In the plasma membrane and other cellular compartments (endosome/lysosome), sphingomyelin can be hydrolyzed to ceramide by sphingomyelinases. Ceramide generated by this pathway is further degraded into sphingosine by ceramidases. Shingosine can also be phosphorylated by sphingosine kinases to sphingosine-1-phosphate. Changes in the profiles of sphingomyelin and its metabolites ceramide, sphingosine, and sphingosine-1-phosphate (S1P) can result in a pathological condition triggered by accumulation or by altering cell signaling.
- diabetes
- angiotensin II-induced hypertension
- sphingomyelin
1. Introduction
Numerous human studies have shown that, in cardiovascular, renal, and metabolic diseases, the profiles of sphingomyelin [1][2][3][4] and its metabolites ceramide [5][6][7][8][9][10][11], sphingosine [12], and sphingosine-1-phosphate (S1P) [13][14] are altered (reduction or elevation) in the plasma, organs (liver and heart), and tissues (skeletal muscle and adipose). Most of these studies focused on the determination of ceramide in plasma. However, it is necessary to perform preclinical studies to determine the content of sphingomyelin and its bioactive metabolites in plasma and organs such as the brain, liver, heart, and kidney, because the sphingolipid metabolism imbalance can be affected directly or indirectly in various organs.
On the other hand, changes in the expression or activity of the enzymes that participate in sphingolipid metabolism may explain the alterations in their profile.
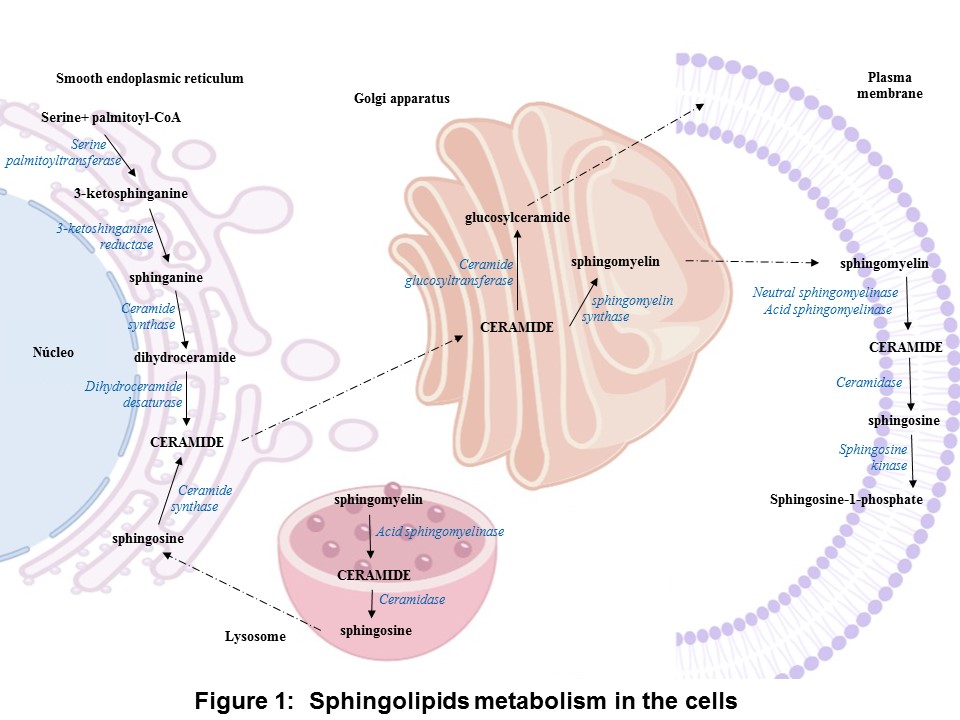
In the anabolic pathway, the synthesis of sphingolipids stars by the condensation of serine and palmitoyl-CoA into 3-ketosphinganine by the enzyme serine palmitoyl transferase, and it is followed by a reduction yielding sphinganine. The sphinganine is acylate by ceramide synthase resulting dihydroceramides. Finally, ceramide is formed by the dehydrogenation of dihydroceramide by dihydroceramide desaturase. The ceramide is converted into sphingomyelin by sphingomyelin synthase or glycosphingolipid by ceramide glucosyltransferase. In the catabolic pathway, sphingomyelinases (SMases) hydrolyzes sphingomyelin to release ceramide, which is hydrolyzed into sphingosine and S1P by ceramidase (CDase) and sphingosine kinase (SK), respectively (Figure 1) [15].
Concerning the expression at the mRNA level of the enzymes involved in the synthesis (serine palmitoyltransferase) and degradation of ceramide (SMase, CDase, and SK-1), the levels of these enzymes were increased in intra-abdominal adipose tissue and the myocardium of obese patients with or without type 2 diabetes [16][17].
Regarding enzyme activity, secretory SMase activity increased in the serum of patients with type 2 diabetes, chronic heart failure, or acute coronary syndromes [18][19][20]. In the adipose tissue of obese non-diabetic or diabetic patients, the activity of serine palmitoyltransferase, neutral and acid CDase (nCDase and aCDase) was increased, while the aSMase activity was decreased [7].
Changes in the profiles of sphingomyelin and its metabolites ceramide, sphingosine, and sphingosine-1-phosphate (S1P) can result in a pathological condition triggered by accumulation or by altering cell signaling.
In a previous study, researchers demonstrated that in the isolated perfused rat kidney of diabetic rats, the vasoconstriction produced by S1P increases [21]. Additionally, in the isolated perfused rat kidney, angiotensin II (Ang II) stimulates ceramide formation via the activation of nSMase (Figure 2) [22].
2. Applications
Therefore, drugs that modify the expression or activity of the enzymes involved in sphingolipid metabolism are attractive candidates for the treatment of cardiovascular, renal, and metabolic disease.
OurThe results suggest that empagliflozin downregulates the interaction of the de novo pathway and the catabolic pathway of sphingolipid metabolism in the diabetes, whereas in Ang II-dependent hypertension, it only downregulates the sphingolipid catabolic pathway (Figure 2).

References
- Spijkers, L.J.; Van den Akker, R.F.; Janssen, B.J.; Debets, J.J.; De Mey, J.G.; Stroes, E.S.; Van den Born, B.J.; Wijesinghe, D.S.; Chalfant, C.E.; MacAleese, L.; Eijkel, G.B.; Heeren, R.M.; Alewijnse, A.E.; Peters, S.L. Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PLoS One. 2011; 6(7):218-17, https://doi.org/10.1371/journal.pone.0021817.
- Blachnio-Zabielska, A.U.; Koutsari, C.; Tchkonia, T.; Jensen, M.D. Sphingolipid Content of Human Adipose Tissue: Relationship to Adiponectin and Insulin Resistance. Obesity2012, 20, 2341–2347, https://doi.org/10.1038/oby.2012.126.
- Barlovic, D.P.; Harjutsalo, V.; Sandholm, N.; Forsblom, C.; Groop, P-H. on behalf of the FinnDiane Study Group Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia2020, 63, 1847–1856,https://doi.org/10.1007/s00125-020-05201-9.
- Jensen, P.N.;Fretts, A. M.; Hoofnagle, A. N.;Sitlani, C. M.; McKnight, B.; King, I. B.;Siscovick, D. S.;Psaty, B. M.;Heckbert, S. R.;Mozaffarian, D.;et al. Plasma Ceramides and Sphingomyelins in Relation to Atrial Fibrillation Risk: The Cardiovascular Health Study. J Am Heart Assoc. 2020 Feb 18;9(4): e012853, https://doi: 10.1161/JAHA.119.012853.
- Haus, J.M.;Kashyap, S.R.;Kasumov, T.; Zhang, R.; Kelly, K.R.;Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes2009, 58, 337–343.
- Longato, L.; Tong, M.; Wands, J.R.; de la Monte, S.M. High fat diet induced hepatic steatosis and insulin resistance: Role of dysregulated ceramide metabolism. Res.2012, 42, 412–427, https://doi.org/10.1111/j.1872-034x.2011.00934. x.
- Błachnio-Zabielska, A.; Pułka, M.; Baranowski, M.; Nikołajuk, A.; Zabielski, P.; Górska, M.; Górski, J. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. Cell. Physiol.2012, 227, 550–557, https://doi.org/10.1002/jcp.22745.
- Lopez, X.; Goldfine, A.; Holland, W.L.; Gordillo, R.; Scherer, P.E. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. Pediatr. Endocrinol. Metab.2013, 26, 995–998, https://doi.org/10.1515/jpem-2012-0407.
- Mitsnefes, M.; Scherer, P.E.; Friedman, L.A.; Gordillo, R.; Furth, S.; A Warady, B.; CKiD Study Group; the CKiD study group Ceramides and cardiac function in children with chronic kidney disease. Nephrol.2014, 29, 415–422,https://doi.org/10.1007/s00467-013-2642-1.
- Klein, R.L.; Hammad, S.M.; Baker, N.L.; Hunt, K.J.; Al Gadban, M.M.; Cleary, P.A.; Virella, G.; Lopes-Virella, M.F. Decreased plasma levels of select very long chain ceramide species Are associated with the development of nephropathy in type 1 diabetes. Metabolism2014, 63, 1287–1295https://doi.org/10.1016/j.metabol.2014.07.001.
- de la Maza, M.P.;Rodriguez, J.M.; Hirsch, S.; Leiva, L.; Barrera, G.; Bunout, D. Skeletalmuscleceramidespecies in menwith abdominal obesity. Nutr. Health Aging2015, 19, 389–396.
- Górska, M.; Dobrzyn, A.; Baranowski, M. Concentrations of sphingosine and sphinganine in plasma of patients with type 2 diabetes. Med Sci. Monit.2005, 11, CR35–CR38.
- Deutschman, D.H.; Carstens, J.S.; Klepper, R.L.; Smith, W.S.; Page, M.; Young, T.R.; A Gleason, L.; Nakajima, N.; A Sabbadini, R. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Hear. J.2003, 146, 62–68,https://doi.org/10.1016/s0002-8703(03)00118-2.
- Kowalski, G.M.; Carey, A.L.; Selathurai, A.; Kingwell, B.A.; Bruce, C.R. Plasma Sphingosine-1-Phosphate Is Elevated in Obesity. PLoS ONE2013, 8, e72449, https://doi.org/10.1371/journal.pone.0072449.
- Hannun, Y.A.; Luberto, C.; Argraves, K.M. Enzymes of Sphingolipid Metabolism: From Modular to Integrative Signaling. Biochemistry2001, 40, 4893–4903, https://doi.org/10.1021/bi002836k.
- Baranowski, M.; Blachnio-Zabielska, A.; Hirnle, T.; Harasiuk, D.; Matlak, K.; Knapp, M.; Zabielski, P.; Górski, J. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. Lipid Res.2010, 51, 74–80, https://doi.org/10.1194/jlr.m900002-jlr200.
- Kolak, M.; Gertow, J.; Westerbacka, J.; Summers, A.S.; Liska, J.; Franco-Cereceda, A.; Orešič, M.; Yki-Järvinen, H.; Eriksson, P.; Fisher, R.M. Expression of ceramide-metabolising enzymes in subcutaneous and intra-abdominal human adipose tissue. Lipids Heal. Dis.2012, 11, 115–115, https://doi.org/10.1186/1476-511x-11-115.
- Górska, M.; Barańczuk, E.; Dobrzyń, A. Secretory Zn2+-dependent Sphingomyelinase Activity in the Serum of Patients with Type 2 Diabetes is Elevated. Metab. Res.2003, 35, 506–507, https://doi.org/10.1055/s-2003-41810.
- Doehner, W.; Bunck, A.C.; Rauchhaus, M.; von Haehling, S.; Brunkhorst, F.M.; Cicoira, M.; Tschope, C.; Ponikowski, P.; Claus, R.A.; Anker, S.D. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Heart J. 2007, 28, 821–828.
- Pan, W.; Yu, J.; Shi, R.; Yan, L.; Yang, T.; Li, Y.; Zhang, Z.; Yu, G.; Bai, Y.; Schuchman, H.; et al. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron Artery Dis. 2014; 25(3):230-5, https://doi.org/10.1097/mca.0000000000000079.
- Bautista-Pérez, R.; Arellano, A.; Franco, M.; Osorio, H.; Coronel, I. Sphingosine-1-phosphate induced vasoconstriction is increased in the isolated perfused kidneys of diabetic rats. Diabetes Res. Clin. Pr.2011, 94, e8–e11, https://doi.org/10.1016/j.diabres.2011.06.023.
- Bautista-Pérez, R.; Del Valle-Mondragón, L.; Cano-Martínez, A.; Pérez-Méndez, O.; Escalante, B.; Franco, M. Involvement of neutral sphingomyelinase in theangiotensin II signalingpathway. J. Physiol. Physiol.2015, 308, F1178–F1187, https:// doi.org/10.1152/ajprenal.00079.2014.
