Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Lindsay Dong and Version 2 by Beatrix Zheng.
Migraine is a primary headache disorder characterized by a unilateral, throbbing, pulsing headache, which lasts for hours to days, and the pain can interfere with daily activities. It exhibits various symptoms, such as nausea, vomiting, sensitivity to light, sound, and odors, and physical activity consistently contributes to worsening pain.
- primary headache
- migraine
- trigeminal system
- neuropeptides
- neurogenic inflammation
- animal model
- inflammatory soup
- dura mater
- immune system
- migraine treatment
1. Neurogenic Inflammation
The localized form of inflammation is neuroinflammation, which occurs in both the peripheral and CNSs. The main features of NI are the increased vascular permeability, leukocyte infiltration, glial cell activation, and increased production of inflammatory mediators, such as cytokines and chemokines [1]. NI increases the permeability of the blood to the brain barrier, thus allowing an increased influx of peripheral immune cells into the CNS [2] (Figure 1A).
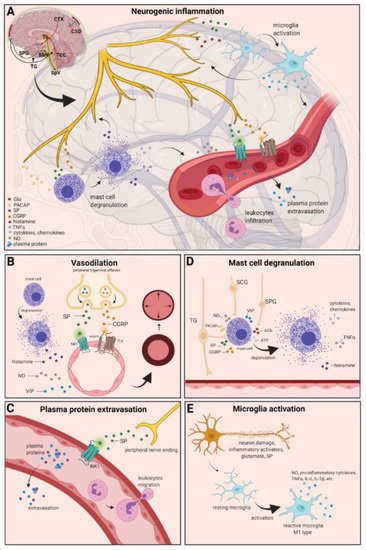
Figure 1. Neurogenic inflammation and its main features. (A) Stimulation of the trigeminal nerve causes the release of neuropeptides, including CGRP, SP, NO, VIP, and 5-HT, leading to neurogenic inflammation, which has four main features: the increased vascular permeability, leukocyte infiltration, glial cell activation, and increased production of inflammatory mediators, such as cytokines and chemokines. (B) Vasoactive peptides, such as CGRP and SP, bind their receptors on smooth muscle of dural vessels and cause vasodilation. The released neuropeptides induce mast cell degranulation, resulting in the release of histamine, which leads endothelium-dependent vasodilation. (C) Binding of the released SP to the NK1 receptors expressed on the microvascular blood vessels disrupts the membrane and causes plasma protein leakage and leukocyte extravasation. (D) Mast cells are in close association with neurons, especially in the dura, where they can be activated following trigeminal nerve and cervical or sphenopalatine ganglion stimulation. Release of neuropeptides causes mast cell degranulation, which leads to release of histamine and serotonin and selectively can cause the release of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6. (E) Under the influence of inflammatory stimuli, microglia can become reactive microglia. Microglia activation leads to the production of inflammatory mediators and cytotoxic mediators.
The concept of NI was introduced by the experiment of Goltz and Bayliss, in which skin vasodilation was observed during electrical stimulation of the dorsal horn, which could not be linked to the immune system [3][4]. Dalessio was the first who hypothesized a connection between NI and migraine and believed that a headache is a result of vasodilation of cranial vessels associated with local inflammation [5]. This theory was later reworked by Moskowitz, who believed that upon activation, the neuropeptide release from trigeminal neurons has a role in increasing vascular permeability and vasodilation [6].
There are several theories concerning the mechanism of NI. Hormonal fluctuations or cortical spreading depression can initiate two types of processes: activating the TS to trigger the liberation of neuropeptides from the peripheral trigeminal afferents and/or degranulating the mast cells that can lead to the release of neuropeptides by activating and sensitizing the nociceptors [7]. In rats, Bolay and colleagues demonstrated that after local electrical stimulation of the cerebral cortex, CSD is generated, and it can trigger trigeminal activation, which causes meningeal inflammation occurring after the CSD disappearance [8]. Both CGRP and SP play an important role in the development of NI. Released peptides, such as CGRP, bind to its receptor on smooth muscle cells, eliciting a vasodilatory response, thereby increasing meningeal blood flow in the dural vasculature. In contrast to CGRP, binding of the released SP to the NK1 receptors expressed on the microvascular blood vessels disrupts the membrane and causes plasma protein leakage. Both neuropeptides can induce mast cell degranulation through their specific receptors and further sensitize meningeal nociceptors [7]. The meningeal nerve fibers also contain neurotransmitters (e.g., glutamate, serotonin) and hormones (e.g., prostaglandins) that can affect the activation and release of neuropeptides, causing neurogenic inflammation [5]. Moreover, several cell types (e.g., endothelial cells, mast cells, and dendritic cells) can release tumor necrosis factor alpha (TNFα), interleukins, nerve-growth factor (NGF), and VIP, also causing plasma protein extravasation (PPE) [9][10], which is a key characteristic of NI. In addition, neuronal nitric oxide synthase (nNOS) enzyme can be detected in the trigeminal nerve endings, the dural mastocytes, and also the TNC and the TG [11], which catalyzes the synthesis of retrograde signaling molecule nitric oxide (NO). NO has a major role in mediating many aspects of inflammatory responses; NO can affect the release of various inflammatory mediators from cells participating in inflammatory responses (e.g., leukocytes, macrophages, mast cells, endothelial cells, and platelets) [12]. Through its retrograde signaling action, astrocytes can influence the release of CGRP, SP, and glutamate [13][14]. Beside this, bradykinin and histamine induce NO release from vascular endothelial cells, suggesting a strong interaction between NO and inflammation [15]. The inflammation can lead to CGRP release from the activated primary afferent neurons, which force satellite glial cells to release NO. NO can induce nNOS, which can be considered a significant marker of the sensitization process of the TS. TRPV channels permit afferent nerves to detect thermal, mechanical, and chemical stimuli, thereby regulating NI and nociception [16]. TRPV1 was identified in dorsal root ganglion (DRG), TG neurons, and spinal and peripheral nerve terminals [17]. Inflammatory mediators remarkably up-regulate TRPV1 through activation of phospholipase C (PLC) and protein kinase A (PKA) and protein kinase C (PKC) signaling pathways [18][19][20][21]. Increased TRPV1 expression in peripheral nociceptors contributes to maintaining inflammatory hyperalgesia [17]. In an experimental injury model, Vergnolle et al. demonstrated that a decrease in osmolarity of extracellular fluid could induce neurogenic inflammation, which TRPV4 can mediate [22]. Furthermore, plasma and cerebrospinal fluid levels of neuropeptides, histamine, proteases, and pro-inflammatory cytokines (e.g., TNFα, IL-1β) are elevated during migraine attacks [23][24], suggesting that neuroimmune interactions contribute to migraine pathogenesis.
1.1. Vasodilation
There are various cell types in blood vessels that both release and respond to numerous mediators that can contribute to migraine; this includes growth factors, cytokines, adenosine triphosphate (ATP), and NO [25][26][27][28]. In the central system, NO may be involved in the regulation of cerebral blood flow and neurotransmission [29]. NO can stimulate the release of neuropeptides and causes neurogenic vasodilation [30]. In addition to NO, NGF also increases the expression of CGRP and enhances the production and release of neuropeptides, including SP and CGRP, in sensory neurons [31]. CGRP, a potent vasodilator, is released from intracranial afferents during migraine attacks. This vasodilatory effect of CGRP is mediated by its action on CGRP receptors, which stimulates the adenyl cyclase and increases cyclic adenosine monophosphate (cAMP), thus producing potent vasodilation via the direct relaxation of vascular smooth muscle [32][33]. In response to prolonged noxious stimuli, SP is released from trigeminal sensory nerve fibers around dural blood vessels, leading to endothelium-dependent vasodilation [34]. VIP also contributes to neurogenic inflammation by inducing vasodilation [35] (Figure 1B).
1.2. Plasma Protein Extravasation
Another critical feature of neurogenic inflammation is PPE. Based on preclinical studies, the neurogenic PPE plays a role in the pathogenesis of migraine [36]. In several studies, following electrical stimulation of the trigeminal neurons or intravenous capsaicin, the peripheral nerve endings in the dural vasculature released SP, which caused plasma protein leakage and vasodilation through the NK-1 receptors [37]. Transduction of the SP signal through the NK1 receptor occurs via G protein signaling and the secondary messenger cAMP, ultimately leading to the regulation of ion channels, enzyme activity, and alterations in gene expression [38]. SP can indirectly influence plasma extravasation by activating mast cell degranulation, which results in histamine release [39]. In addition, NKA is able to induce plasma protein efflux and activate inflammatory cells [40] (Figure 1C).
1.3. Mast Cell Degranulation
It is well known that dural mast cells play a role in the pathophysiology of migraine [41]. Meningeal mast cells are in close association with neurons, especially in the dura, where they can be activated following trigeminal nerve and cervical or sphenopalatine ganglion stimulation [42]. The release of neuropeptides, such as CGRP, PACAP, and SP, from meningeal nociceptors can cause the degranulation of mast cells [43], resulting in the release of histamine and serotonin and selectively can cause the release of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6 [44][45][46]. The plasma and CSF levels of these mediators (e.g., CGRP, TNFα, and IL-1β) are enhanced during migraine attacks [20]. VIP promotes degranulation of mast cells [47], similar to the effects of SP [39]. It was found that CSD can induce intracranial mast cell degranulation and promote the activation of meningeal nociceptors [48][49]. Besides these, according to several studies, mast cells can be activated by acute stress [42][50][51], which is known to precipitate or exacerbate migraines [52][53]. Based on these findings, mast cells in themselves may promote a cascade of associated inflammatory events resulting in trigeminovascular activation (Figure 1D).
1.4. Microglia Activation
Microglia appears in the CNS and can exert neuroprotective and neurotoxic effects as well. Under the influence of inflammatory stimuli, microglia can become efficient mobile effector cells [54]. Microglia activation leads the production of inflammatory mediators and cytotoxic mediators (e.g., NO, reactive oxygen species, prostaglandins) [55][56], which might disrupt the integrity of the blood brain barrier, thereby allowing leukocyte migration into the brain [57]. Microglia express receptors for neurotransmitters, such as glutamate, gamma- aminobutyric acid, noradrenaline, purines, and dopamine [58]. It has been described that activation of ion channels is related to the activation of microglia; therefore, neurotransmitters probably influence microglia function [59]. Glutamate leads to neuronal death but is also an activation signal for microglia [60]. Activation of glutamate receptors causes the release of TNF-α, which, with microglia-derived Fas ligand, leads to neurotoxicity [61]. Besides this, Off signals from neurons appear important in maintaining tissue homeostasis and limiting microglia activity under inflammatory conditions, presumably preventing damage to intact parts of the brain [62]. Endothelin B-receptor-mediated regulation of astrocytic activation was reported to improve brain disorders, such as neuropathic pain [63]. SP also directly activates microglia and astrocytes and contributes to microglial activation [64][65], initiating signaling via the nuclear factor kappa B pathway, leading to pro-inflammatory cytokines production [66] (Figure 1E).
1.5. Cytokines, Chemokines
Cytokines are small proteins produced by most cells in the body, which possess multiple biologic activities to promote cell-cell interaction [67]. There is evidence that cytokines play an important role in several physiological and pathological settings, such as immunology, inflammation, and pain. [68]. The most important pro-inflammatory cytokines include IL-1, IL-6, and TNFα, and the key chemokine is IL-8 [68]. Cytokines and chemokines are released by neurons, microglia, astrocytes, macrophages, and T cells, and these factors might activate nociceptive neurons [69]. TNFα can trigger tissue edema and immune cell infiltration [70] and can influence the reactivity of signal nociceptors to the brain and increase blood levels during headaches, playing a crucial role in the genesis of migraine [71]. Cytokines are considered to be pain mediators in neurovascular inflammation, which generates migraine pain [72]. They can induce sterile inflammation of meningeal blood vessels in migraines [73]. Besides this, elevated levels of chemokines can stimulate the activation of trigeminal nerves and the release of vasoactive peptides; thereby, they can induce inflammation [74]. Based on these, cytokines and chemokines might contribute to migraine.
2. Current Treatments and Advances in Preclinical Research
Triptans are widely used to relieve migraine attacks; acting as agonists on 5-hydroxytryptamine receptors (5-HT1B/1D), they can cause the constriction of dilated cranial arteries and the inhibition of CGRP release [75]. In an animal model of migraine, after electrical stimulation of the TG, sumatriptan attenuates PPE by preventing the release of CGRP [76]. In knockout mice and guinea pigs, it has been shown that 5-HT1D receptors have a role in the inhibition of neuropeptide release, thereby modifying the dural neurogenic inflammatory response [77]. The use of triptans is limited by their vasoconstrictive properties. As triptans are not effective in everyone, they often lead to medication overuse, triggering migraine to become chronic (Table 1).
Table 1. Current treatments and advances in preclinical research.
| Drug Class | Drug | Target | FDA Appoved |
|---|---|---|---|
| NSAIDs | Acetylsalicylic acid | COX1–2 | yes |
| Ibuprofen | yes | ||
| Diclofenac potassium | yes | ||
| Paracetamol | yes | ||
| Triptans | Sumatriptan | 5-HT1D receptor | yes |
| Zolmitriptan | yes | ||
| Almotriptan | yes | ||
| Rizatriptan | yes | ||
| Frovatriptan | yes | ||
| Naratriptan | yes | ||
| Eletriptan | 5-HT1B/1D receptor | yes | |
| Ditans | Lasmiditan | 5-HT1F receptor | yes |
| Gepants | Ubrogepant | CGRP receptor | yes |
| Rimegepant | yes | ||
| Atogepant | no | ||
| Vazegepant | no | ||
| Ergot alkaloids | Ergotamine tartrate | α-adrenergic receptor,5-HT receptors | yes |
| CGRP/CGRP receptor monoclonal antibody | Erenumab | CGRP receptor | yes |
| Eptinezumab | CGRP ligand | yes | |
| Fremanezumab | yes | ||
| Galcenezumab | yes | ||
| NK1R antagonists | Aprepitant | NK1 receptor | yes |
| PACAP/PAC1 receptor monoclonal antibody | ALD1910 | PACAP38 | no |
| AMG-301 | PAC1 receptor | no | |
| Endocannabinoids | 2-Arachidonoylglycerol | CB1 receptor | no |
| Anandamide | CB1 receptor | no |
Ditans target the 5-HT1F receptor, which is expressed in the cortex, the hypothalamus, the trigeminal ganglia, the locus coeruleus, the middle cerebral artery, and the upper cervical cord. Lasmiditan is the first drug approved for clinical use. Contrary to triptans, Lasmiditan does not cause vasoconstriction. The activation of 5-HT1F receptor inhibits the release of CGRP and probably SP from the peripheral trigeminal endings of the dura and acts on the trigeminal nucleus caudalis or the thalamus [78].
Besides triptans and ditans, acute treatments of migraine headaches, i.e., ergot alkaloids and nonsteroidal anti-inflammatory agents (NSAIDs), may decrease the neurogenic inflammatory response [79]. NSAIDs have anti-inflammatory, analgesic, and anti-pyretic properties. Their primary effect is to block the enzyme cyclooxygenase and hence mitigate prostaglandin synthesis from arachidonic acid [80]. Both acetaminophen and ibuprofen, which can reduce pain intensity, can also be used in children. Magnesium pretreatment increases the effectiveness of these treatments and reduces the frequency of pain [81]. Ergotamine has been recommended to abort migraine attacks by eliminating the constriction of dilated cranial blood vessels and carotid arteriovenous anastomoses, reducing CGRP release from perivascular trigeminal nerve endings, and inhibit the nociceptive transmission on peripheral and central ends of trigeminal sensory nerves [82].
An alternative treatment strategy is the use of CGRP-blocking monoclonal antibodies. Monoclonal antibodies have a number of positive properties: (1) a long half-life, (2) long duration of action, and (3) high specificity [83]. Four monoclonal antibodies are currently developing for migraine prevention: three against CGRP and one against the CGRP receptor. The safety and tolerability of these antibodies are promising; no clinically significant change in vitals, ECGs, or hepatic enzymes was observed. Blocking of CGRP function by monoclonal antibodies has demonstrated efficacy in the prevention of migraine with minimal side effects in multiple Phase II and III clinical trials [84].
Another alternative approach to treating the migraine attack by limiting neurogenic inflammatory vasodilation is the blockade of CGRP receptors by selective antagonists. Gepants were designed to treat acute migraines [85]. These bind to CGRP receptors and reverse CGRP-induced vasodilation but were not vasoconstrictors per se [86]. Based on these, gepants may be an alternative if triptans are contraindicated. Currently, two gepants (Ubrogepant, Rimegepant) are available on the market, but several are in development.
In gene-knockout studies, the hypothesis the tachykinins are the primary mediators of the PPE component of NI has been strengthened [87][88]. Following topical application of capsaicin to the ear, the PPE was decreased in Tac1-deficient mice compared to wild-type mice [89]. Following activation of the trigeminal system by chemical, mechanical, or electrical stimulation, tachykinin Receptor 1 (TACR1) antagonists seem to be adequate to blocking dural PPE [90]. However, lanepitant, a selective TACR1 antagonist, has no significant effect on migraine-associated symptoms [91]; moreover, it was found ineffective in a migraine prevention study [92]. The only currently available and clinically approved NK1 receptor antagonist is aprepitant, which is used as an antiemetic to chemotherapy-induced nausea in cancer patients [93].
In animal models, blockage of TRPV1 receptors was effective to reverse inflammatory pain; however, TRPV1 antagonists produce some serious side effects, e.g., hyperthermia [94]. Clinical data suggest that TRPV1 antagonists might be effective as therapeutic options for certain conditions, such as migraine and pain related to several types of diseases. Hopefully, current clinical trials with TRPV1 receptor antagonists and future studies provide an answer as to the role of TRPV1 in inflammatory and neuropathic pain syndromes.
The anti-nociceptive effects of endocannabinoids are thought to be mediated mainly through the activation of cannabinoid receptor type 1 (CB1) [95]. Localization of CB1 receptors along the trigeminal tract and trigeminal afferents [96][97] suggests that the endocannabinoid system can modulate the neurogenic-induced migraine [98]. Clinical data suggested that in migraine patients, the endocannabinoid levels are lower [99][100]. In animal models of migraine, endocannabinoids can reduce neurogenic inflammation. Akerman et al. reported that capsaicin-induced vasodilation is less after intravenous administration of anandamide (AEA); in addition, AEA significantly prevented CGRP- and NO-induced vasodilation in the dura [101]. In a previous study, Nagy-Grócz and colleagues observed that NTG and AEA alone or combined treatment of them affects 5-HTT expression, which points out a possible interaction between the serotonergic and endocannabinoid system on the NTG-induced trigeminal activation and sensitization phenomenon, which are essential during migraine attacks [102]. These results raise the possibility that the AEA has a CB1 receptor-mediated inhibitory effect on neurogenic vasodilation of trigeminal blood vessels. Based on these, anandamide may be a potential therapeutic target for migraine. Besides these, the presence of CB1 receptors in the brain makes them a target for the treatment of migraine, blocking not only peripheral but also central nociceptive traffic and reducing CSD. CB2 receptors in immune cells may be targeted to reduce the inflammatory component associated with migraine.
PACAP and its G-protein-coupled receptors, pituitary adenylate cyclase 1 receptor (PAC1) and vasoactive intestinal peptide receptor 1/2 (VPAC1/2), are involved in various biological processes. Activation of PACAP receptors has an essential role in the pathophysiology of primary headache disorders, and PACAP plays an excitatory role in migraine [103]. There are two pharmacology options to inhibit PACAP: PAC1 receptor antagonists/antibodies directed against the receptor or antibodies directed against the peptide PACAP [104]. Studies of the PAC1 receptor antagonist PACAP (6–38) have proved that antagonism of this receptor may be beneficial even during migraine progression [105]. PACAP38 and PAC1 receptor blockade are promising antimigraine therapies, but results from clinical trials are needed to confirm their efficacy and side effect profile.
The tryptophan-kynurenine metabolic pathway (KP) is gaining growing attention in search of potential biomarkers and possible therapeutic targets in various illnesses, including migraine [106][107]. KYNA is a neuroactive metabolite of the KP, which affects several glutamate receptors, playing a relevant role in pain processing and neuroinflammation [78]. KYNA may block the activation of trigeminal neurons, affect the migraine generators, and modulate the generation of CSD [106][108]. An abnormal decrease or increase in the KYNA level can cause an imbalance in the neurotransmitter systems, and it is associated with several neurodegenerative and neuropsychiatric disorders [109][110][111][112]. Based on human and animal data, the KP is downregulated under different headaches; thus, possibly less KYNA is produced [113]. It is consistent with the theory of hyperactive NMDA receptors, which play a key role in the development of central sensitization [114] and thus in migraine pathophysiology. In an NTG-induced rodent model of migraine, Nagy-Grócz et al. demonstrated a decrease in the expression of KP enzymes after NTG administration in rat TNC [115]. Interferons can control the transcription expression of indoleamine 2,3-dioxygenase (IDO), kynurenine 3-monooxygenase (KMO), and kynureninase (KYNU); therefore, the pro-inflammatory cytokines may affect the kynurenine pathway [116]. It is difficult for KYNA to cross the blood-brain barrier (BBB); therefore, synthetic KYNA analogs may provide an additional alternative for synthesizing compounds that have neuroprotective effects comparable to KYNA that can cross the BBB effectively. Preclinical studies have shown the effectiveness of KYNA analogs in animal models of dural stimulation [117][118]. Further preclinical studies are required to understand the role of KYNA analogs in migraine and clinical studies that assess their effectiveness in acute or prophylactic treatment (Figure 2).
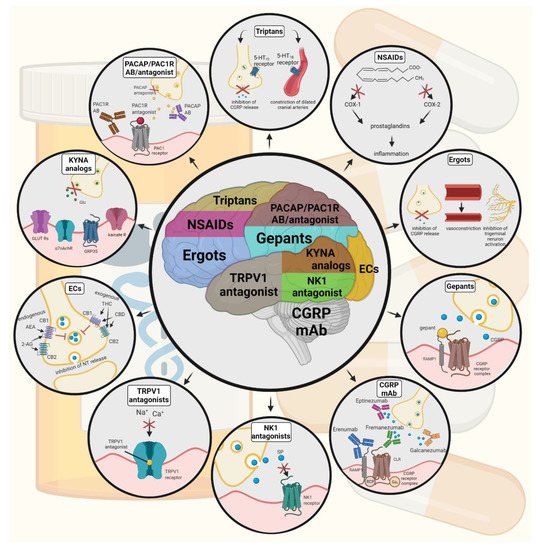

Figure 2. Possible treatments of neurogenic inflammation and migraine. NSAIDs, non-steroidal anti-inflammatory drugs; 5-HT, serotonin; CGRP, calcitonin gene-related peptide; COX, cyclooxygenase; Ab, antibody; NK1, neurokinin 1; TRPV1, transient receptor potential vanilloid receptor; SP, substance P; EC, endocannabinoids; AEA, anandamide; 2-AG, 2-arachidonoylglycerol; CB, cannabinoid receptor; THC, tetrahidrokanabinol; CBD, cannabidiol; NT, neurotransmitter; GLUT R, glutamate receptors; α7AchR, alpha-7 nicotinic receptor; GPR35, G protein-coupled receptor 35; PACAP, pituitary adenylate cyclase-activating polypeptide; PAC1R, pituitary adenylate cyclase 1 receptor.
In animal models of chronic pain and inflammation and several clinical trials, palmitoylethanolamide (PEA), endogenous fatty acid amide, has been influential on various pain states [119][120][121]. In a pilot study, for patients suffering from migraine with aura, ultra-micronized PEA treatment has been shown effective and safe [122]. Based on these, PEA is a new therapeutic option in the treatment of pain and inflammatory conditions.
References
- Goadsby, P.J.; Knight, Y.E.; Hoskin, K.L.; Butler, P. Stimulation of an intracranial trigeminally-innervated structure selectively increases cerebral blood flow. Brain Res. 1997, 751, 247–252.
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548.
- Goltz, F. Uber gefasserweiternde nerven. Pflueger Arch. Ges. Physiol. 1874, 9, 185.
- Bayliss, W.M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J. Physiol. 1901, 26, 173–209.
- Dalessio, D.J. Vascular permeability and vasoactive substances: Their relationship to migraine. Adv. Neurol. 1974, 4, 395–401.
- Moskowitz, M.A. The neurobiology of vascular head pain. Ann. Neurol. 1984, 16, 157–168.
- Ramachandran, R. Neurogenic inflammation and its role in migraine. Semin. Immunopathol. 2018, 40, 301–314.
- Bolay, H.; Reuter, U.; Dunn, A.K.; Huang, Z.; Boas, D.A.; Moskowitz, M.A. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 2002, 8, 136–142.
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53.
- Tajti, J.; Szok, D.; Majláth, Z.; Tuka, B.; Csáti, A.; Vécsei, L. Migraine and neuropeptides. Neuropeptides 2015, 52, 19–30.
- Berger, R.J.; Zuccarello, M.; Keller, J.T. Nitric oxide synthase immunoreactivity in the rat dura mater. Neuroreport 1994, 5, 519–521.
- Wallace, J.L. Nitric oxide as a regulator of inflammatory processes. Mem. Inst. Oswaldo Cruz 2005, 100 (Suppl. 1), 5–9.
- Bellamy, J.; Bowen, E.J.; Russo, A.F.; Durham, P.L. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur. J. Neurosci. 2006, 23, 2057–2066.
- Strecker, T.; Dux, M.; Messlinger, K. Increase in meningeal blood flow by nitric oxide--interaction with calcitonin gene-related peptide receptor and prostaglandin synthesis inhibition. Cephalalgia 2002, 22, 233–241.
- Leston, J.M. Anatomie fonctionnelle du nerf trijumeau . Neurochirurgie 2009, 55, 99–112. (In French)
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP channels: An overview. Cell Calcium 2005, 38, 233–252.
- Planells-Cases, R.; Garcìa-Sanz, N.; Morenilla-Palao, C.; Ferrer-Montiel, A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005, 451, 151–159.
- Bhave, G.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W., Iv. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 2002, 35, 721–731.
- Crandall, M.; Kwash, J.; Yu, W.; White, G. Activation of protein kinase C sensitizes human VR1 to capsaicin and to moderate decreases in pH at physiological temperatures in Xenopus oocytes. Pain 2002, 98, 109–117.
- Premkumar, L.S.; Ahern, G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature 2000, 408, 985–990.
- Vellani, V.; Mapplebeck, S.; Moriondo, A.; Davis, J.B.; McNaughton, P.A. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 2001, 534 Pt 3, 813–825.
- Vergnolle, N.; Cenac, N.; Altier, C.; Cellars, L.; Chapman, K.; Zamponi, G.W.; Materazzi, S.; Nassini, R.; Liedtke, W.; Cattaruzza, F.; et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br. J. Pharmacol. 2010, 159, 1161–1173.
- Perini, F.; D’Andrea, G.; Galloni, E.; Pignatelli, F.; Billo, G.; Alba, S.; Bussone, G.; Toso, V. Plasma cytokine levels in migraineurs and controls. Headache 2005, 45, 926–931.
- Sarchielli, P.; Alberti, A.; Baldi, A.; Coppola, F.; Rossi, C.; Pierguidi, L.; Floridi, A.; Calabresi, P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006, 46, 200–207.
- Jacobs, B.; Dussor, G. Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 2016, 338, 130–144.
- Breier, G.; Risau, W. The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol. 1996, 6, 454–456.
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666.
- Mantovani, A.; Sozzani, S.; Introna, M. Endothelial activation by cytokines. Ann. N. Y. Acad. Sci. 1997, 832, 93–116.
- Malhotra, R. Understanding migraine: Potential role of neurogenic inflammation. Ann. Indian Acad. Neurol. 2016, 19, 175–182.
- Yonehara, N.; Yoshimura, M. Effect of nitric oxide on substance P release from the peripheral endings of primary afferent neurons. Neurosci. Lett. 1999, 271, 199–201.
- Price, T.J.; Louria, M.D.; Candelario-Soto, D.; Dussor, G.O.; Jeske, N.A.; Patwardhan, A.M.; Diogenes, A.; Trott, A.A.; Hargreaves, K.M.; Flores, C.M. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: Effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005, 6, 4.
- Miyoshi, H.; Nakaya, Y. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Res. Cardiol. 1995, 90, 332–336.
- Hanko, J.; Hardebo, J.E.; Kåhrström, J.; Owman, C.; Sundler, F. Calcitonin gene-related peptide is present in mammalian cerebrovascular nerve fibres and dilates pial and peripheral arteries. Neurosci. Lett. 1985, 57, 91–95.
- Heatley, R.V.; Denburg, J.A.; Bayer, N.; Bienenstock, J. Increased plasma histamine levels in migraine patients. Clin. Allergy 1982, 12, 145–149.
- Wilkins, B.W.; Chung, L.H.; Tublitz, N.J.; Wong, B.J.; Minson, C.T. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J. Appl. Physiol. (1985) 2004, 97, 1291–1298.
- Williamson, D.J.; Hargreaves, R.J. Neurogenic inflammation in the context of migraine. Microsc. Res. Tech. 2001, 53, 167–178.
- Markowitz, S.; Saito, K.; Moskowitz, M.A. Neurogenically mediated plasma extravasation in dura mater: Effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia 1988, 8, 83–91.
- Lundy, F.T.; Linden, G.J. Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Crit. Rev. Oral Biol. Med. 2004, 15, 82–98.
- Foreman, J.C.; Jordan, C.C.; Oehme, P.; Renner, H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J. Physiol. 1983, 335, 449–465.
- De Swert, K.O.; Joos, G.F. Extending the understanding of sensory neuropeptides. Eur. J. Pharmacol. 2006, 533, 171–181.
- Koyuncu Irmak, D.; Kilinc, E.; Tore, F. Shared Fate of Meningeal Mast Cells and Sensory Neurons in Migraine. Front. Cell Neurosci. 2019, 13, 136.
- Theoharides, T.C.; Spanos, C.; Pang, X.; Alferes, L.; Ligris, K.; Letourneau, R.; Rozniecki, J.J.; Webster, E.; Chrousos, G.P. Stress-induced intracranial mast cell degranulation: A corticotropin-releasing hormone-mediated effect. Endocrinology 1995, 136, 5745–5750.
- Rozniecki, J.J.; Dimitriadou, V.; Lambracht-Hall, M.; Pang, X.; Theoharides, T.C. Morphological and functional demonstration of rat dura mater mast cell-neuron interactions in vitro and in vivo. Brain Res. 1999, 849, 1–15.
- Theoharides, T.C.; Donelan, J.; Kandere-Grzybowska, K.; Konstantinidou, A. The role of mast cells in migraine pathophysiology. Brain Res. Brain Res. Rev. 2005, 49, 65–76.
- Schwartz, L.B. Mediators of human mast cells and human mast cell subsets. Ann. Allergy 1987, 58, 226–235.
- Aich, A.; Afrin, L.B.; Gupta, K. Mast Cell-Mediated Mechanisms of Nociception. Int. J. Mol. Sci. 2015, 16, 29069–29092.
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta. 2012, 1822, 21–33.
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013, 339, 1092–1095.
- Zhao, J.; Levy, D. Modulation of intracranial meningeal nociceptor activity by cortical spreading depression: A reassessment. J. Neurophysiol. 2015, 113, 2778–2785.
- Kempuraj, D.; Selvakumar, G.P.; Thangavel, R.; Ahmed, M.E.; Zaheer, S.; Raikwar, S.P.; Iyer, S.S.; Bhagavan, S.M.; Beladakere-Ramaswamy, S.; Zaheer, A. Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer’s Disease Pathogenesis. Front. Neurosci. 2017, 11, 703.
- Baldwin, A.L. Mast cell activation by stress. Methods Mol. Biol. 2006, 315, 349–360.
- Sauro, K.M.; Becker, W.J. The stress and migraine interaction. Headache 2009, 49, 1378–1386.
- Radat, F. Stress et migraine . Rev. Neurol (Paris) 2013, 169, 406–412.
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758.
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394.
- Colton, C.A.; Gilbert, D.L. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987, 223, 284–288.
- de Vries, H.E.; Blom-Roosemalen, M.C.; van Oosten, M.; de Boer, A.G.; van Berkel, T.J.; Breimer, D.D.; Kuiper, J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 1996, 64, 37–43.
- Färber, K.; Kettenmann, H. Physiology of microglial cells. Brain Res. Brain Res. Rev. 2005, 48, 133–143.
- Pannasch, U.; Färber, K.; Nolte, C.; Blonski, M.; Yan Chiu, S.; Messing, A.; Kettenmann, H. The potassium channels Kv1.5 and Kv1.3 modulate distinct functions of microglia. Mol. Cell Neurosci. 2006, 33, 401–411.
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535.
- Taylor, D.L.; Jones, F.; Kubota, E.S.; Pocock, J.M. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. 2005, 25, 2952–2964.
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602.
- Koyama, Y. Endothelin ETB Receptor-Mediated Astrocytic Activation: Pathological Roles in Brain Disorders. Int. J. Mol. Sci. 2021, 22, 4333.
- Fiebich, B.L.; Schleicher, S.; Butcher, R.D.; Craig, A.; Lieb, K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J. Immunol. 2000, 165, 5606–5611.
- Lin, R.C. Reactive astrocytes express substance-P immunoreactivity in the adult forebrain after injury. Neuroreport 1995, 7, 310–312.
- Carthew, H.L.; Ziebell, J.M.; Vink, R. Substance P-induced changes in cell genesis following diffuse traumatic brain injury. Neuroscience 2012, 214, 78–83.
- Bruno, P.P.; Carpino, F.; Carpino, G.; Zicari, A. An overview on immune system and migraine. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 245–248.
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014, 1843, 2563–2582.
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019, 15, 483–490.
- Roach, D.R.; Bean, A.G.; Demangel, C.; France, M.P.; Briscoe, H.; Britton, W.J. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 2002, 168, 4620–4627.
- Conti, P.; D’Ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur. J. Pharmacol. 2019, 844, 87–94.
- Empl, M.; Sostak, P.; Riedel, M.; Schwarz, M.; Müller, N.; Förderreuther, S.; Straube, A. Decreased sTNF-RI in migraine patients? Cephalalgia 2003, 23, 55–58.
- Mueller, L.; Gupta, A.K.; Stein, T.P. Deficiency of tumor necrosis factor alpha in a subclass of menstrual migraineurs. Headache 2001, 41, 129–137.
- Christopherson, K., II; Hromas, R. Chemokine regulation of normal and pathologic immune responses. Stem Cells 2001, 19, 388–396.
- Saxena, P.R.; De Vries, P.; Villalón, C.M. 5-HT1-like receptors: A time to bid goodbye. Trends Pharmacol. Sci. 1998, 19, 311–316.
- Buzzi, M.G.; Moskowitz, M.A. The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br. J. Pharmacol. 1990, 99, 202–206.
- Cutrer, F.M.; Yu, X.J.; Ayata, G.; Moskowitz, M.A.; Waeber, C. Effects of PNU-109,291, a selective 5-HT1D receptor agonist, on electrically induced dural plasma extravasation and capsaicin-evoked c-fos immunoreactivity within trigeminal nucleus caudalis. Neuropharmacology 1999, 38, 1043–1053.
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert Opin. Pharmacother. 2021, 22, 1221–1225.
- Moskowitz, M.A. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol. Sci. 1992, 13, 307–311.
- Pardutz, A.; Schoenen, J. NSAIDs in the Acute Treatment of Migraine: A Review of Clinical and Experimental Data. Pharmaceuticals 2010, 3, 1966–1987.
- Gallelli, L.; Avenoso, T.; Falcone, D.; Palleria, C.; Peltrone, F.; Esposito, M.; De Sarro, G.; Carotenuto, M.; Guidetti, V. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache 2014, 54, 313–324.
- Goadsby, P.J.; Lipton, R.B.; Ferrari, M.D. Migraine--current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270.
- Tso, A.R.; Goadsby, P.J. Anti-CGRP Monoclonal Antibodies: The Next Era of Migraine Prevention? Curr. Treat. Options Neurol. 2017, 19, 27.
- Yuan, H.; Lauritsen, C.G.; Kaiser, E.A.; Silberstein, S.D. CGRP Monoclonal Antibodies for Migraine: Rationale and Progress. BioDrugs 2017, 31, 487–501.
- Negro, A.; Martelletti, P. Novel synthetic treatment options for migraine. Expert Opin. Pharmacother. 2021, 22, 907–922.
- Olesen, J.; Diener, H.C.; Husstedt, I.W.; Goadsby, P.J.; Hall, D.; Meier, U.; Pollentier, S.; Lesko, L.M. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004, 350, 1104–1110.
- Peroutka, S.J. Neurogenic inflammation and migraine: Implications for the therapeutics. Mol. Interv. 2005, 5, 304–311.
- Zimmer, A.; Zimmer, A.M.; Baffi, J.; Usdin, T.; Reynolds, K.; König, M.; Palkovits, M.; Mezey, E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc. Natl. Acad. Sci. USA 1998, 95, 2630–2635.
- Cao, Y.Q.; Mantyh, P.W.; Carlson, E.J.; Gillespie, A.M.; Epstein, C.J.; Basbaum, A.I. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 1998, 392, 390–394.
- Lee, W.S.; Moussaoui, S.M.; Moskowitz, M.A. Blockade by oral or parenteral RPR 100893 (a non-peptide NK1 receptor antagonist) of neurogenic plasma protein extravasation within guinea-pig dura mater and conjunctiva. Br. J. Pharmacol. 1994, 112, 920–924.
- Goldstein, D.J.; Wang, O.; Saper, J.R.; Stoltz, R.; Silberstein, S.D.; Mathew, N.T. Ineffectiveness of neurokinin-1 antagonist in acute migraine: A crossover study. Cephalalgia 1997, 17, 785–790.
- Goldstein, D.J.; Offen, W.W.; Klein, E.G.; Phebus, L.A.; Hipskind, P.; Johnson, K.W.; Ryan, R.E., Jr. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia 2001, 21, 102–106.
- Herrstedt, J.; Muss, H.B.; Warr, D.G.; Hesketh, P.J.; Eisenberg, P.D.; Raftopoulos, H.; Grunberg, S.M.; Gabriel, M.; Rodgers, A.; Hustad, C.M.; et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer 2005, 104, 1548–1555.
- Jara-Oseguera, A.; Simon, S.A.; Rosenbaum, T. TRPV1: On the road to pain relief. Curr. Mol. Pharmacol. 2008, 1, 255–269.
- Leimuranta, P.; Khiroug, L.; Giniatullin, R. Emerging Role of (Endo)Cannabinoids in Migraine. Front. Pharmacol. 2018, 9, 420.
- Tsou, K.; Brown, S.; Sañudo-Peña, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998, 83, 393–411.
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564.
- Russo, E.B. Clinical endocannabinoid deficiency (CECD): Can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro. Endocrinol. Lett. 2004, 25, 31–39.
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Piomelli, D.; Tassorelli, C. Endocannabinoid System and Migraine Pain: An Update. Front. Neurosci. 2018, 12, 172.
- Cupini, L.M.; Bari, M.; Battista, N.; Argiró, G.; Finazzi-Agró, A.; Calabresi, P.; Maccarrone, M. Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia 2006, 26, 277–281.
- Akerman, S.; Kaube, H.; Goadsby, P.J. Anandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociception. J. Pharmacol. Exp. Ther. 2004, 309, 56–63.
- Nagy-Grócz, G.; Bohár, Z.; Fejes-Szabó, A.; Laborc, K.F.; Spekker, E.; Tar, L.; Vécsei, L.; Párdutz, Á. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J. Chem. Neuroanat. 2017, 85, 13–20.
- Tajti, J.; Tuka, B.; Botz, B.; Helyes, Z.; Vécsei, L. Role of pituitary adenylate cyclase-activating polypeptide in nociception and migraine. CNS Neurol. Disord. Drug Targets 2015, 14, 540–553.
- Rubio-Beltrán, E.; Correnti, E.; Deen, M. PACAP38 and PAC1 receptor blockade: A new target for headache? J. Headache Pain 2018, 19, 64.
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778.
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338.
- Liao, C.; de Molliens, M.P.; Schneebeli, S.T.; Brewer, M.; Song, G.; Chatenet, D.; Braas, K.M.; May, V.; Li, J. Targeting the PAC1 Receptor for Neurological and Metabolic Disorders. Curr. Top. Med. Chem. 2019, 19, 1399–1417.
- Fejes, A.; Párdutz, Á.; Toldi, J.; Vécsei, L. Kynurenine metabolites and migraine: Experimental studies and therapeutic perspectives. Curr. Neuropharmacol. 2011, 9, 376–387.
- Tajti, J.; Majlath, Z.; Szok, D.; Csati, A.; Toldi, J.; Fülöp, F.; Vécsei, L. Novel kynurenic acid analogues in the treatment of migraine and neurodegenerative disorders: Preclinical studies and pharmaceutical design. Curr. Pharm. Des. 2015, 21, 2250–2258.
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431.
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734.
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Trypto-phan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897.
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2015, 17, 47.
- Sarchielli, P.; Di Filippo, M.; Nardi, K.; Calabresi, P. Sensitization, glutamate, and the link between migraine and fibromyalgia. Curr. Pain Headache Rep. 2007, 11, 343–351.
- Nagy-Grócz, G.; Laborc, K.F.; Veres, G.; Bajtai, A.; Bohár, Z.; Zádori, D.; Fejes-Szabó, A.; Spekker, E.; Vécsei, L.; Párdutz, Á. The Effect of Systemic Nitroglycerin Administration on the Kynurenine Pathway in the Rat. Front. Neurol. 2017, 14, 278.
- Mándi, Y.; Vécsei, L. The kynurenine system and immunoregulation. J. Neural Trans. 2012, 119, 197–209.
- Lukács, M.; Tajti, J.; Fülöp, F.; Toldi, J.; Edvinsson, L.; Vécsei, L. Migraine, Neurogenic Inflammation, Drug Development - Pharmacochemical Aspects. Curr. Med. Chem. 2017, 24, 3649–3665.
- Jovanovic, F.; Candido, K.D.; Knezevic, N.N. The Role of the Kynurenine Signaling Pathway in Different Chronic Pain Conditions and Potential Use of Therapeutic Agents. Int. J. Mol. Sci. 2020, 21, 6045.
- Hesselink, J.M.K. New targets in pain, non-neuronal cells, and the role of palmitoylethanolamide. Open Pain J. 2012, 5, 2–23.
- Chirchiglia, D.; Paventi, S.; Seminara, P.; Cione, E.; Gallelli, L. N-Palmitoyl Ethanol Amide Pharmacological Treatment in Patients With Nonsurgical Lumbar Radiculopathy. J. Clin. Pharmacol. 2018, 58, 733–739.
- Chirchiglia, D.; Della Torre, A.; Signorelli, F.; Volpentesta, G.; Guzzi, G.; Stroscio, C.A.; Deodato, F.; Gabriele, D.; Lavano, A. Administration of palmitoylethanolamide in combination with topiramate in the preventive treatment of nummular headache. Int Med. Case Rep. J. 2016, 18, 193–195.
- Chirchiglia, D.; Cione, E.; Caroleo, M.C.; Wang, M.; Di Mizio, G.; Faedda, N.; Giacolini, T.; Siviglia, S.; Guidetti, V.; Gallelli, L. Effects of Add-On Ultramicronized N-Palmitol Ethanol Amide in Patients Suffering of Migraine With Aura: A Pilot Study. Front. Neurol. 2018, 17, 674.
More
