You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Brandon Lucke-Wold and Version 3 by Vicky Zhou.
High altitude illness in its most severe form can lead to high altitude cerebral edema (HACE). The strategies have focused on prevention with graduated ascents, pharmacologic prophylaxis, and descent at first signs of symptoms. Little is understood regarding treatment with steroids and oxygenation being commonly utilized. Pre-clinical studies with turmeric derivatives have offered promise due to its anti-inflammatory and antioxidant properties, but they warrant validation clinically.
- acute mountain sickness
- high altitude cerebral edema
- prevention
- glymphatic system
1. Introduction
High altitude illness (HAI) encompasses a group of conditions that are thought to occur secondary to hypoxemia, which develops at high altitude environments [1][2][1,2]. High altitude cerebral edema (HACE) is considered to be a HAI in one of its most severe forms [1][3][1,3]. It is a rare, yet potentially fatal, neurologic condition that warrants prompt attention and medical management [1].
Established risk factors for HACE include rapid ascent, inadequate acclimatization, extreme altitudes, physical exertion, and a prior history of HAI [4]. Among those traveling to high altitudes, such as tourists, trekkers and mountain climbers, the estimated incidence of HACE is reported to be approximately 0.5–1% [4][5][6][4,5,6]. Although less common, HACE also occurs in high-altitude residents and well-acclimatized climbers, most often in the setting of increased ascension, heavy physical exertion, or ingestion of substances, such as alcohol [7]. In addition to such risk factors, the presence of HACE in well-acclimatized populations could also be secondary to a “re-entry” phenomenon, as postulated to occur in high altitude pulmonary edema [8][9][8,9]. Although not yet studied in the setting of HACE, individuals well acclimatized to high altitudes may be primed to undergo abrupt physiologic adaptions that predispose to HACE upon return from excursions to lower altitude environments.
Patients with HACE present with encephalopathy and declining neurologic function, such as altered mental status, slurred speech, cranial nerve palsies, papilledema, and ataxia [3][10][3,10]. It most often occurs following prolonged exposure to altitudes greater than 4000 m, although HACE at lower altitudes have been reported [3][6][10][3,6,10]. Acute mountain sickness (AMS) is another important form of HAI that is often found to precede HACE (Figure 1) [4][11][4,11]. It is characterized by non-specific symptoms, such as headache, confusion, dizziness, nausea, and vomiting, and presents at altitudes lower than those observed in HACE [3]. The symptoms of AMS peak around 24–48 h, around the time that HACE develops [1][11][12][1,11,12]. Due to disease time course and symptomatology, HACE is often proposed to be an end-stage presentation of AMS [4][6][11][13][4,6,11,13]. However, HACE can also present without preceding symptoms of AMS and has also been reported to present acutely within a matter of hours [1][14][1,14]. Thus, HACE may represent a distinct pathology unique from AMS and further study is warranted to clarify this matter.
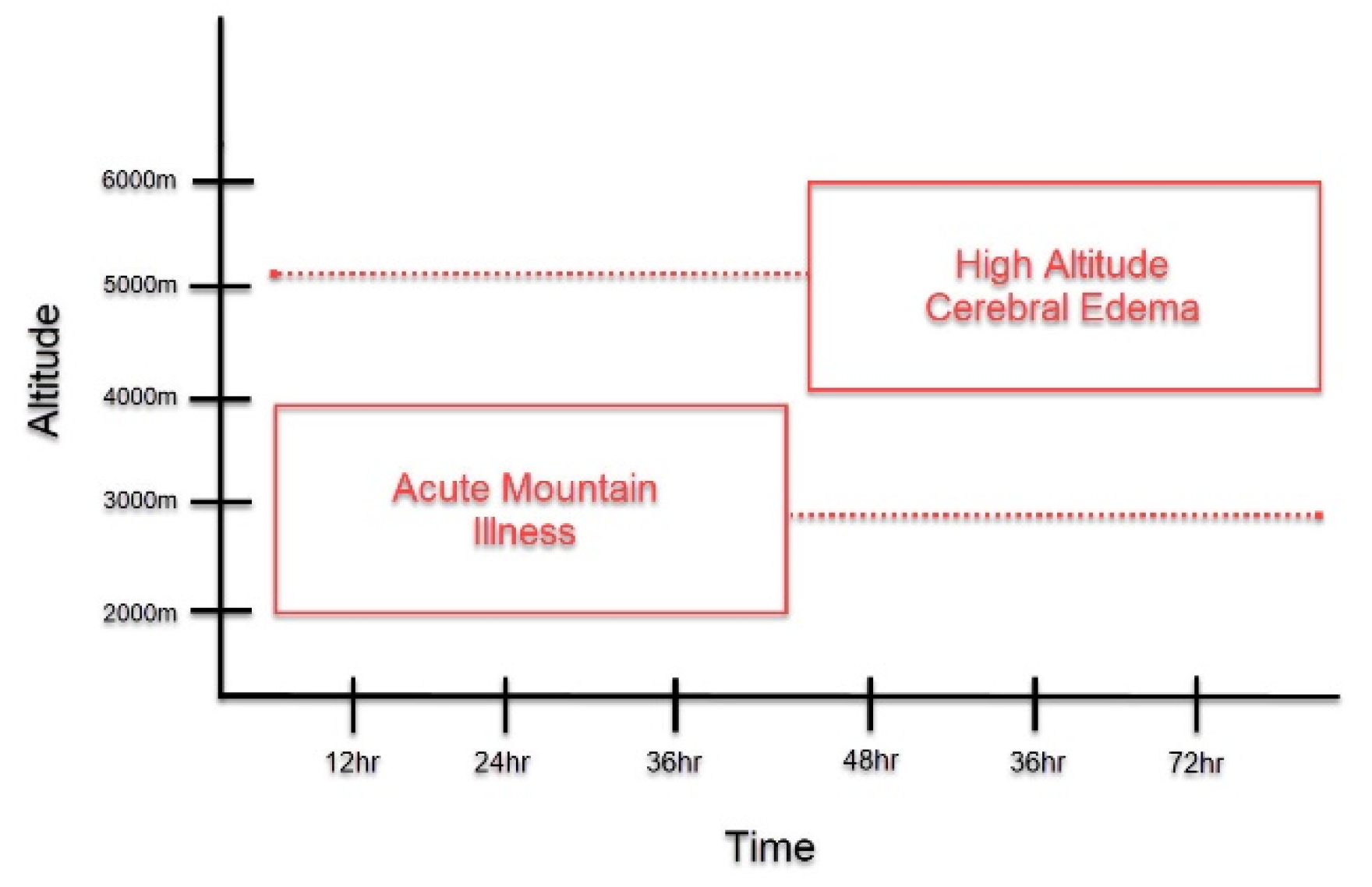
Figure 1. Symptom Onset of Acute Mountain Illness (AMS) and High Altitude Cerebral Edema (HACE). This graph depicts the symptom onset of AMS and HACE as it relates to altitude and length of exposure to high altitudes. As compared to HACE, AMS generally presents at lower altitudes HACE and with earlier symptom onset. At the time that AMS symptoms peak, if altitude is sufficient, the onset of HACE may occur. However, as depicted in the figure, this time course is not absolute. AMS may present in a delayed fashion, while HACE could present acutely.
2. Current Medical Management
2.1. Prevention
Due to its associated morbidity and mortality, preventing HACE should take priority over treatment [15][20]. Clinically, HACE presents as a more severe form of AMS. As such, current guidelines utilize similar measures for both AMS and HACE prevention [13]. The primary focus of prevention is to improve acclimatization [16][21]. Inadequate acclimation is typically the result of rapid ascent, low vital capacity, or an intrinsic poor hypoxic ventilatory response [17][22]. Those who are unacclimated at altitudes greater than 2500 m are at greatest risk for HACE [13]. A variety of nonpharmacological and pharmacological strategies are implemented to aid in acclimatization [13][18][13,23]. The strategies chosen should depend on the anticipated altitude, prior performance at high altitudes, rate of ascent, and the availability of acclimatization days [13].
2.1.1. Nonpharmacologic Prevention
Graded Ascent
Graded ascent is an effective method of prevention for all HAI, including HACE [19][24]. A slow, staged ascent provides the body with an adequate amount of time to properly acclimate to greater altitudes [15][19][20,24]. It is defined by a staged increase in the altitude at which one sleeps [13]. Although randomized controlled studies are lacking, based on clinical studies, a slow, graded ascent is strongly recommended for HACE prevention [5][13][20][5,13,25]. In particular, above 3000 m, climbers should not increase sleeping elevation at rates greater than 500 m a day [13].
Pre-Acclimatization
Pre-acclimatization can be accomplished through multiple methods, including intermittent exposure to hypobaric hypoxia or normobaric hypoxia using hypoxic tents, chambers, or masks [21][26]. However, each of these devices varies in hypoxic “dose” and duration of exposure [21][26]. As such, only a few pre-acclimatization programs of intermittent exposure have shown decreases in AMS [13][21][22][23][13,26,27,28]. Thus, pre-acclimatization can be considered, but is not strongly recommended, in the setting of HACE prevention [13].
2.1.2. Pharmacologic Prevention
Prophylactic medication is not recommended in climbers with a low risk of HACE; however, for those with moderate-to-high risk, as defined by the Wilderness Medical Society Clinical Practice Guidelines, prophylactic treatment is crucial [13]. Despite this, it should be noted that sufficient acclimatization remains the best strategy in HACE prevention [21][26].
Acetazolamide
Acetazolamide is the only medication proven to aid in acclimatization and is the gold-standard for AMS and HACE prophylaxis (Table 1) [13][17][13,22].
Table 1.
Drugs Used in Prevention and Treatment of HACE (recommendations based on Wilderness Medical Society Clinical Practice Guidelines) [13].
| Drug | Indication | Route | Dose | Adverse Effects |
|---|---|---|---|---|
| Acetazolamide | Prevention | Oral | Prevention: 125 mg/12 h (begin 24 h before ascent and continue at least 2 days at arrival of target altitude) Pediatric: 2.5 mg/kg/12 h |
Paresthesia, polyuria, nausea, fatigue, Stevens–Johnson syndrome or anaphylaxis |
| Dexamethasone | Prevention and Treatment | Prevention: Oral Treatment: Oral, intravenous, or intramuscular |
Prevention: 2 mg/6 h or 4 mg/12 h Treatment: 8 mg once, then 4 mg/6 h |
Mood changes, insomnia, dyspepsia, adrenal suppression, hyperglycemia |
Dexamethasone
Dexamethasone is the recommended alternative in those who cannot tolerate acetazolamide (Table 1) [13][19][13,24]. The mechanism by which dexamethasone prevents AMS and HACE is unclear; however, reduction in vascular permeability, inflammatory pathway inhibition, antioxidant balance, aquaporin-4 channel (AQP4) modulation and sympathetic blockade have all been proposed [24][30]. Prospective studies have shown dexamethasone to be effective in AMS prevention, which has been further confirmed in a meta-analysis [13][25][26][27][13,31,32,33].
2.2. Treatment
2.2.1. Nonpharmacologic Treatment Strategies
Descent
Descent is the gold standard treatment in those who develop HACE, and the decision to descend should be made as soon as possible [13]. Descent should be at least 300 to 1000 m or continued until the patient is asymptomatic [13]. As descent is the main treatment option, other treatment options should not delay descent and should only be used in settings where descent is not possible or may be delayed [28][19].
Oxygen
Supplemental oxygen should be given in cases of severe HACE with a goal saturation of 90% [13]. It should only be used in combination with descent or while awaiting descent [13]. It is important to note that there are currently no controlled studies that have studied the benefit of supplemental oxygen therapy in HACE patients. Despite this, based on clinical experience, many authors suggest the use of supplemental oxygen to raise oxygen saturation and to help to resolve symptoms in HACE [13][15][21][13,20,26]. However, clinicians should be sure to avoid prolonged hyperoxia due to new associations with increases in mortality in critically ill patients [13].
Portable Hyperbaric Chambers
Portable hyperbaric chambers are reserved for patients with severe HACE in situations where evacuation is delayed [13]. Hyperbaric chambers can raise the ambient pressure around the patient [29][38]. Two psi, or 105 mmhg, is the pressure setting commonly utilized; however, chambers are capable of generating pressures up to 130 mmhg [10][30][10,39]. This increase in pressure may replicate a descent of 1800 to 2400 m, or greater, depending on starting elevation [10][30][10,39]. The chamber also contains a valve system that supplies enough ventilation to avoid carbon dioxide accumulation [21][26].
2.2.2. Pharmacologic Treatment Strategies
Dexamethasone
Despite the emphasis on immediate descent and evacuation to a medical facility, when possible, such interventions may not always be feasible depending on altitude, terrain, and geographic location. Thus, if descent or evacuation must be delayed, dexamethasone should be administered (Table 1) [13]. Although controlled clinical studies have yet to study the efficacy of dexamethasone in the treatment of HACE, decades of clinical experience continue to support its use and it remains the mainstay of pharmaceutical intervention [13]. Of note, administration should include a loading dose of 8 mg, followed by a 4 mg dose every 6 h. It can be given intravenously, intramuscularly, or orally [13].
Acetazolamide
Although acetazolamide has been studied in the setting of AMS treatment, there is currently no evidence to support its efficacy in treating HACE [15][31][20,44]. Thus, the routine use of acetazolamide in the setting of HACE is not recommended and should not replace dexamethasone [13].
2.2.3. Re-Ascent
The safety of climbers continuing their ascent after an episode of HACE remains controversial. However, if ascent is pursued, the person must be symptom-free without medication for several days before proceeding [18][23]. It is also strongly recommended that those who continue ascent after HACE utilize dexamethasone prophylaxis for the remainder of ascent [18][23].
3. Conclusions
HACE remains a serious, life-threatening form of HAI with a poorly understood pathophysiology that continues to be explored. It is proposed by some to be an end-stage presentation of AMS, reflecting acute decompensation in cerebral fluid homeostasis. However, it is also known to occur without preceding symptoms of AMS and, thus, could represent a distinct pathophysiology unique from AMS and other HAIs.
Current medical management relies heavily on both clinical experience and clinical studies. The mainstay of prevention includes graded ascent, as well as acetazolamide prophylaxis to assist with acclimatization when needed. Definitive treatment entails rapid descent, as well as the administration of dexamethasone and supportive measures, such as hyperbaric chambers and oxygen supplementation, if descent is not possible or must be delayed.
Despite the efficacy of current medical management, the incidence of HACE remains and current treatment modalities are not always feasible or absolute. Thus, further research is needed. As thwe group continues to explore new avenues of research, several opportunities for therapeutic development emerge. Pre-clinical studies investigating novel anti-inflammatory agents have shown great promise and could soon transition to a clinical research setting. Further, the exploration of emerging concepts regarding cerebral fluid homeostasis, such as the glymphatic system and cerebral venous obstruction, have advanced theour current understanding of HACE pathophysiology, enabling us to approach HACE from innovative perspectives. As new avenues of research continue to open, scientistswe can further advance medical management and the development of new strategies to address HACE from high altitude environments.
