Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 3 by Dean Liu.
Surface modification of electrospun products has been an attractive method for increasing multifunctionality and biocompatibility properties.
- electrospinning
- gelatin
- nanofibers
- plasma
- skin tissue engineering
1. Introduction
Gelatin is a natural biopolymer obtained from collagen under controlled hydrolysis. It has well-known properties for drug delivery, wound healing, and tissue regeneration applications [1][2][3][4][5][6][7]. Despite several advantages in biomedical engineering and regenerative medicine, gelatin has some major problems including melting at temperatures about or above 37 °C in water and hardening into gel at room temperatures. Therefore, specific pre-treatments are required for gelatin prior to applications in tissue engineering. Studies showed that gelatin can mimic different properties of collagen after the crosslinking approach with chemical reagents or physical processes [1]. In this context, aldehyde-based chemicals have been proposed by some researchers despite their moderate toxicity in tissue regeneration studies. Therefore, researchers have explored bioactive, non-toxic, and natural compounds to stabilize gelatin biomacromolecules [8]. Tannic acid is a plant polyphenol and a glucose derivative which is widely used as a natural reagent in the moisture, antioxidant, antimicrobial, antiviral, and anti-inflammatory products. Recent studies have approved that tannic acid can be effectively applied as a crosslinker for gelatin [9][10].
Electrospinning is a simple and effective method for producing long, uniform, and fine diameter nanofibers. Resultant scaffolds from different nanofibers have shown specific properties such as high surface-to-volume ratio, high porosity, and long lengths. Notably, they have been used in various products such as artificial tissues, biomedical products, electronics, sensors, and nanocomposites [11][12][13][14][15]. In the electrospinning process, different biomaterials can be utilized with effective mechanical properties in order to adapt and functionalize a biological environment [13][14][15]. On the other hand, surface modification of electrospun products has been an attractive method for increasing multifunctionality and biocompatibility properties [10]. A great number of studies demonstrated that different techniques can be applied for biofunctionalization of polymers including plasma, corona discharge, flame, photons, electron beam, ion beam, X-ray, and Gama-ray functionalization. However, plasma process of polymers has been known as a safe, cost-effective, and simple approach without affecting the bulk properties [16][17][18][19].
2. Microscopic Assessment
The surface morphology of gelatin nanofiber scaffolds was assessed before and after treatment with air pressure plasma, using SEM and AFM methods. Figure 1A,B indicates the results of SEM data for the untreated and plasma-treated gelatin nanofibers. There were no considerable changes on the surface, morphology, and the average diameter of nanofibers after plasma treatment. As a result of using acetic acid solvent, rwesearchers previously found that several factors prevent bead-like patterns on electrospun nanofibers including electrostatic repulsion, surface tension, and viscoelastic properties. Therefore, researherswe used optimized electrospinning conditions from theour previous studies to generate smooth gelatin nanofibers [9][10]. In theour latest study on argon and argon–oxygen plasma-treated gelatin nanofibers, SEM did not indicate any surface changes which is in agreement with the results from air pressure plasma-treated nanofibers.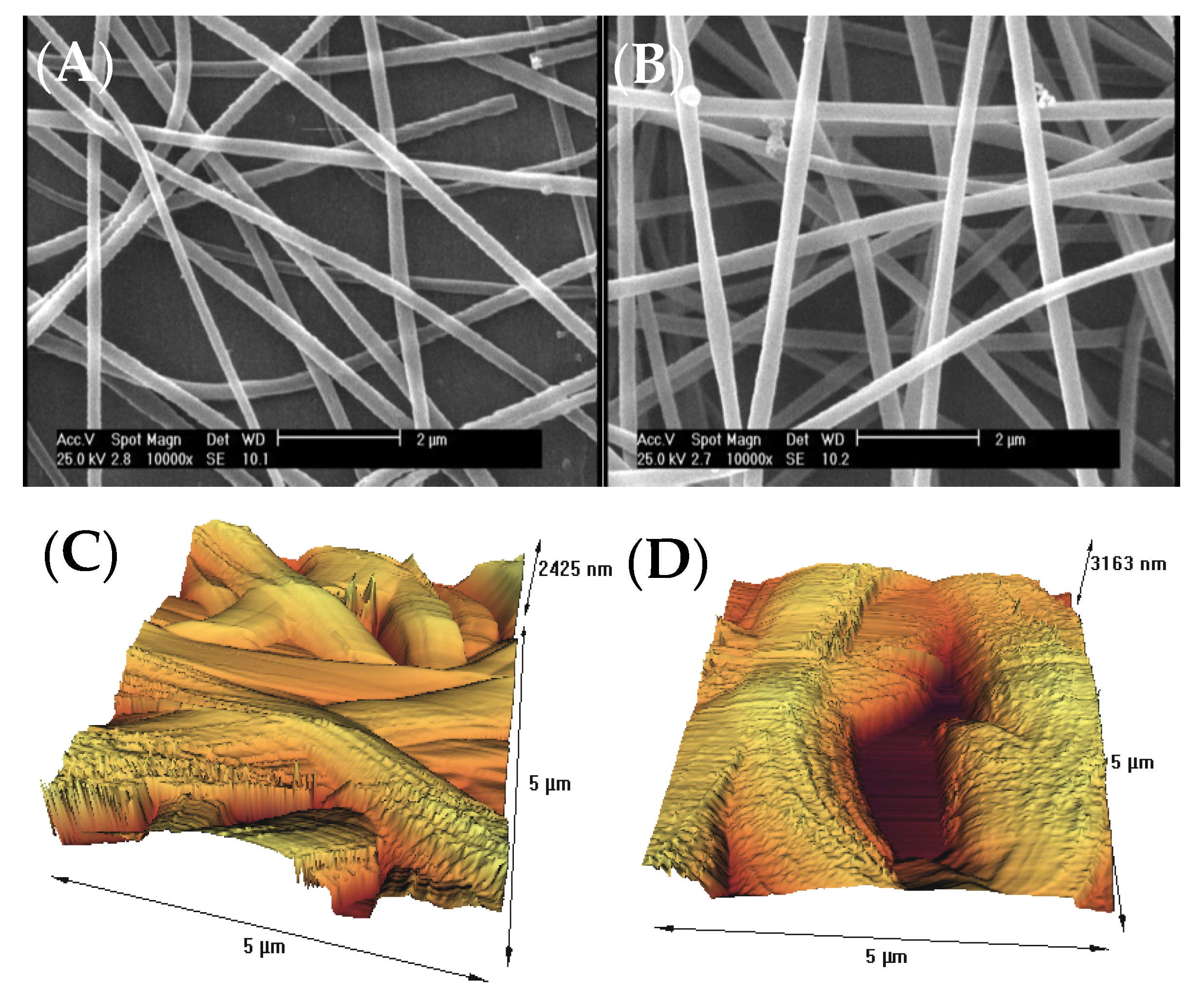
Figure 1. (A) SEM image of untreated gelatin nanofibers, (B) SEM image of atmospheric-air plasma-treated gelatin nanofibers, (C) AFM image of untreated gelatin nanofibers, (D) AFM image of atmospheric-air plasma-treated gelatin nanofibers.
Table 1. Different parameters extracted from AFM analysis for the untreated and atmospheric-air plasma-treated gelatin nanofibers (standard deviations in parenthesis).
]. Several studies postulated that the introduction of oxygen-polar domains on the nanofiber surfaces can be conducted by the atmospheric-air plasma method, which can subsequently lead to a drastic change in their hydrophilicity [22][23][24][25]. This observation is in good agreement with theour ATR-FTIR and AFM results.
Table 2. The average water CA (°) of (A) untreated, (B) atmospheric air plasma-treated gelatin nanofibers.
| Samples | Water CA (°) | Image from Camera |
|---|---|---|
| A | 20.6 |  |
| B | 11.8 |  |
| Samples | Plasma Exposure Time (s) | Maximum Peak Height, Ra (nm) | Maximum Valley Depth, Rz (nm) | Average Peak-to-Valley Height, Rq (nm) |
RMS (nm) |
|---|---|---|---|---|---|
| Untreated nanofibers |
0 | 6.5 (0.02) | 276.8 (2.7) | 47.4 (1.1) | 5.1 (0.01) |
| Plasma-treated nanofibers | 90 | 30.8 (0.1) | 479.8 (5.3) | 222.6 (2.5) | 954.9 (4.3) |
3. Water Contact Angle Properties of Nanofibers
One of the most important properties of biomedical scaffolds is their hydrophilic/hydrophobic functionalities [22]. For this purpose, the contact angle (CA) of water droplets was measured on nanofibers using the device software. Table 2 represented the results of CA for the untreated and atmospheric-air plasma-treated gelatin nanofibers. As expected, gelatin indicated excellent wettability in comparison with different hydrophobic biopolymers including polylactic acid and poly caprolactone, with a water CA of 20.65. This is owing to its hydrophilic nature [23]. On the other hand, the water CA decreased to 11.8 for the atmospheric-air plasma-treated gelatin nanofibers due to the oxidation or attachment of polar groups [244. Effect of Atmospheric-Air Plasma on the Fibroblast Cell Culture
Fibroblast cells were put on the scaffolds and analyzed with inverted optical microscope along with SEM after 24 h.
As it can be seen from the inverted microscope (Figure 2A with a magnification of 200×), fibroblast cells were flat. However, the microscopic examination did not reveal any major change in cell shape and adhesion to the atmospheric-air plasma-treated gelatin film (Figure 2B). According to Figure 2C from SEM, fibroblast cells were connected to the untreated gelatin film and their natural shapes were maintained after 24-h treatment with trypsin. On the other hand, there was no change in the shape of fibroblast cells on the plasma-treated gelatin film (Figure 2D). Interestingly, itwe is found that the number of cells were increased on gelatin due to plasma functionalization. This result was in agreement with the our recent study on argon and argon-oxygen plasma-treated gelatin nanofibers [10]. Several studies stated that plasma modification has a critical role in improving the hydrophilicity of scaffold surfaces which can further enhance the proliferation and differentiation of osteoblastic cells [26][27][28][29][30]. These researchers found that the number of oxygen atoms on tissue scaffolds was increased after atmospheric-air plasma treatment, thus the cell growth was improved.
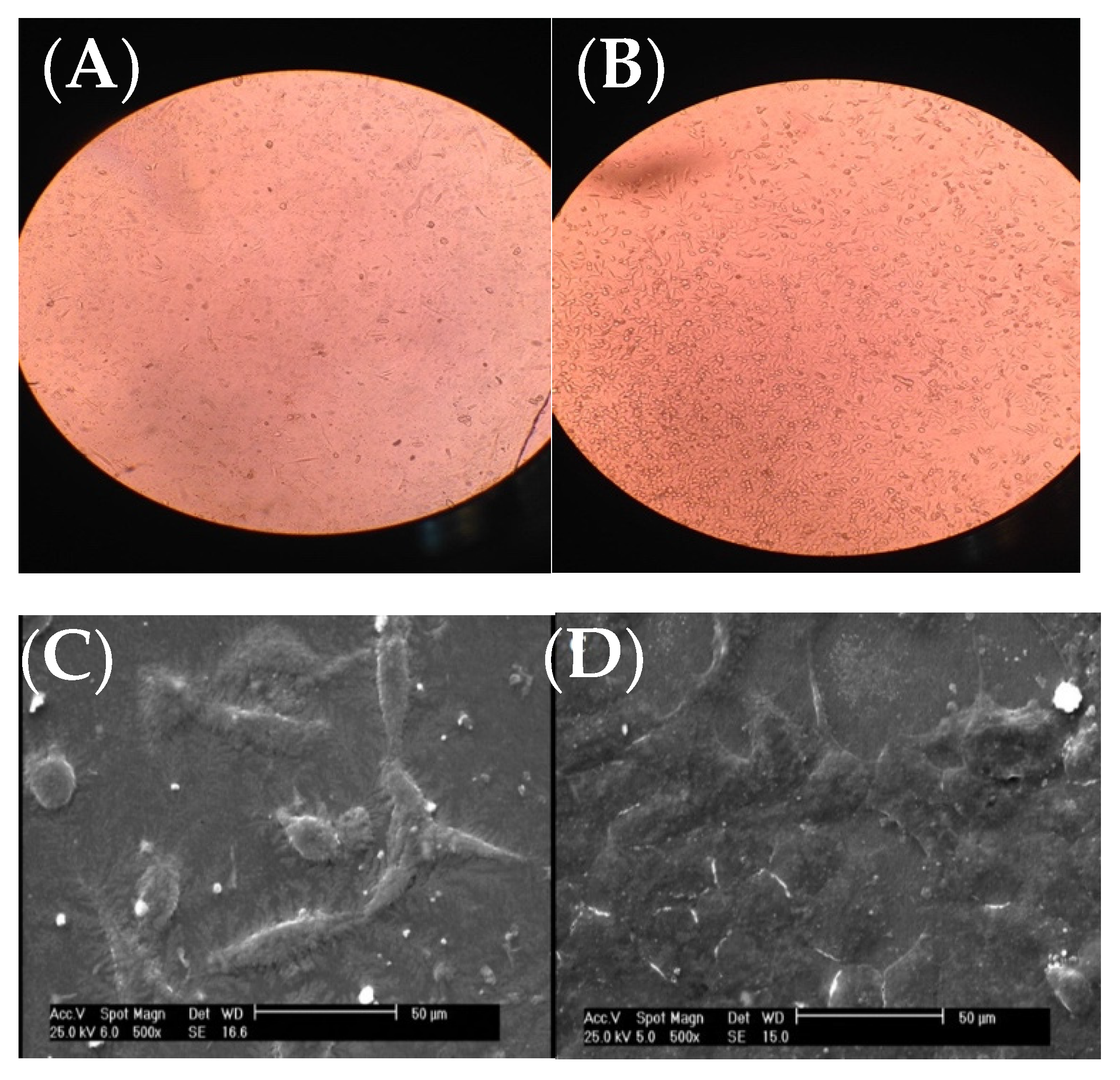

Figure 2. Images of fibroblast cells on gelatin films, (A) inverted optical microscope image from the untreated gelatin film, (B) inverted optical microscope image from the atmospheric-air plasma-treated gelatin film, (C) SEM image from the untreated gelatin film, (D) SEM image from the atmospheric-air plasma-treated gelatin film.
References
- Chiu, C.-M.; Nootem, J.; Santiwat, T.; Srisuwannaket, C.; Pratumyot, K.; Lin, W.-C.; Mingvanish, W.; Niamnont, N. Enhanced Stability and Bioactivity of Curcuma comosa Roxb. Extract in Electrospun Gelatin Nanofibers. Fibers 2019, 7, 76.
- Chaochai, T.; Imai, Y.; Furuike, T.; Tamura, H. Preparation and Properties of Gelatin Fibers Fabricated by Dry Spinning. Fibers 2016, 4, 2.
- Yamaguchi, S.; Akeda, K.; Shintani, S.A.; Sudo, A.; Matsushita, T. Drug-Releasing Gelatin Coating Reinforced with Calcium Titanate Formed on Ti–6Al–4V Alloy Designed for Osteoporosis Bone Repair. Coatings 2022, 12, 139.
- Cuevas-Acuña, D.A.; Plascencia-Jatomea, M.; Santacruz-Ortega, H.d.C.; Torres-Arreola, W.; Ezquerra-Brauer, J.M. Development of Chitosan/Squid Skin Gelatin Hydrolysate Films: Structural, Physical, Antioxidant, and Antifungal Properties. Coatings 2021, 11, 1088.
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Zalewska, M.; Poltorak, A. Application of Propolis Extract in Gelatin Coatings as Environmentally Friendly Method for Extending the Shelf Life of Pork Loin. Coatings 2021, 11, 979.
- Zhao, S.; Chen, Z.; Dong, Y.; Lu, W.; Zhu, D. The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase. Gels 2022, 8, 146.
- Tejo-Otero, A.; Fenollosa-Artés, F.; Achaerandio, I.; Rey-Vinolas, S.; Buj-Corral, I.; Mateos-Timoneda, M.Á.; Engel, E. Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms. Gels 2022, 8, 40.
- Zhang, Y.; Ouyang, H.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Electrospinning of Gelatin Fibers and Gelatin/PCL Composite Fibrous Scaffolds. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2005, 72, 156–165.
- Mirjalili, M.; Mozaffari, A.; Parvinzadeh Gashti, M.; Parsania, M. Effect of Tannic Acid on Properties of Electrospun Gelatin Nanofibers. Indian J. Fibre Text. Res. 2020, 45, 289–298.
- Mozaffari, A.; Parvinzadeh Gashti, M.; Mirjalili, M.; Parsania, M. Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications. Membranes 2021, 11, 31.
- Bodillard, J.; Pattappa, G.; Pilet, P.; Weiss, P.; Réthoré, G. Functionalisation of Polysaccharides for the Purposes of Electrospinning: A Case Study Using HPMC and Si-HPMC. Gels 2015, 1, 44–57.
- Szewczyk, P.K.; Ura, D.P.; Stachewicz, U. Humidity Controlled Mechanical Properties of Electrospun Polyvinylidene Fluoride (PVDF) Fibers. Fibers 2020, 8, 65.
- Lubasova, D.; Netravali, A.N. A Novel Method for Electrospinning Nanofibrous 3-D Structures. Fibers 2020, 8, 27.
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased Mechanical Properties of Carbon Nanofiber Mats for Possible Medical Applications. Fibers 2019, 7, 98.
- Borojeni, I.A.; Gajewski, G.; Riahi, R.A. Application of Electrospun Nonwoven Fibers in Air Filters. Fibers 2022, 10, 15.
- Thibodeaux, N.; Guerrero, D.E.; Lopez, J.L.; Bandelt, M.J.; Adams, M.P. Effect of Cold Plasma Treatment of Polymer Fibers on the Mechanical Behavior of Fiber-Reinforced Cementitious Composites. Fibers 2021, 9, 62.
- Pornwannachai, W.; Horrocks, A.R.; Kandola, B.K. Surface Modification of Commingled Flax/PP and Flax/PLA Fibres by Silane or Atmospheric Argon Plasma Exposure to Improve Fibre–Matrix Adhesion in Composites. Fibers 2022, 10, 2.
- Parvinzadeh Gashti, M.; Hegemann, D.; Stir, M.; Hulliger, J. Thin Film Plasma Functionalization of Polyethylene Terephthalate to Induce Bone-Like Hydroxyapatite Nanocrystals. Plasma Process. Polym. 2014, 11, 37–43.
- Parvinzadeh, M.; Ebrahimi, I. Influence of Atmospheric-Air Plasma on the Coating of a Nonionic Lubricating Agent on Polyester Fiber. Radiat. Eff. Def. Solid. 2011, 166, 408–416.
- Ferreira, B.; Pinheiro, L.M.P.; Nascente, P.A.P.; Ferreira, M.J.; Duek, E.A.R. Plasma Surface Treatments of Poly (l-Lactic Acid)(PLLA) and Poly (Hydroxybutyrate-co-Hydroxyvalerate)(PHBV). Mat. Sci. Eng. C 2009, 29, 806–813.
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-Surface Modification of Biomaterials. Mat. Sci. Eng. R Rep. 2002, 36, 143–206.
- Zhao, Y.; Liu, J.; Zhang, M.; He, J.; Zheng, B.; Liu, F.; Zhao, Z.; Liu, Y. Use of Silver Nanoparticle–Gelatin/Alginate Scaffold to Repair Skull Defects. Coatings 2020, 10, 948.
- Char, C.; Padilla, C.; Campos, V.; Pepczynska, M.; Díaz-Calderón, P.; Enrione, J. Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin. Coatings 2019, 9, 595.
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41.
- Yilma, B.B.; Luebben, J.F.; Nalankilli, G. The Effect of Air, Ar and O2 Plasmas on the Electrical Resistivity and Hand-Feel Properties of Polyester/Cotton Blend Fabric. Fibers 2020, 8, 17.
- Prabhakaran, M.P.; Venugopal, J.; Chan, K.C.; Ramakrishna, S. Surface Modified Electrospun Nanofibrous Scaffolds for Nerve Tissue Engineering. Nanotechnology 2008, 19, 455102.
- Ko, Y.M.; Choi, D.Y.; Jung, S.C.; Kim, B.H. Characteristics of Plasma Treated Electrospun Polycaprolactone (PCL) Nanofiber Scaffold for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2015, 15, 192–195.
- Schnell, E.; Kinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of Glial Cell Migration and Axonal Growth on Electrospun Nanofibers of Poly-ε-Caprolactone and a Collagen/Poly-ε-Caprolactone Blend. Biomaterials 2007, 28, 3012–3025.
- Kiyotani, T.; Nakamura, T.; Shimizu, Y.; Endo, K. Experimental Study of Nerve Regeneration in a Biodegradable Tube Made from Collagen and Polyglycolic Acid. ASAIO J. 1995, 41, M657–M661.
- Bender, M.D.; Bennett, J.M.; Waddell, R.L.; Doctor, J.S.; Marra, K.G. Multi-Channeled Biodegradable Polymer/CultiSpher Composite Nerve Guides. Biomaterials 2004, 25, 1269–1278.
More
