Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Laila A. Gharzai.
Radiotherapy is an integral component of head/neck squamous cell carcinomas (HNSCCs) treatment, and technological developments including advances in image-guided radiotherapy over the past decades have offered improvements in the technical treatment of these cancers. Integration of magnetic resonance imaging (MRI) into image guidance through the development of MR-guided radiotherapy (MRgRT) offers further potential for refinement of the techniques by which HNSCCs are treated.
- radiotherapy
- magnetic resonance imaging
- head and neck cancer
- MR-Linac
1. Introduction
The delivery of radiotherapy (RT) for cancer treatment was revolutionized in the 1990s with the development of computed tomography (CT)-based three-dimensional RT planning and image-guided RT (IGRT). This allowed for better targeting of tumors and areas at risk while sparing nearby normal tissues. Early approaches to IGRT replaced the utilization of external skin markings and included first fluoroscopy then later portal imaging, including kilovoltage and megavoltage imaging, which had limited soft tissue delineation but allowed for anatomic targeting.
The development of cone beam computed tomography (CBCT) around 2000 ushered in an era of further precision in RT, allowing for dose escalation aiming to eradicate tumors while sparing nearby tissues. Refinement of IGRT techniques has allowed for the proliferation of advanced radiotherapy techniques. Treatment of head and neck squamous cell carcinomas (HNSCCs) in particular has benefited from improvements in technology. Modern approaches to RT for HNSCC include salivary-sparing [1,2][1][2] and pharyngeal constrictor-sparing [3,4][3][4] approaches, which require more conformal dose distributions made possible by the improvement in technology used to delivery radiotherapy.
Integration of CBCT into RT has also allowed for better visualization of changes seen during the course of treatment for HNSCC, which typically lasts 6–7 weeks. Geometric plan adaptation in HNSCC is typically driven by large tumor response or large anatomic shifts due to weight loss and is important due to unintended dosimetric changes that may occur during the course of treatment that may cause unintended toxicities or affect tumor control during the course of treatment [5] (example of patient with significant tumor regression in Figure 1). Early studies assessing adaptation driven by changes seen on CBCT suggest that this approach is feasible and efficacious [6]. However, the structures that define modern approaches to RT (salivary glands, pharyngeal constrictors, lymph nodes) remain poorly visualized on CBCTs. CBCTs are limited in their ability to differentiate the varying soft tissues relevant to HNSCC.
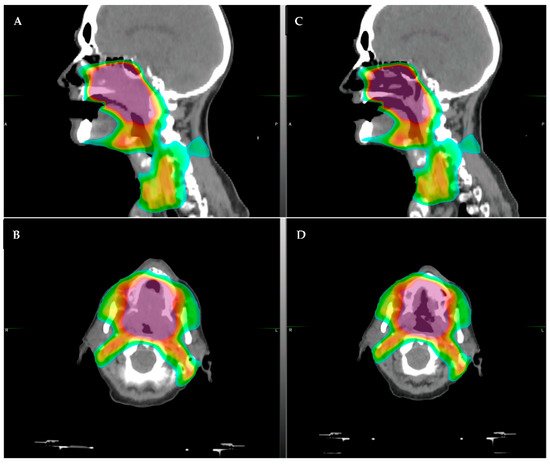
Figure 1. Example of a patient who had significant tumor regression after only 30 Gy of radiotherapy (of planned 70 Gy course). The patient had a large soft palate primary with extension into the nasal cavity. (A,B): original CT simulation, sagittal (A) and axial (B), respectively. (C,D): repeat CT simulation after approximately 36 Gy showing significant tumor response in the area of high dose, (C) sagittal and (D) axial. The color shading represents the radiotherapy dose distribution, with the purple/red representing areas of highest dose.
Magnetic resonance imaging (MRI) has become widely used clinically in HNSCC. MRIs allow for better soft tissue delineation and are of particular use in visualizing perineural invasion, extracapsular extension [7[7][8],8], and muscle invasion, all of which have importance in the diagnosis and treatment of HNSCC. Integrating MRI into IGRT (MRgRT) offers an opportunity to utilize these features and allow for further improvements in RT precision. Importantly, better integration of MRI into IGRT may also expand the role of adaptive RT [9]. Early studies assessing the feasibility of off-line adaptation using MRgRT suggest that this approach is efficacious [10] and may allow for improvements in radiotherapy planning.
Early attempts to integrate MRI into IGRT was hampered by the effect of the magnetic field used to create proton spin on the secondary electrons generated by RT [11,12][11][12]. However, recent advances in technology allowed for the development of an MRgRT linear accelerator. Currently, two machines are Food and Drug Administration (FDA) approved and commercially available—one with a 0.35 Tesla (T) MRI (ViewRay MRIdian) and one with a 1.5 T MRI (Elekta Unity). These machines show promise in improving IGRT, with their better delineation of soft tissue relative to CBCT; however, significant work is needed to further the clinical use of MRgRT for HNSCC.
2. Current Use of MR-Linac in HNSCC
MRgRT is currently in limited use clinically, with only approximately 150 machines out in clinical practice in the world—46 with 0.35 T and approximately 100 with 1.5 T. Publications examining the use of MRgRT are mostly in gastrointestinal and genitourinary cancers [13[13][14][15][16][17],14,15,16,17], where mobile organs at risk, such as bowel, substantially impact RT planning and delivery. Within the head/neck region, incorporating MRI prior to RT could potentially allow for full utilization of the imaging benefits of MRI noted above (improved soft tissue delineation, etc.). Additionally, the imaging obtained during MRgRT is obtained in the treatment position immediately prior to delivery of RT, potentially allowing for better delineation of nearby organs. Limited publications on the use of MRgRT in HNSCC exist; the current data are summarized below.
A retrospective review of the use of the first clinically implemented machine for MRgRT, an older 0.35 T Cobalt 60 machine, included treatment of 17 HNSCC patients (6%, in a study describing 316 patients) [18]. A single institution experience of 13 patients with recurrent or second primary HNSCC treated using the older 0.35 T machine with Cobalt 60 source showed effective disease control with relatively low toxicity [19]. A description of prospective treatment of 10 patients utilizing the Elekta 1.5 T system following the Radiotherapy predicate studies, Idea, Development, Exploration, Assessment, Long-term evaluation conceptual framework for technical development (R-IDEAL) [20] showed that use of adaptive MRgRT is safe and feasible for HNSCC [21]. Prospective treatment with MRgRT within the multi-institutional MR-Linac Consortium on the MOMENTUM study (NCT04075305) evaluating use of the 1.5 T system included treatment of 13 patients with HNSCC and showed good tolerability of the MRgRT approach [22]. A single-institution registry study of 18 patients treated with MRgRT showed that the clinical outcomes were similar to standard RT approaches [23]. Thus, the existing limited data suggest that the use of MRgRT to adapt treatment for HNSCC is safe and feasible; however, it remains unknown whether MRgRT offers improved outcomes without compromising tumor control as compared with standard IGRT.
There are multiple ongoing or planned trials integrating MRgRT into treatment of HNSCC. The MR-ADAPTOR trial (NCT03224000) led by MD Anderson is an ongoing randomized trial utilizing the 1.5 T system, comparing standard treatment to MRI-adapted treatment in patients with human papillomavirus-related oropharyngeal squamous cell carcinoma [24]. The MARTHA trial in Switzerland (NCT03972072) aims to assess the feasibility of reducing xerostomia using the 0.35 T system. The INSIGHT-2 trial in the United Kingdom (NCT04242459) aims to personalize HNSCC dose using MRgRT. A Canadian trial is planned to assess the potential of implementing stereotactic body RT in HNSCC (NCT04809792) utilizing the 1.5 T system. Thus, while there is currently a paucity of data in utilization of MRgRT for treatment of HNSCC, multiple ongoing trials are aiming to further the use of this system and provide additional information. However, challenges to wider implementation in the treatment of HNSCC exist.
References
- Hawkins, P.G.; Lee, J.; Mao, Y.; Li, P.; Green, M.; Worden, F.P.; Swiecicki, P.L.; Mierzwa, M.L.; Spector, M.E.; Schipper, M.J.; et al. Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiother. Oncol. 2018, 126, 68–74.
- Vainshtein, J.M.; Moon, D.H.; Feng, F.Y.; Chepeha, D.; Eisbruch, A.; Stenmark, M.H. Long-Term Quality of Life After Swallowing and Salivary-Sparing Chemo–Intensity Modulated Radiation Therapy in Survivors of Human Papillomavirus–Related Oropharyngeal Cancer. Int. J. Radiat. Oncol. 2015, 91, 925–933.
- Eisbruch, A.; Kim, H.M.; Feng, F.Y.; Lyden, T.H.; Haxer, M.J.; Feng, M.; Worden, F.P.; Bradford, C.R.; Prince, M.E.; Moyer, J.S.; et al. Chemo-IMRT of Oropharyngeal Cancer Aiming to Reduce Dysphagia: Swallowing Organs Late Complication Probabilities and Dosimetric Correlates. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e93–e99.
- Feng, F.Y.; Kim, H.M.; Lyden, T.H.; Haxer, M.J.; Worden, F.P.; Feng, M.; Moyer, J.S.; Prince, M.E.; Carey, T.E.; Wolf, G.T.; et al. Intensity-Modulated Chemoradiotherapy Aiming to Reduce Dysphagia in Patients With Oropharyngeal Cancer: Clinical and Functional Results. J. Clin. Oncol. 2010, 28, 2732–2738.
- Morgan, H.; Sher, D.J. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck 2020, 5, 1–16.
- Schwartz, D.L.; Garden, A.S.; Shah, S.J.; Chronowski, G.; Sejpal, S.; Rosenthal, D.; Chen, Y.; Zhang, Y.; Zhang, L.; Wong, P.-F.; et al. Adaptive radiotherapy for head and neck cancer—Dosimetric results from a prospective clinical trial. Radiother. Oncol. 2013, 106, 80–84.
- Park, S.I.; Guenette, J.P.; Suh, C.H.; Hanna, G.J.; Chung, S.R.; Baek, J.H.; Lee, J.H.; Choi, Y.J. The diagnostic performance of CT and MRI for detecting extranodal extension in patients with head and neck squamous cell carcinoma: A systematic review and diagnostic meta-analysis. Eur. Radiol. 2020, 31, 2048–2061.
- Sumi, M.; Nakamura, T. Extranodal spread in the neck: MRI detection on the basis of pixel-based time-signal intensity curve analysis. J. Magn. Reson. Imaging 2011, 33, 830–838.
- Boeke, S.; Mönnich, D.; van Timmeren, J.E.; Balermpas, P. MR-Guided Radiotherapy for Head and Neck Cancer: Current Developments, Perspectives, and Challenges. Front. Oncol. 2021, 11, 616156.
- Chuter, R.W.; Pollitt, A.; Whitehurst, P.; Mackay, R.I.; Van Herk, M.; McWilliam, A. Assessing MR-linac radiotherapy robustness for anatomical changes in head and neck cancer. Phys. Med. Biol. 2018, 63, 125020.
- Raaymakers, B.W.; Raaijmakers, A.J.E.; Lagendijk, J.J.W. Feasibility of MRI guided proton therapy: Magnetic field dose effects. Phys. Med. Biol. 2008, 53, 5615–5622.
- Pollard, J.M.; Wen, Z.; Sadagopan, R.; Wang, J.; Ibbott, G.S. The future of image-guided radiotherapy will be MR guided. Br. J. Radiol. 2017, 90, 20160667.
- Bruynzeel, A.M.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.; Spoelstra, F.O.; Piet, A.H.; Meijnen, P.; van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1086–1094.
- Bohoudi, O.; Bruynzeel, A.M.; Meijerink, M.R.; Senan, S.; Slotman, B.J.; Palacios, M.A.; Lagerwaard, F. Identification of patients with locally advanced pancreatic cancer benefitting from plan adaptation in MR-guided radiation therapy. Radiot. Oncol. 2019, 132, 16–22.
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Sicignano, G.; De Simone, A.; Naccarato, S.; et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: Feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat. Oncol. 2020, 15, 1–9.
- Cao, M.; Gao, Y.; Yoon, S.M.; Yang, Y.; Sheng, K.; Ballas, L.K.; Basehart, V.; Sachdeva, A.; Felix, C.; Low, D.A.; et al. Interfractional Geometric Variations and Dosimetric Benefits of Stereotactic MRI Guided Online Adaptive Radiotherapy (SMART) of Prostate Bed after Radical Prostatectomy: Post-Hoc Analysis of a Phase II Trial. Cancers 2021, 13, 2802.
- Chuong, M.D.; Bryant, J.; Mittauer, K.E.; Hall, M.; Kotecha, R.; Alvarez, D.; Romaguera, T.; Rubens, M.; Adamson, S.; Godley, A.; et al. Ablative 5-Fraction Stereotactic Magnetic Resonance–Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract. Radiat. Oncol. 2021, 11, 134–147.
- Fischer-Valuck, B.W.; Henke, L.; Green, O.; Kashani, R.; Achraya, S. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Adv. Radiat. Oncol. 2017, 2, 485–493.
- Chen, A.M.; Cao, M.; Hsu, S.; Lamb, J.; Mikaeilian, A.; Yang, Y.; Agazaryan, N.; Low, D.A.; Steinberg, M.L. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv. Radiat. Oncol. 2017, 2, 167–175.
- Verkooijen, H.M.; Kerkmeijer, L.G.W.; Fuller, C.D.; Huddart, R.; Faivre-Finn, C.; Verheij, M.; Mook, S.; Sahgal, A.; Hall, E.; Schultz, C. R-IDEAL: A Framework for Systematic Clinical Evaluation of Technical Innovations in Radiation Oncology. Front. Oncol. 2017, 7, 59.
- McDonald, B.A.; Vedam, S.; Yang, J.; Wang, J.; Castillo, P.; Lee, B.; Sobremonte, A.; Ahmed, S.; Ding, Y.; Mohamed, A.S.; et al. Initial Feasibility and Clinical Implementation of Daily MR-Guided Adaptive Head and Neck Cancer Radiation Therapy on a 1.5 T MR-Linac System: Prospective R-IDEAL 2a/2b Systematic Clinical Evaluation of Technical Innovation. Int. J. Radiat. Oncol. 2020, 109, 1606–1618.
- De Mol Van Otterloo, S.R.; Christodouleas, J.P.; Blezer, E.L.; Akhiat, H.; Brown, K.; Choudhury, A.; Eggert, D.; Erickson, B.A.; Daamen, L.A.; Faivre-Finn, C.; et al. Patterns of Care, Tolerability, and Safety of the First Cohort of Patients Treated on a Novel High-Field MR-Linac Within the MOMENTUM Study: Initial Results From a Prospective Multi-Institutional Registry. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 867–875.
- Chen, A.M.; Hsu, S.; Lamb, J.; Yang, Y.; Agazaryan, N.; Steinberg, M.L.; Low, D.A.; Cao, M. MRI-guided radiotherapy for head and neck cancer: Initial clinical experience. Clin. Transl. Oncol. 2017, 20, 160–168.
- Bahig, H.; Yuan, Y.; Mohamed, A.; Brock, K.K.; Ng, S.P.; Wang, J.; Ding, Y.; Hutcheson, K.; McCulloch, M.; Balter, P.A.; et al. Magnetic Resonance-based Response Assessment and Dose Adaptation in Human Papilloma Virus Positive Tumors of the Oropharynx treated with Radiotherapy (MR-ADAPTOR): An R-IDEAL stage 2a-2b/Bayesian phase II trial. Clin. Transl. Radiat. Oncol. 2018, 13, 19–23.
More
