Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Lindsay Dong and Version 2 by Jessie Wu.
This entry covers major breakthroughs of MXene and applications.
- MXene
- hard laminates
- applications
1. Background
Two-dimensional materials have secured a novel area of research in material science after the emergence of graphene. Now, a new family of 2D material-MXene is gradually growing and making its mark in this field of study. MXenes since 2011 have been synthesized and experimented on in several ways. The HF treatment although successful poses some serious problems that gradually propelled the ideas of new synthesis methods.
2. Applications and Recent Works
The exclusive properties of MXenes are indication enough that they can have their own spot in manufacturing industries and device applications. The electronic, optical and mechanical properties discussed above have their individual areas of applications for MXenes and numerous works are going on to overcome the challenges to produce optimum results. The passing of the experimental stage is crucial though to make them commercially successful and so we need to find out the sectors where they can have the most use. Through the years there are several ways MXene is being researched on in order to get a picture of their usability. Having the unique combination of properties like mechanical properties contributed by the transition metal carbides/nitrides, high electrical conductivity; hydrophilicity due to functionalization of surfaces; ability to absorb electromagnetic waves efficiently and high negative zeta potential enabling formation of stable colloidal solutions are the explored genres till date and can give way to various applications. The pie chart depicts the sectors of applications (Centre pie chart) Figure 1. On the second ring the years when they were first explored is shown. It appears that MXenes have found their niche in energy storage applications and recently in environmental sectors as well. As per the data collected, the initial stages of experiments were focused on the structural mapping of the MXenes. The energy storage field was the first thing that was sought for regarding MXenes and remains the large portion of MXene activities. Recently other fields are also considered like the Biomedical and Photo catalysis applications in the last couple of years are gaining importance rapidly. The energy sector includes use of MXenes in electrochemical capacitors, micro-super-capacitors, Li-ion batteries and others. Back in 2012, Naguib and team first experimented on MXene based Li-ion battery. It was established that due to a higher exposed surface area and easy intercalation between weakly bound MX layers, the capacity was 5 times higher than that of Ti2AlC. Exfoliated Ti2C demonstrated a stable capacity of 225 mAhg−1 at C/25 rate. This showed that MXenes can be used for energy storage other than the bulky counterparts [1].
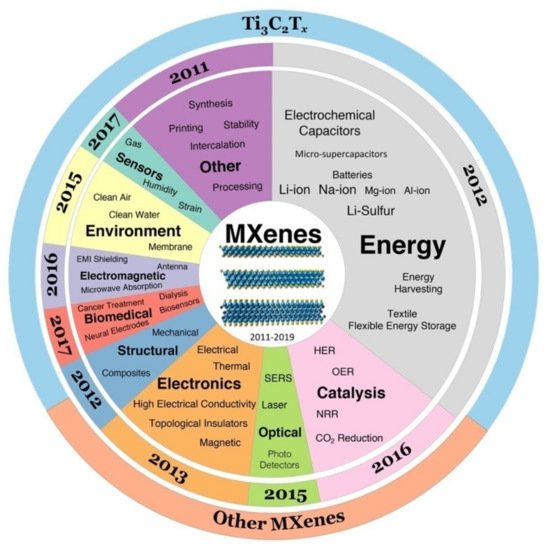
Figure 1. Mxene applications: The pie chart depicts the MXene applications in various sectors explored. Center pie chart depicts the no. of publications made for the applications mentioned. The middle ring shows the year of onset of those publications; i.e., the breakthrough works with most impact. The last ring shows the ratio of explored Ti3C2Tx to other MXenes. Adapted with permission from [2] (Gogotsi, 2019). Copyright © 2019 American Chemical Society.
After that work, other research teams also lead review studies aiming at establishing MXene as a superior electrode material. Aierken and his fellow researchers produced a research paper in 2018 about MXene/graphene heterostructures in Li batteries. The physical properties of the system in consideration were derived from DFT calculations. The MXene/graphene bilayer vertical heterostructures (M2CX2 + Gr, where M = Ti, Sc, V and X = OH, O) were investigated with respect to the strongest Li binding sites. Ti2CO2 + Gr and V2CO2 + Gr emerged as promising systems for Li intercalation. There was 100% Li intercalation in these two systems, which means all the sites were occupied resulting in an open circuit voltage of 1.49 V for Ti2CO2 + Gr and 1.93 V for V2CO2 + Gr bilayer. The molecular weight of the heterostructure bilayer was lower than exposed MXene bilayer and so offered higher storage density [3] (Aierken, Sevik, Gülseren, Peeters, & Çakır, 2018). Tang et al. also gave a review regarding MXene’s suitability as an electrode material for storing energy. The vast review covered the use of simple MXene as well as MXene-based composites in Li-ion batteries, Na-ion batteries, Li-S batteries, supercapacitors, hybrid-capacitors, and sodium-ion hybrid capacitors in a systematic way [4] (Tang et al., 2018). The results pointed out the pros and cons of MXene use in batteries and also the amount of experimental work needed to further increase the scope. The paper also pointed out the fact that the stacked nature of MXenes makes it capable of energy storing via intercalating charges in between. So, if the interlayer distance can somehow be increased without hampering the stability of the structure, then the capacity to store more charge will increase. A recent review in 2020 also discussed the same topic, solely focused on the effect of the interlayer distance in MXenes [5] (Garg, Agarwal, & Agarwal, 2020).
The interlayer distance is very crucial and so is the choice of cations to be intercalated. Larger cations can lower conductivity thus affecting the transfer of charges and ions. By intercalating potassium ion there was a small increase in interlayer distance contrary to intercalating lithium, sodium, magnesium which led to electrode contraction. The lack of experimental evidence regarding MXene-polymer composites for energy storage purpose makes it difficult to compare and determine which one is better. Lin et al. also published a paper regarding MXenes as high rate electrodes in both aqueous and non-aqueous electrolytes as well as in organic electrolytes containing metal ions [6] (Z. Lin, Shao, Xu, Taberna, & Simon, 2020). The choice of solvent is equally important as the surface terminations of MXenes. The electrochemical performance in acidic aqueous electrolytes shows an ultra-high volumetric capacitance of 1500 Fcm−3 titanium carbide serving as electrodes [7] (Lukatskaya et al., 2013). This value is definitely greater than what offered by conventionally used porous carbon and its derivatives. The only limitation was the narrow potential window resulting from the rapid oxidation of MXene at higher negative potential significantly lowering the energy density. That is why the focus shifted to non-aqueous electrolytes (organic or ionic) having a stable potential window ranging from 3V to higher values. This can change the entire picture but the specific capacitance remains limited in this case compared to the aqueous electrolyte which was exceptionally higher. This is due to the shrinkage of MXenes reducing the interlayer distance and thus cutting off the access to cations or anions. Thus the possibility remains of finding a point of stability for MXenes to avoid restacking and dictating a specific design with tuned interlayer spacing. In 2021, a perspective was laid forward by Balach and Giebeler about the progress and use of MXenes in Li-S batteries [8] (Balach & Giebeler, 2021). The paper theoretically develops to extend applications in micro scale industry and generate autonomous high power reservoirs without compromising the size by significantly enhancing the Li-S battery features using MXene properties. The idea of miniature Li-S batteries is not yet entertained due to the shuttle effect (the formation of polysulfides in the electrolyte while reaction that can poison the anode or depletion of cathode via rigorous redox reactions). Also it is important to establish the superiority of Li-S batteries experimentally over the already existing Li-ion batteries. The idea of MXenes as electrodes curbing the shuttle effect while meeting the criteria of reliability fits perfectly. To make the electrodes last longer in a manner of self-healing via making the broken bonds during the ongoing reaction, the proposal to use MXene based polymer composites is also coined. The ambitious study poses solid questions and opens many areas of research of synthesis of MXenes not just in its simple form but with other binders serving as stable matrix.
Microsupercapacitors are now most probably the most exciting devices in energy storage applications. The era now and upcoming will always strive to miniaturize and elongate the lifetime of batteries without constantly having to replace or replenish them. Electrochemical capacitors or supercapacitors are emerging as alternatives for batteries as they are theoretically meant to have unlimited lifetime and provide high power density. The only issue is their size and bulky nature, which is not suitable for micro devices. Thus, the urge to shift towards microsupercapacitors (MSC) is rational and is being adopted to be integrated with electric circuits in a micro level. According to a review, recently the MSC available is of two types: microelectrodes arranged in an array in microscale sizes and thin film electrodes having sandwich-like structure and thickness less than 10 µm [9] (Beidaghi & Gogotsi, 2014), [10] (S. Zheng, Shi, Das, Wu, & Bao, 2019). Through the years, carbon and its derivatives are used for constructing microsupercapacitors like carbon-derived carbon [11] (Chmiola, Largeot, Taberna, Simon, & Gogotsi, 2010), carbon nanotubes [12] (J. Lin et al., 2012); graphene [13][14] (J. Liang, Mondal, Wang, & Iacopi, 2019), (G. Zhang et al., 2018) and others [15][16][17] (Kim, Hsia, Carraro, & Maboudian, 2014), (Kim et al., 2014), (Pech et al., 2010), (S. Wang, Hsia, Carraro, & Maboudian, 2014). However, they provided low-energy density that overshadowed their high conductivity. The alternate use of pseudocapacitive materials like transition metal oxides and conducting polymers showed poor conductivity [18][19][20][21] (Kurra, Hota, & Alshareef, 2015) (Meng, Maeng, John, & Irazoqui, 2014), (Augustyn et al., 2013), (Choi, Blomgren, & Kumta, 2006). This is why the MXenes with their 2D morphology and experimentally tunable surface terminations are claimed to be a better replacement. The surface terminations actually install the property of hydrophilicity in MXenes that in turn significantly influence the Fermi level densities. Peng et al. in 2016 fabricated Ti3C2TX MXene MSC by spray coating technique and painting a PVA/H2SO4 gel electrolyte. The volumetric capacitance was found to be 356.8 Fcm−3 at 0.2 mA cm−2along with a high areal capacitance of 27.3 mF cm−2 at 20mVs−1 [22][23] (Y.-Y. Peng et al., 2016). (Jiang et al., 2019) found out that Ti3C2TXMXene exhibits superior capacitate nature comparable to commercially available 4 mF capacitor. This shows that the bulky electrolytic capacitors are replaceable with MXene based MSCs [24] (Jiang et al., 2019). Li et al. fabricated double-sided MSC’s (DMSC) using MXene ink attached to the window of 7.2 V working potential in various series and parallel configurations. It was seen that by decreasing the electrode gap, the capacitance rose steeply with 10 µm gap showing the highest volumetric capacitance of 308 Fcm−3 at 5 mVs−1 [25] (Q. Li et al., 2020). Another study by Sharma and Rout illustrates the electrochemical properties and also the various fabrication techniques of microsupercapacitors pointing MXenes as a promising material. MXene-based hybrids and MXene/CNT-based MSCs are also discussed in this paper along with a detailed table reporting other hybrids too [26] (Sharma & Rout, 2021). There is a clear lack of experimentation to further enhance the use of MXene in MSCs as this needs careful regulation and optimization of working electrode materials. Also, the ambition to get higher areal capacitance and higher energy density should be the main point to keep in mind while developing the electrodes.
Photocatalysts originated in the 20th century only propelled by the need of developing environment-friendly technologies and devices. The prospects of clean energy, nontoxic wastes, and environmental wellbeing are the prime concern of any new advancement regarding technology and photocatalysts are able to fulfill all these criteria. Their nature is semiconducting and when light falls on them and the electrons from valence band leaps to the conduction band leaving behind a hole at initial position. The process is guided by oxidation and redox reactions that need to be understood and explored more. This migration of the charge carriers’ photogenerated by the incident light to the catalyst surface is the main step, also the rate determining step of the entire reaction. The inhibition of the recombination of the charge carriers aids in the photocatalysis process [27] (B. Wang, Zhang, & Huang, 2017), [28] (Y. Li, Jin, Zhang, & Fan, 2019). The reaction mechanisms typically involve photocatalytic hydrogen evolution reaction (HER), reduction reaction of CO2 (CO2RR), and degradation reaction of photocatalysts. Veering towards nanomaterial specially in two dimensions, the thickness goes down compared to the three-dimensional ones, which makes the Fermi level discrete and the energy gap wider, enabling more excitation of photons [29] (X. Li et al., 2021). A recent review by Kuang and team displayed the MXene-based photocatalysts showing light on the use of MXenes and their role in photocatalysis. Being a 2D material puts MXenes as the tool to be used in photocatalysts. The paper establishes other factors also in a very organized way that proves the usability of MXene in this type of emerging technology. The abundance of surface functional groups and large surface area enhances MXene’s possibility and makes them infuse the photo activated separation of charge carriers at the same time, acting as stable support or surface for the reactions to occur. MXenes also limits the size of the photocatalysts and thus has been used in various photocatalyst-based applications like water splitting, nitrogen fixation, and carbon dioxide reduction [30] (Kuang, Low, Cheng, Yu, & Fan, 2020). In 2016, a study came out claiming MXene as a promising photocatalyst for water splitting [31] (Guo, Zhou, Zhu, & Sun, 2016). All three types of transition metal carbides (M2C, M3C2, M4C3) and the analysis were done by XPS and EDS. A varied range of transition metal elements were experimented with, out of which 2D Zr2CO2 and Hf2CO2 was successful in water splitting. It has been already established that Ti3C2TX is the most used MXene as photocatalyst in HER [32] (Takanabe, 2017), [33] (Kudo & Miseki, 2009). The hydrophilicity promotes the reaction and the Gibbs free energy aids in the adsorption of hydrogen. Ti3C2TX acts as a base catalyst or a co-catalyst enhancing the performance of the base catalyst by doubling yield of H2. A single 2D flake of Ti3C2TX is more suitable for this job; however, extracting a 2D flake is complex. One major setback is instability of MXenes for applications in aqueous media. MXenes are not highly resistant to oxidation despite ambient conditions and is considered to be thermodynamically metastable. Ti3C2TX can even oxidize in normal air under atmospheric pressure with Ti turning to TiO2 leaving behind a sandwich-like structure of carbon layers and TiO2 layers. Also, it has been determined that Ti3C2TX contains Ti defects intrinsically that aid in further instability. This instability contradicts the accepted behavior of long lifetime in photocatalysts. Despite this inching towards the environmental remediation, MXenes are turning out to be a good choice in need of modifications. MXene surface can adsorb target pollutants via photodegradation, which can be quite complex [34] (Sinopoli et al., 2019). MXenes can adsorb CO2 at elevated temperatures and low partial pressures. Garcia and group put forward the idea of MXene carbides as CO2 absorbers and the effect of thickness in the adsorption. DFT calculations are employed to evaluate the results theoretically and thus opening a gate for experimental endeavors [33] (Morales-García, Mayans-Llorach, Viñes, & Illas, 2019). Adsorption is directly linked with waste water treatment and pollutant management in a very efficient low cost way and the search for a better material to serve the purpose is very important. The paper determines that surface of MXene carbides undergoes exothermic activation of CO2 molecule and can be easily manipulated by changing stoichiometry, composition, and thickness. This paper also points to the structural similarity between transition metal carbides (TMCs) (111) surface and MXene (0001) surface. Now, TMCs are highly unstable and the formation energy of the bulk form is also high. MXenes having the same structural mapping and atomic sites arraying can be considered as a replacement for TMCs. Chen et al. investigated on CO2 capture as well as its possible conversion to products of value like fuel by using MXene-based products. They exclusively focused on CO2 capture, sensing, and conversion of CO2 and also the recent territories that are being explored in this area [35] (Y. Chen et al., 2020). Also, MXene-based composites with suitable functionalization and introduced to CO2-philic substances can enhance the chance of CO2 capture more. The CO2 adsorption capacity is directly linked to the increased specific surface area and can reach 44.2 mmol g−1 [36] (Ding et al., 2018). MXene at this stage is still being explored in terms of synthesizing and stability, so most of the work is focused on energy storage. A lot of experimental work is pending in order to materialize the theoretical predictions being made so far. In a review article regarding the progress in environmental applications of MXene, scientists found that MXene polymer matrices and covalent, non-covalent modifications can provide diverse opportunities of applications. The covalent modifications offer more stable properties and functions when compared to the non-covalent ones. Apart from adsorbing toxic gases, it has been theoretically proved by Zhang et al. that MXene can successfully adsorb and remove uranyl too, which in turn suggests that MXenes can be used in removing radioactive waste [37] (Zexiang He, Huang, Yue, Zhu, & Zhao, 2021). Somehow, the optical and electronic properties of MXene tend to blend, forming a middle ground that actually opens up multiple areas of applications. In biomedical industry, biotoxicity is another hazard to battle and theories of using MXene is being coined provided that the MXene itself does not produce any impurity and maintains stability [38] (J. Chen et al., 2020).
References
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969.
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494.
- Aierken, Y.; Sevik, C.; Gülseren, O.; Peeters, F.M.; Çakır, D. MXenes/graphene heterostructures for Li battery applications: A first principles study. J. Mater. Chem. A 2018, 6, 2337–2345.
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene–2D layered electrode materials for energy storage. Prog. Nat. Sci. 2018, 28, 133–147.
- Garg, R.; Agarwal, A.; Agarwal, M. A review on MXene for energy storage application: Effect of interlayer distance. Mater. Res. Express 2020, 7, 022001.
- Lin, Z.; Shao, H.; Xu, K.; Taberna, P.-L.; Simon, P. MXenes as High-Rate Electrodes for Energy Storage. Trends Chem. 2020, 2, 654–664.
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505.
- Balach, J.M.; Giebeler, L. MXenes and the progress of Li–S battery development—A perspective. J. Phys. Energy 2020, 3, 021002.
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884.
- Zheng, S.; Shi, X.; Das, P.; Wu, Z.; Bao, X. The Road Towards Planar Microbatteries and Micro-Supercapacitors: From 2D to 3D Device Geometries. Adv. Mater. 2019, 31, e1900583.
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic Carbide-Derived Carbon Films for Micro-Supercapacitors. Science 2010, 328, 480–483.
- Lin, J.; Zhang, C.; Yan, Z.; Zhu, Y.; Peng, Z.; Hauge, R.H.; Natelson, D.; Tour, J.M. 3-Dimensional Graphene Carbon Nanotube Carpet-Based Microsupercapacitors with High Electrochemical Performance. Nano Lett. 2012, 13, 72–78.
- Liang, J.; Mondal, A.K.; Wang, D.; Iacopi, F. Graphene-Based Planar Microsupercapacitors: Recent Advances and Future Challenges. Adv. Mater. Technol. 2018, 4, 1800200.
- Zhang, G.; Han, Y.; Shao, C.; Chen, N.; Sun, G.; Jin, X.; Gao, J.; Ji, B.; Yang, H.; Qu, L. Processing and manufacturing of graphene-based microsupercapacitors. Mater. Chem. Front. 2018, 2, 1750–1764.
- Kim, M.S.; Hsia, B.; Carraro, C.; Maboudian, R. Flexible micro-supercapacitors with high energy density from simple transfer of photoresist-derived porous carbon electrodes. Carbon 2014, 74, 163–169.
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654.
- Wang, S.; Hsia, B.; Carraro, C.; Maboudian, R. High-performance all solid-state micro-supercapacitor based on patterned photoresist-derived porous carbon electrodes and an ionogel electrolyte. J. Mater. Chem. A 2014, 2, 7997–8002.
- Kurra, N.; Hota, M.; Alshareef, H.N. Conducting polymer micro-supercapacitors for flexible energy storage and Ac line-filtering. Nano Energy 2015, 13, 500–508.
- Meng, C.; Maeng, J.; John, S.W.M.; Irazoqui, P.P. Ultrasmall Integrated 3D Micro-Supercapacitors Solve Energy Storage for Miniature Devices. Adv. Energy Mater. 2013, 4, 1301269.
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522.
- Choi, D.; Blomgren, G.E.; Kumta, P.N. Fast and Reversible Surface Redox Reaction in Nanocrystalline Vanadium Nitride Supercapacitors. Adv. Mater. 2006, 18, 1178–1182.
- Peng, Y.-Y.; Akuzum, B.; Kurra, N.; Zhao, M.-Q.; Alhabeb, M.; Anasori, B.; Kumbur, E.C.; Alshareef, H.N.; Ger, M.-D.; Gogotsi, Y. All-MXene (2D titanium carbide) solid-state microsupercapacitors for on-chip energy storage. Energy Environ. Sci. 2016, 9, 2847–2854.
- Jiang, Q.; Kurra, N.; Maleski, K.; Lei, Y.; Liang, H.; Zhang, Y.; Gogotsi, Y.; Alshareef, H.N. On-Chip MXene Microsupercapacitors for AC-Line Filtering Applications. Adv. Energy Mater. 2019, 9, 1901061.
- Li, Q.; Wang, Q.; Li, L.; Yang, L.; Wang, Y.; Wang, X.; Fang, H. Microsupercapacitors: Femtosecond Laser-Etched MXene Microsupercapacitors with Double-Side Configuration via Arbitrary On-and Through-Substrate Connections (Adv. Energy Mater. 24/2020). Adv. Energy Mater. 2020, 10.
- Sharma, A.; Rout, C.S. Two-Dimensional MXene Based Materials for Micro-Supercapacitors; Headquarters IntechOpen Limited: London, UK, 2021.
- Wang, B.; Zhang, J.; Huang, F. Enhanced visible light photocatalytic H2 evolution of metal-free g-C3N4 /SiC heterostructured photocatalysts. Appl. Surf. Sci. 2017, 391, 449–456.
- Li, Y.; Jin, Z.; Zhang, L.; Fan, K. Controllable design of Zn-Ni-P on g-C3N4 for efficient photocatalytic hydrogen production. Chin. J. Catal. 2019, 40, 390–402.
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3C2Tx) in photocatalysis: A review. Mater. Adv. 2021, 2, 1570–1594.
- Kuang, P.; Low, J.; Cheng, B.; Yu, J.; Fan, J. MXene-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 18–44.
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 11446–11452.
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022.
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2008, 38, 253–278.
- Morales-García, Á.; Mayans-Llorach, M.; Viñes, F.; Illas, F. Thickness biased capture of CO2 on carbide MXenes. Phys. Chem. Chem. Phys. 2019, 21, 23136–23142.
- Sinopoli, A.; Othman, Z.; Rasool, K.; Mahmoud, K.A. Electrocatalytic/photocatalytic properties and aqueous media applications of 2D transition metal carbides (MXenes). Curr. Opin. Solid State Mater. Sci. 2019, 23, 100760.
- Chen, J.; Huang, Q.; Huang, H.; Mao, L.; Liu, M.; Zhang, X.; Wei, Y. Recent progress and advances in the environmental applications of MXene related materials. Nanoscale 2020, 12, 3574–3592.
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.-X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 155.
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horizons 2019, 5, 235–258.
- Chen, Y.; Liu, C.; Guo, S.; Mu, T.; Wei, L.; Lu, Y. CO2 capture and conversion to value-added products promoted by MXene-based materials. Green Energy Environ. 2020.
More
