 1993, 20, 158–169.
- Betchel, N.T.; Fariba, K.; Saadabadi, A. Lamotrigine. In StatPearls ; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022.
- Benarroch, E.E. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology 2013, 81, 1079–1088.
- Roubille, F.; Tardif, J.C. New therapeutic targets in cardiology: Heart failure and arrhythmia: HCN channels. Circulation 2013, 127, 1986–1996.
- Zaccara, G.; Lattanzi, S. Comorbidity between epilepsy and cardiac arrhythmias: Implication for treatment. Epilepsy Behav. 2019, 97, 304–312.
- Anderson, J.L.; Prystowsky, E.N. Sotalol. An important new antiarrhythmic. Am. Heart J. 1999, 137, 388–409.
- Lei, M.; Wu, L.; Terrar, D.A.; Huang, C.L. Modernized classification of cardiac antiarrhythmic drugs. Circulation 2018, 138, 1879–1896.
- Rivolta, I.; Binda, A.; Masi, A.; DiFrancesco, J.C. Cardiac and neuronal HCN channelopathies. Pflugers Arch. 2020, 472, 931–951.
- Biel, M.; Wahl-Schott, C.; Michalakis, S.; Zong, X. Hyperpolarization-activated cation channels: From genes to function. Physiol. Rev. 2009, 89, 847–885.
- Wahl-Schott, C.; Biel, M. HCN channels: Structure, cellular regulation and physiological function. Cell. Mol. Life Sci. 2009, 66, 470–494.
- Luszczki, J.J.; Prystupa, A.; Andres-Mach, M.; Marzeda, E.; Florek-Luszczki, M. Ivabradine (a hyperpolarization activated cyclic nucleotide-gated channel blocker) elevates the threshold for maximal electroshock-induced tonic seizures in mice. Pharmacol. Rep. 2013, 65, 1407–1414.
- Sawicka, K.M.; Wawryniuk, A.; Zwolak, A.; Daniluk, J.; Szpringer, M.; Florek-Luszczki, M.; Drop, B.; Zolkowska, D.; Luszczki, J.J. Influence of ivabradine on the anticonvulsant action of four classical antiepileptic drugs against maximal electroshock-induced seizures in mice. Neurochem. Res. 2017, 42, 1038–1043.
- Sawicka, K.M.; Załuska, K.; Wawryniuk, A.; Załuska-Patel, K.; Szczyrek, M.; Drop, B.; Daniluk, J.; Szpringer, M.; Żółkowska, D.; Łuszczki, J.J. Ivabradine attenuates the anticonvulsant potency of lamotrigine, but not that of lacosamide, pregabalin and topiramate in the tonic-clonic seizure model in mice. Epilepsy Res. 2017, 133, 67–70.
- Banach, M.; Piskorska, B.; Borowicz-Reutt, K.K. Propafenone enhances the anticonvulsant action of classical antiepileptic drugs in the mouse maximal electroshock model. Pharmacol. Rep. 2016, 68, 555–560.
- Borowicz-Reutt, K.K.; Popławska, M.; Banach, M.; Wróblewska, D. Influence of propafenone on the anticonvulsant activity of various novel antiepileptic drugs in the mouse maximal electroshock model. Pharmacol. Rep. 2018, 70, 481–487.
- Borowicz-Reutt, K.K.; Banach, M.; Piskorska, B. Mexiletine and its interactions with classical antiepileptic drugs: An isobolographic analysis. Neurochem. Res. 2016, 41, 1185–1191.
- Wróblewska, D.; Rudkowska, M.; Banach, M.; Borowicz-Reutt, K.K. Interactions of mexiletine with novel antiepileptic drugs in the maximal electroshock test in mice: An isobolographic analysis. Neurochem. Res. 2018, 43, 1887–1896.
- Akkan, A.G.; Yillar, D.O.; Eşkazan, E.; Akcasu, A.; Ozüner, Z. The effect of propranolol on maximal electroshock seizures in mice. Int. J. Clin. Pharmacol. Ther. Toxicol. 1989, 27, 255–257.
- Raju, S.S.; Gopalakrishna, H.N.; Venkatadri, N. Effect of propranolol and nifedipine on maximal electroshock-induced seizures in mice: Individually and in combination. Pharmacol. Res. 1998, 38, 449–452.
- Fischer, W.; Müller, M. Pharmacological modulation of central monoaminergic systems and influence on the anticonvulsant effectiveness of standard antiepileptics in maximal electroshock seizure. Biomed. Biochim. Acta 1988, 47, 631–645.
- Khanna, N.; Ray, A.; Alkondon, M.; Sen, P. Effect of beta-adrenoceptor antagonists and some related drugs on maximal electroshock seizures in mice. Indian J. Exp. Biol. 1989, 27, 128–130.
- Luchowska, E.; Luchowski, P.; Wielosz, M.; Kleinrok, Z.; Czuczwar, S.J.; Urbańska, E.M. Propranolol and metoprolol enhance the anticonvulsant action of valproate and diazepam against maximal electroshock. Pharmacol. Biochem. Behav. 2002, 71, 223–231.
- Borowicz-Reutt, K.K.; Banach, M.; Rudkowska, M. Nebivolol attenuates the anticonvulsant action of carbamazepine and phenobarbital against the maximal electroshock-induced seizures in mice. Pharmacol. Rep. 2020, 72, 80–86.
- Banach, M.; Poplawska, M.; Borowicz-Reutt, K.K. Amiodarone, a multi-channel blocker, enhances anticonvulsive effect of carbamazepine in the mouse maximal electroshock model. Epilepsy Res. 2018, 140, 105–110.
- Banach, M.; Rudkowska, M.; Sumara, A.; Borowicz-Reutt, K. Amiodarone enhances anticonvulsive Effect of oxcarbazepine and pregabalin in the mouse maximal electroshock model. Int. J. Mol. Sci. 2021, 22, 1041.
- Sawicka, K.M.; Wawryniuk, A.; Daniluk, J.; Karwan, S.; Florek-Łuszczki, M.; Chmielewski, J.; Luszczki, J. Influence of dronedarone (a class III antiarrhythmic drug) on the anticonvulsant potency of four classical antiepileptic drugs in the tonic–clonic seizure model in mice. J. Neural Transm. 2018, 126, 115–122.
- Sawicka, K.M.; Florek-Łuszczki, M.; Wawryniuk, A.; Daniluk, J.; Wróblewska-Łuczka, P.; Chmielewski, J.; Karwan, S.; Łuszczki, J. Dronedarone (a multichannel blocker) enhances the anticonvulsant potency of lamotrigine, but not that of lacosamide, pregabalin and topiramate in the tonic-clonic seizure model in mice. Epilepsy Res. 2019, 154, 62–68.
- Banach, M.; Popławska, M.; Borowicz-Reutt, K.K. Sotalol enhances the anticonvulsant action of valproate and diphenylhydantoin in the mouse maximal electroshock model. Pharmacol. Rep. 2017, 69, 1173–1177.
- Borowicz-Reutt, K.K.; Banach, M.; Rudkowska, M.; Stachniuk, A. Sotalol does not interfere with the antielectroshock action of selected second-generation antiepileptic drugs in mice. Pharmacol. Rep. 2021, 73, 516–524.
- Czuczwar, S.J.; Chodkowska, A.; Kleinrok, Z.; Małek, U.; Jagiełło-Wójtowicz, E. Effects of calcium channel inhibitors upon the efficacy of common antiepileptic drugs. Eur. J. Pharmacol. 1990, 176, 75–83.
- Luszczki, J.J.; Trojnar, M.K.; Trojnar, M.P.; Kimber-Trojnar, Z.; Szostakiewicz, B.; Zadrozniak, A.; Borowicz, K.K.; Czuczwar, S.J. Effects of three calcium channel antagonists (amlodipine, diltiazem and verapamil) on the protective action of lamotrigine in the mouse maximal electroshock-induced seizure model. Pharmacol. Rep. 2007, 59, 672–682.
- Luszczki, J.J.; Trojnar, M.K.; Trojnar, M.P.; Kimber-Trojnar, Z.; Szostakiewicz, B.; Zadrozniak, A.; Borowicz, K.K.; Czuczwar, S.J. Effects of amlodipine, diltiazem, and verapamil on the anticonvulsant action of topiramate against maximal electroshock-induced seizures in mice. Can. J. Physiol. Pharmacol. 2008, 86, 113–121.
- Mori, K.; Ito, H.; Toda, Y.; Hashimoto, T.; Miyazaki, M.; Saijo, T.; Kuroda, Y. Successful management of intractable epilepsy with lidocaine tapes and continuous subcutaneous lidocaine infusion. Epilepsia 2004, 45, 1287–1290.
- De Oliveira, G.G.; Borges, M.A. Propranolol action in chronically unstable generalized epilepsy. Am. J. Ther. 1994, 1, 38–41.
- Mayer, T.; Specht, U. Propranolol in startle induced epileptic seizures. J. Neurol. Neurosurg. Psychiatry 1995, 58, 382–383.
- Enoki, H.; Hata, H.; Ohmori, I.; Maniwa, S.; Ohta, H.; Kobayashi, K. Clinical applications and the effect of mexiletine on refractory epilepsies. No Hattatsu = Brain Dev. 2000, 32, 29–34. (In Japanese)
- Miyamoto, A.; Takahashi, S.; Oki, J. A successful treatment with intravenous lidocaine followed by oral mexiletine in a patient with Lennox-Gastaut syndrome. No To Hattatsu 1999, 31, 459–464. (In Japanese)
- Nakazawa, M.; Okumura, A.; Niijima, S.; Yamashita, S.; Shimono, K.; Hirose, S.; Shimizu, T. Oral mexiletine for lidocaine-responsive neonatal epilepsy. Brain Dev. 2013, 35, 667–669.
- Schmitt, F.C.; Dehnicke, C.; Merschhemke, M.; Meencke, H.J. Verapamil attenuates the malignant treatment course in recurrent status epilepticus. Epilepsy Behav. 2010, 17, 565–568.
- Summers, M.A.; Moore, J.L.; McAuley, J.W. Use of verapamil as a potential P-glycoprotein inhibitor in a patient with refractory epilepsy. Ann. Pharmacother. 2004, 38, 1631–1634.
More
