Plant-derived (phyto) carbazole alkaloids are an important class of compounds, presented in the family of Rutaceae (Genera Murraya, Clausena, Glycosmis, Micromelum and Zanthoxylum). Due to several significant biological activities, such as antitumor, antibacterial, antiviral, antidiabetic, anti-HIV and neuroprotective activities of the parent skeleton (3-methylcarbazole), carbazole alkaloids are recognized as an important class of potential therapeutic agents. Neurodegenerative diseases (NDs) may exhibit a vast range of conditions, affecting neurons primarily and leading ultimately to the progressive losses of normal motor and cognitive functions. The main pathophysiological indicators of NDs comprise increasing atypical protein folding, oxidative stresses, mitochondrial dysfunctions, deranged neurotransmissions and neuronal losses. Phyto-carbazole alkaloids can be investigated for exerting multitarget approaches to ameliorating NDs.
- neurodegenerative disease
- Rutaceae
- phyto carbazole alkaloid
- oxidative stress
- neuroinflammation
- Alzheimer’s disease
1. Introduction
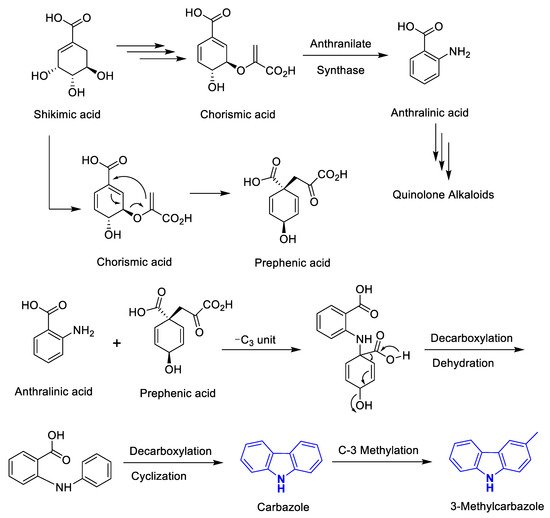
2. Phyto-Carbazole Alkaloids in Neuroprotection
2. Phyto-Carbazole Alkaloids in Neuroprotection
2.1. Phyto-Carbazole Alkaloids from the Genus Murraya
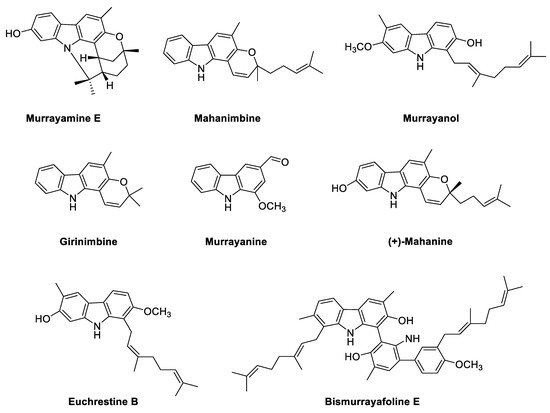
2.2. Phyto-Carbazole Alkaloids from the Genus Clausena
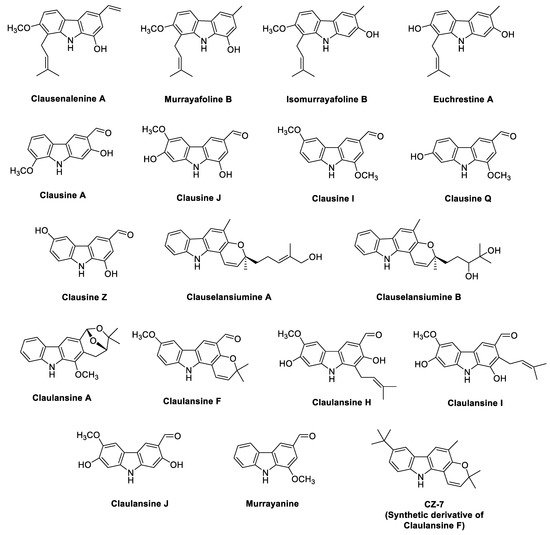
2.3. Phyto-Carbazole Alkaloids from Glycomis pentaphylla
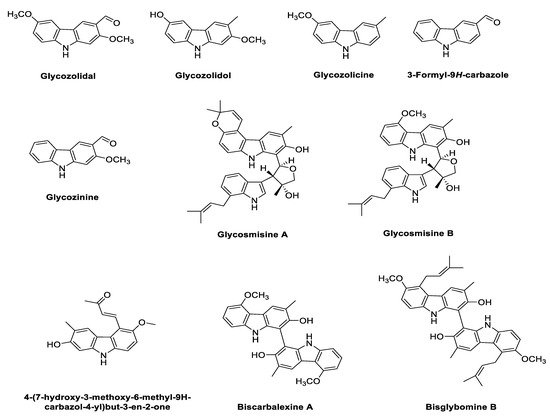
2.4. Phyto-Carbazole Alkaloids from Micromelum
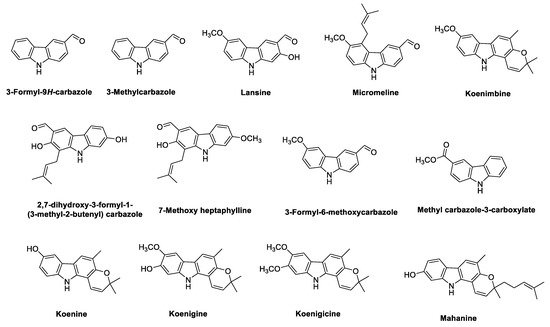
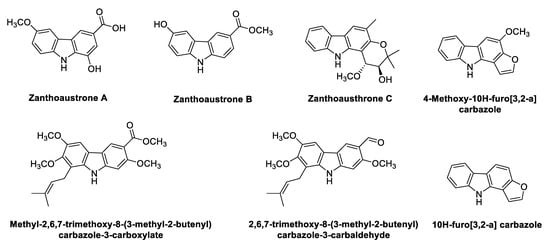
2.5. Carbazole Alkaloids from Zanthoxylum
References
- Winner, B.; Kohl, Z.; Gage, F.H. Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 2011, 33, 1139–1151.
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15.
- Sharma, N.; Tan, M.A.; An, S.S.A. Mechanistic aspects of Apiaceae family spices in ameliorating Alzheimer’s disease. Antioxidants 2021, 10, 1571.
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413.
- Cui, X.; Lin, Q.; Liang, Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front. Aging Neurosci. 2020, 12, 209.
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential metabolic modulators in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 12255.
- Głuszyńska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426.
- Ito, C.; Itoigawa, M.; Aizawa, K.; Yoshida, K.; Ruangrungsi, N.; Furukawa, H. γ-Lactone carbazoles from Clausena anisata. J. Nat. Prod. 2009, 72, 1202–1204.
- Thiratmatrakul, S.; Yenjai, C.; Waiwut, P.; Vajragupta, O.; Reubroycharoen, P.; Tohda, M.; Boonyarat, C. Synthesis, biological evaluation and molecular modeling study of novel tacrine–carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 75, 21–30.
- Börger, C.; Bruetting, C.; Julich-Gruner, K.K.; Hesse, R.; Kumar, V.P.; Kutz, S.K.; Ronnefahrt, M.; Thomas, C.; Wan, B.; Franzblau, S.G.; et al. Anti-tuberculosis activity and structure–activity relationships of oxygenated tricyclic carbazole alkaloids and synthetic derivatives. Bioorg. Med. Chem. 2017, 25, 6167–6174.
- Yang, J.-H.; Wang, X.-Y.; Zhou, Y.-P.; Lu, R.; Chen, C.-H.; Zhang, M.-H.; Cheng, Y.-Y.; Morris-Natschke, S.; Lee, K.-H.; Wang, Y.-S. Carbazole alkaloids from Clausena anisumolens: Isolation, characterization, and anti-HIV evaluation. Molecules 2020, 25, 99.
- Schmidt, A.W.; Reddy, K.R.; Knölker, H.J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012, 112, 3193–3328.
- Greger, H. Phytocarbazoles: Alkaloids with great structural diversity and pronounced biological activities. Phytochem. Rev. 2017, 16, 1095–1153.
- Birari, R.; Roy, S.K.; Singh, A.; Bhutani, K.K. Pancreatic lipase inhibitory alkaloids of Murraya koenigii leaves. Nat. Prod. Commun. 2009, 4, 1089–1092.
- Birari, R.; Javia, V.; Bhutani, K.K. Antiobesity and lipid lowering effects of Murraya koenigii (L.) Spreng leaves extracts and mahanimbine on high fat diet induced obese rats. Fitoterapia 2010, 81, 1129–1133.
- Ito, C.; Itoigawa, M.; Nakao, K.; Murata, T.; Kaneda, N.; Furukawa, H. Apoptosis of HL-60 leukemia cells induced by carbazole alkaloids isolated from Murraya euchrestifolia. J. Nat. Med. 2012, 66, 357–361.
- Huang, L.; Feng, Z.-L.; Wang, Y.-T.; Lin, L.-G. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017, 15, 881–888.
- Guillonneau, C.; Pierré, A.; Charton, Y.; Guilbaud, N.; Kraus-Berthier, L.; Léonce, S.; Michel, A.; Bisagni, E.; Atassi, G. Synthesis of 9-O-substituted derivatives of 9-hydroxy-5, 6-dimethyl-6 H-pyrido carbazole-1-carboxylic acid (2-(Dimethylamino) ethyl) amide and their 10-and 11-methyl analogues with improved antitumor activity. J. Med. Chem. 1999, 42, 2191–2203.
- Chen, Y.; Cao, N.; Lv, H.; Zeng, K.; Yuan, J.; Guo, X.; Zhao, M.; Tu, P.; Jiang, Y. Antiinflammatory and cytotoxic carbazole alkaloids from Murraya kwangsiensis. Phytochemistry 2020, 170, 112186.
- Zhang, F.F.; Gan, L.L.; Zhou, G.H. Synthesis, anti-bacterial and antifungal activities of some carbazole derivatives. Bioorg. Med. Chem. 2010, 20, 1881–1884.
- Thevissen, K.; Marchand, A.; Chaltin, P.; Meert, E.M.K.; Cammue, B.P.A. Antifungal carbazoles. Curr. Med. Chem. 2009, 16, 2205–2211.
- Patel, O.P.; Mishra, A.; Maurya, R.; Saini, D.; Pandey, J.; Taneja, I.; Raju, K.S.R.; Kanojiva, S.; Shukla, S.K.; Srivastava, M.N.; et al. Naturally occurring carbazole alkaloids from Murraya koenigii as potential antidiabetic agents. J. Nat. Prod. 2016, 79, 1276–1284.
- Wei, R.; Ma, Q.; Li, T.; Liu, W.; Sang, Z.; Li, M.; Liu, S. Carbazole alkaloids with antiangiogenic activities from Clausena sanki. Bioorg. Chem. 2018, 77, 387–392.
- Ashok, P.; Chander, S.; Smith, T.K.; Sankaranarayanan, M. Design, synthesis and biological evaluation of piperazinyl-β-carboline derivatives as anti-leishmanial agents. Eur. J. Med. Chem. 2018, 150, 559–566.
- Zang, Y.; Liu, K.; Wang, W.; Li, C.; Ma, J.; Yang, J.; Chen, X.; Wang, X.; Zhang, D. Claulansine F–donepezil hybrids as anti-Alzheimer’s disease agents with cholinergic, free-radical scavenging, and neuroprotective activities. Molecules 2021, 26, 1303.
- Kongkathip, B.; Kongkathip, N.; Sunthitikawinsakul, A.; Napaswat, C.; Yoosook, C. Anti-HIV-1 constituents from Clausena excavata: Part II. Carbazoles and a pyranocoumarin. Phytother. Res. 2005, 19, 728–731.
- Saengkhae, C.; Salerno, M.; Ades, D.; Siove, A.; le Moyec, L.; Migonney, V.; Garnier-Suillerot, A. Ability of carbazole salts, inhibitors of Alzheimer beta-amyloid fibril formation, to cross cellular membranes. Eur. J. Med. Chem. 2007, 559, 124–131.
- Bertini, S.; Asso, V.; Ghilardi, E.; Granchi, C.; Manera, C.; Minutolo, F.; Saccomanni, G.; Bortolato, A.; Mason, J.; Moro, S.; et al. Carbazole-containing arylcarboxamides as bace1 inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 6657–6661.
- Fang, L.; Fang, X.; Gou, S.; Lupp, A.; Lenhardt, I.; Sun, Y.; Huang, Z.; Chen, Y.; Zhang, Y.; Fleck, C. Design, synthesis and biological evaluation of D-ring opened galantamine analogs as multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2014, 76, 376–386.
- Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88.
- Knoelker, H.J.; Reddy, K.R. Chapter 3—Biogenesis of carbazole alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: Leiden-Boston, The Netherlands, 2008; Volume 65, pp. 159–180.
- Meena, S.; Kumar, S.R.; Dwivedi, V.; Singh, A.K.; Chanotiya, C.S.; Akhtar, M.Q.; Kumar, K.; Shasany, A.K.; Nagegowda, D.A. Transcriptomic insight into terpenoid and carbazole alkaloid biosynthesis, and functional characterization of two terpene synthases in curry tree (Murraya koenigii). Sci. Rep. 2017, 7, 44126.
- Iyer, D.; Devi, U. Phyto-pharmacology of Murraya koenigii (L.). Pharmacog. Rev. 2008, 2, 180–184.
- Balakrishnan, R.; Vijayraja, D.; Jo, S.H.; Ganesan, P.; In, S.K.; Choi, D.K. Medicinal profile, phytochemistry, and pharmacological activities of Murraya koenigii and its primary bioactive compounds. Antioxidants 2020, 9, 101.
- Mani, V.; Ramasamy, K.; Ahmad, A.; Wahab, S.N.; Jaafar, S.M.; Kek, T.L.; Salleh, M.Z.; Majeed, A.B.A. Effects of the total alkaloidal extract of Murraya koenigii leaf on oxidative stress and cholinergic transmission in aged mice. Phytother. Res. 2013, 27, 46–53.
- Tachibana, Y.; Kikuzaki, H.; Lajis, N.H.; Nakatani, N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agr. Food Chem. 2001, 49, 5589–5594.
- Vasudevan, M.; Parle, M. Antiamnesic potential of Murraya koenigii leaves. Phytother. Res. 2009, 23, 308–316.
- Nalli, Y.; Khajuria, V.; Gupta, S.; Arora, P.; Riyaz-Ul-Hassan, S.; Ahmed, Z.; Ali, A. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org. Biomol. Chem. 2016, 14, 3322–3332.
- Ramsewak, R.S.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L.; Nitiss, J.L. Biologically active carbazole alkaloids from Murraya koenigii. J. Agric. Food Chem. 1999, 47, 444–447.
- Yano, M.; Nakashima, S.; Kasa, S.; Nakamura, S.; Nishimura, K.; Oda, Y.; Takata, K.; Matsuda, H. Accelerative effects of carbazole-type alkaloids from Murraya koenigii on neurite outgrowth and their derivative’s in vivo study for spatial memory. J. Nat. Med. 2020, 74, 448–455.
- Ma, X.; Chen, H.; Zhu, S.; Tu, P.; Jiang, Y. Trimeric and dimeric carbazole alkaloids from Murraya microphylla. Molecules 2021, 26, 5689.
- Lv, H.; Wen, R.; Zhou, Y.; Zeng, K.; Li, J.; Guo, X.; Tu, P.; Jiang, Y. Nitrogen oxide inhibitory trimeric and dimeric carbazole alkaloids from Murraya tetramera. J. Nat. Prod. 2015, 78, 2432–2439.
- Lyu, H.-N.; Zhou, Y.; Wen, R.; Tu, P.-F.; Jiang, Y. Nitric oxide inhibitory carbazole alkaloids from the folk medicine Murraya tetramera C.C. Huang. Chem. Biodivers. 2020, 17, e2000490.
- Kumar, N.S.; Mukherjee, P.K.; Bhadra, S.; Saha, B.P.; Pal, B.C. Acetylcholinesterase inhibitory potential of a carbazole alkaloid, mahanimbine, from Murraya koenigii. Phytother. Res. 2010, 24, 629–631.
- Azahan, N.S.M.; Mani, V.; Ramasamy, K.; Lim, S.M.; Alhowail, A.; Sajid, S.; Majeed, A.B.A. Neuroprotective potential of mahanimbine against lipopolysaccharides (LPS)-induced neuronal deficits on SK-N-SH cells and antioxidant potentials in ICR mice brain. J. Pharm. Res. Int. 2019, 31, 1–11.
- Azahan, N.S.M.; Mani, V.; Ramasamy, K.; Lim, S.M.; James, R.M.J.; Alsharidah, M.; Alhowail, A.; Majeed, A.B.A. Mahanimbine-induced neuroprotection via cholinergic system and attenuated amyloidogenesis as well as neuroinflammation in lipopolysaccharides-induced mice. Pharmacog. Mag. 2020, 16, 57–63.
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 2020, 25, 4926.
- Liang, S.; Zheng, Y.; Lei, L.; Deng, X.; Ai, J.; Li, Y.; Zhang, T.; Mei, Z.; Ren, Y. Corydalis edulis total alkaloids (CETA) ameliorates cognitive dysfunction in rat model of Alzheimer disease through regulation of the antioxidant stress and MAP2/NF-κB. J. Ethnopharmacol. 2020, 251, 112540.
- Mani, V.; Ramasamy, K.; Ahmad, A.; Parle, M.; Shah, S.A.A.; Majeed, A.B.A. Protective effects of total alkaloidal extract from Murraya koenigii leaves on experimentally induced dementia. Food Chem. Toxicol. 2012, 50, 1036–1044.
- Chakraborty, D.P.; Barman, B.K.; Bose, P.K. On the constitution of murrayanine, a carbazole derivative isolated from Murraya koengii Spreng. Tetrahedron 1965, 21, 681–685.
- Laroux, F.S.; Lefer, D.J.; Kawachi, S.; Scalia, R.; Cockrell, A.S.; Gray, L.; Van der Heyde, H.; Hoffman, J.M.; Grisham, M.B. Role of nitric oxide in the regulation of acute and chronic inflammation. Antioxid. Redox Signal. 2000, 2, 391–396.
- Jha, N.K.; Jha, S.K.; Kar, R.; Nand, P.; Swati, K.; Goswami, V.K. Nuclear factor-kappa β as a therapeutic target for Alzheimer’s disease. J. Neurochem. 2019, 150, 113–137.
- Pandya, P.N.; Kumar, S.P.; Bhadresha, K.; Patel, C.N.; Patel, S.K.; Rawal, R.M.; Mankad, A.U. Identification of promising compounds from curry tree with cyclooxygenase inhibitory potential using a combination of machine learning, molecular docking, dynamics simulations and binding free energy calculations. Mol. Simul. 2020, 46, 812–822.
- Iman, V.; Mohan, S.; Abdelwahab, S.I.; Karimian, H.; Nordin, N.; Fadaeinasab, M.; Noordin, M.H.; Noor, S.M. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug. Des. Devel. Ther. 2017, 11, 103–121.
- Mohan, S.; Hobani, Y.H.; Shaheen, E.; Abou-Elhamd, A.S.; Alhazmi, H.A.; Abdelwahab, S.I. Girinimbine from curry leaves promotes gastro protection against ethanol induced peptic ulcers and improves healing via regulation of anti-inflammatory and antioxidant mechanisms. Food Funct. 2020, 11, 3493–3505.
- Liu, Y.-P.; Guo, J.-M.; Xie, Z.; Suo, X.-Y.; Liu, Z.-Y.; Qiao, Z.-H.; Guan, R.-Q.; Bian, Y.; Qiang, L.; Fu, Y.-H. Clausanisumine, a prenylated bicarbazole alkaloid from the fruits of Clausena anisumolens and its potential anti-HIV activity. J. Org. Chem. 2021, 86, 17722–17726.
- Liu, Y.-P.; Hu, S.; Liu, Y.-Y.; Zhang, M.-M.; Zhang, W.-H.; Qiang, L.; Fu, Y.H. Anti-inflammatory and antiproliferative prenylated carbazole alkaloids from Clausena vestita. Bioorg. Chem. 2019, 91, 103107.
- Liu, Y.-P.; Guo, J.-M.; Wang, X.-P.; Liu, Y.-Y.; Zhang, W.; Wang, T.; Qiang, L.; Fu, Y. Geranylated carbazole alkaloids with potential neuroprotective activities from the stems and leaves of Clausena lansium. Bioorg. Chem. 2019, 92, 103278.
- Xia, H.-M.; Yang, G.-Q.; Li, C.-J.; Yang, J.-Z.; Ma, J.; Zhang, D.; Li, Y.; Li, L.; Zhang, D.-M. Clauemarazoles A–G, seven carbazole alkaloids from the stems of Clausena emarginata. Fitoterapia 2015, 103, 83–89.
- Cao, N.; Chen, Y.; Ma, X.; Zeng, K.; Zhao, M.; Tu, P.; Li, J.; Jiang, Y. Bioactive carbazole and quinoline alkaloids from Clausena dunniana. Phytochem. 2018, 151, 1–8.
- Phachonpai, W.; Tongun, T. Neuroprotective and cognitive enhancing effects of Clausena lansium (Lour.) skeels peels extract in ischemic rat brains. J. Applied Pharm. Sci. 2021, 11, 001–008.
- Yan, G.; Li, Y.-J.; Zhao, Y.-Y.; Guo, J.-M.; Zhang, W.-H.; Zhang, M.-M.; Fu, Y.-H.; Liu, Y.-P. Neuroprotective carbazole alkaloids from the stems and leaves of Clausena lenis. Nat. Prod. Res. 2021, 35, 2002–2009.
- Potterat, O.; Puder, C.; Bolek, W.; Wagner, K.; Ke, C.; Ye, Y.; Gillardon, F. Clausine Z, a new carbazole alkaloid from Clausena excavata with inhibitory activity on CDK5. Pharmazie 2005, 60, 637–639.
- Weishaupt, J.H.; Kussmaul, L.; Grotsch, P.; Heckel, A.; Rohde, G.; Romig, H.; Bahr, M.; Gillardon, F. Inhibition of CDK5 is protective in necrotic and apoptotic paradigms of neuronal cell death and prevents mitochondrial dysfunction. Mol. Cell. Neurosci. 2003, 24, 489–502.
- Pao, P.-C.; Tsai, L.H. Three decades of Cdk5. J. Biomed. Sci. 2021, 28, 79.
- Tseng, H.-C.; Zhou, Y.-Z.; Shen, Y.; Tsai, L.-H. A survey of Cdk5 activator p35 and p25 levels in Alzheimer’s disease brains. FEBS Lett. 2002, 523, 58–62.
- Lee, M.S.; Kwon, Y.T.; Li, M.; Peng, J.; Friedlander, R.M.; Tsai, L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 2000, 405, 360–364.
- Patrick, G.N.; Zukerberg, L.; Nikolic, M.; de la Monte, S.; Dikkes, P.; Tsai, L.-H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402, 615–622.
- Shelton, S.B.; Johnson, G.V. Cyclin-dependent kinase-5 in neurodegeneration. J. Neurochem 2004, 88, 1313–1326.
- Liu, Y.-P.; Guo, J.-M.; Liu, Y.-Y.; Hu, S.; Yan, G.; Qiang, L.; Fu, Y.-H. Carbazole alkaloids with potential neuroprotective activities from the fruits of Clausena lansium. J. Agric. Food Chem. 2019, 67, 5764–5771.
- Sun, X.; Li, C.; Ma, J.; Zang, Y.; Huang, J.; Chen, N.; Wang, X.; Zhang, D. New amide alkaloids and carbazole alkaloid from the stems of Clausena lansium. Fitoterapia 2021, 154, 104999.
- Liu, H.; Li, C.-J.; Yang, J.-Z.; Ning, N.; Si, Y.-K.; Li, L.; Chen, N.-H.; Zhao, J.; Zhang, D.-M. Carbazole alkaloids from the stems of Clausena lansium. J. Nat. Prod. 2012, 75, 677–682.
- Liu, D.-D.; Yuan, X.; Chu, S.-F.; Chen, C.; Ren, Q.; Luo, P.; Lin, M.-Y.; Wang, S.S.; Zhu, T.B.; Ai, Q.D.; et al. CZ-7, a new derivative of Claulansine F, ameliorates 2VO-induced vascular dementia in rats through a Nrf2-mediated antioxidant responses. Acta Pharmacol. Sin. 2019, 40, 425–440.
- Li, J.-W.; Ning, N.; Ma, Y.-Z.; Zhang, R.; Tan, F.; Chen, N.-H. Claulansine F suppresses apoptosis induced by sodium nitroprusside in PC12 cells. Free Radic. Res. 2013, 47, 488–497.
- Zang, Y.; Song, X.; Li, C.; Ma, J.; Chu, S.; Liu, D.; Ren, Q.; Li, Y.; Chen, N.; Zhang, D. Pyranocarbazole alkaloids as effective agents against ischemic stroke in vitro and in vivo. Eur. J. Med. Chem. 2018, 143, 438–448.
- Sivakumar, M.; Chamundeeswari, D. Identification of active phytocomponents in the ethyl acetate extract of Glycosmis pentaphylla (Retz.) DC. by using GC-MS. Int. J. Med. Health Biomed. Bioeng. Pharm. Eng. 2016, 10, 453–457.
- Murugan, N.; Natarajan, D. Phytochemical, antioxidant and antibacterial activities of Glycosmis pentaphylla (Rutaceae) leaf extracts against selected multi-drug resistant bacteria. J. Chem. Pharm. Res. 2016, 8, 737–744.
- Chowdhury, M.; Sultana, I.; Tasneem, Z.; Jubair, A.A. Phytochemical and pharmacological screening of Glycosmis pentaphylla (Retz.) DC. (fam. Rutaceae). Int. J. Sci. Eng. Res. 2015, 6, 928–934.
- Raju, N.J.; Rao, B.G. Evaluation of hepatoprotective activity of Glycosmis pentaphylla roots against CCl4–induced acute liver injury in rats. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 81–86.
- Bhattacharyya, P.; Chowdhury, B.K. Glycozolidal, a new carbazole alkaloid from Glycosmis pentaphylla. J. Nat. Prod. 1985, 48, 465–466.
- Bhattacharyya, P.; Chakrabartty, P.K.; Chowdhury, B.K. Glycozolidol, an antibacterial carbazole alkaloid from Glycosmis pentaphylla. Phytochemistry 1985, 24, 882–883.
- Chakraborty, A.; Chowdhury, B.K.; Jash, S.S.; Biswas, G.K.; Bhattacharyya, S.K.; Bhattacharyya, P. Carbazole alkaloids from Glycosmis pentaphylla. Phytochemistry 1992, 31, 2503–2505.
- Chen, Y.; Tang, C.; Wu, Y.; Mo, S.; Wang, S.; Yang, G.; Mei, Z. Glycosmisines A and B: Isolation of two new carbazole–indole-type dimeric alkaloids from Glycosmis pentaphylla and an evaluation of their antiproliferative activities. Org. Biomol. Chem. 2015, 13, 6773–6781.
- Yang, G.-Z.; Wu, Y.; Chen, Y. Alkaloids from the stems of Glycosmis pentaphylla. Helv. Chim. Acta 2012, 95, 1449–1454.
- Rahman, R.; Islam, T.; Faruquee, H.M.; Akhter, S.; Haque, M.A. Evaluation of antioxidant, cholinesterase inhibitory properties, and antibacterial potentials of Glycomis pentaphylla leaf extract relevant to the treatment of Alzheimer’s disease. J. Appl. Pharm. 2017, 9, 17–30.
- Prakasia, P.P.; Nair, A.S. Evaluation of in vitro antioxidant potential of the total crude alkaloid extract of Glycosmis pentaphylla (Retz.) Correa leaves. Int. J. Pharm. Pharm. Sci. 2016, 8, 85–91.
- Ma, C.; Case, R.J.; Wang, Y.; Zhang, H.-J.; Tan, G.T.; Van Hung, N.; Cuong, N.M.; Franzblau, S.G.; Soejarto, D.D.; Fong, H.H.S.; et al. Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med. 2005, 71, 261–267.
- Siridechakorn, I.; Ritthiwigrom, T.; Laphookhieo, S. Coumarins and carbazole alkaloids from the roots of Micromilum glanduliferum. Biochem. Syst. Ecol. 2012, 40, 69–70.
- Bowen, I.H.; Perera, K. Alkaloids, coumarins and flavonoids of Micromelum zeylanicum. Phytochemistry 1982, 21, 433–437.
- Nakahara, K.; Trakoontivakorn, G.; Alzoreky, N.S.; Ono, H.; Onishi-Kameyama, M.; Yoshida, M. Antimutagenicity of some edible Thai plants, and a bioactive carbazole alkaloid, mahanine, isolated from Micromelum minutum. J. Agric. Food Chem. 2002, 50, 4796–4802.
- Roy, M.K.; Thalang, V.N.; Trakoontivakorn, G.; Nakahara, K. Mechanism of mahanine-induced apoptosis in human leukemia cells (HL-60). Biochem. Pharmacol. 2004, 67, 41–51.
- Yang, X.-L.; Xie, Z.-H.; Jiang, X.-J.; Huang, Y.-B.; Liu, J.-K. A new acridone alkaloid from Micromelum integerrimum. Chem. Pharm. Bull. 2009, 57, 734–735.
- Thant, T.M.; Aminah, N.S.; Kristanti, A.N.; Ramadhan, R.; Aung, H.T.; Takaya, Y. Phytoconstituents of Genus Micromelum and their bioactivity—A Review. Nat. Prod. Commun. 2020, 15, 1–15.
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Udenigwe, C.C. Zanthoxylum Species: A comprehensive review of traditional uses, phytochemistry, pharmacological and nutraceutical applications. Molecules 2021, 26, 4023.
- Fu, Y.-H.; Guo, J.-M.; Xie, Y.-T.; Hua, J.; Dai, Y.-Y.; Zhang, W.; Lin, T.C.; Liu, Y.-P. Structural characterization, antiproliferative and anti-inflammatory activities of alkaloids from the roots of Zanthoxylum austrosinense. Bioorg. Chem. 2020, 102, 104101.
- Samad, A.; Badshah, S.; Khan, D.; Ali, F.; Amanullah, M.; Hanrahan, J. New prenylated carbazole alkaloids from Zanthoxylum armatum. J. Asian Nat. Prod. Res. 2014, 16, 1126–1131.
- Macías, V.; Coy, E.; Cuca, L.E. Novel furocarbazole a lkaloids and antibacterial activity of ethanol extract from Zanthoxylum fagara (L.) Sargent. Rev. Colomb. Quím. 2010, 39, 333–341.
- Han, H.J.; Park, S.K.; Kim, M.J.; An, J.W.; Lee, S.J.; Kang, J.Y.; Kim, J.M.; Heo, H.J. Industrial potential of domestic Zanthoxylum piperitum and Zanthoxylum schinifolium: Protective effect of both extracts on high glucose-induced neurotoxicity. Korean J. Food Sci. Technol. 2020, 52, 274–283.
- Hsu, Y.-H.; Chen, Y.-W.; Wu, M.-H.; Tu, L.-H. Protein glycation by glyoxal promotes amyloid formation by islet amyloid polypeptide. Biophys. J. 2019, 116, 2304–2313.
- Kim, J.G.; Lim, J.J.; You, J.S.; Kwon, H.J.; Lim, H.B. Comparative study of bioactivity and safety evaluation of ethanolic extracts of Zanthoxylum schinifolium fruit and pericarp. Molecules 2021, 26, 5919.
- Seoposengwe, K.; Van Tonder, J.J.; Steenkamp, V. In vitro neuroprotective potential of four medicinal plants against rotenone-induced toxicity in SH-SY5Y neuroblastoma cells. BMC Complement. Altern. Med. 2013, 13, 353.
- Menozzi-Smarrito, C.; Riera, C.E.; Munari, C.; Le Coutre, J.; Robert, F. Synthesis and evaluation of new alkylamides derived from α-hydroxysanshool, the pungent molecule in Szechuan pepper. J. Agric. Food Chem. 2009, 57, 1982–1989.
- Deng, S.; Rong, H.; Tu, H.; Zheng, B.; Mu, X.; Zhu, L.; Zhou, X.; Peng, W.; Xu, M.; Zhang, E.; et al. Molecular basis of neurophysiological and antioxidant roles of Szechuan pepper. Biomed. Pharmacother. 2019, 112, 108696.
- Zhao, M.; Tang, X.; Gong, D.; Xia, P.; Wang, F.; Xu, S. Bungeanum improves cognitive dysfunction and neurological deficits in D-galactose-induced aging mice via activating PI3K/Akt/Nrf2 signaling pathway. Front. Pharmacol. 2020, 11, 71.
- Kerr, F.; Sofola-Adesakin, O.; Ivanov, D.K.; Gatliff, J.; Perez-Nievas, B.G.; Bertrand, H.C.; Martinez, P.; Callard, R.; Snoeren, I.; Cocheme, H.M.; et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017, 13, e1006593.
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases 2019, 7, 22.
- Zeng, K.-W.; Wang, X.-M.; Ko, H.; Kwon, H.C.; Cha, J.W.; Yang, H.O. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid β-protein via the PI3K/Akt/Bad/BclXL-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2011, 672, 45–55.
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 619.
- Wang, R.; Yang, S.; Nie, T.; Zhu, G.; Feng, D.; Yang, Q. Transcription factors: Potential cell death markers in Parkinson’s disease. Neurosci. Bull. 2017, 33, 552–560.
- Zhong, K.; Li, X.-J.; Gou, A.-N.; Huang, Y.-N.; Bu, Q.; Gao, H. Antioxidant and cytoprotective activities of flavonoid glycosides-rich extract from the leaves of Zanthoxylum bungeanum. J. Food Nutr. Res. 2014, 2, 349–356.
- Jeong, C.H.; Kwak, J.H.; Kim, J.H.; Choi, G.N.; Kim, D.O.; Heo, H.J. Neuronal cell protective and antioxidant effects of phenolics obtained from Zanthoxylum piperitum leaf using in vitro model system. Food Chem. 2011, 125, 417–422.
- Plazas, E.A.; Avila, M.C.; Delgado, W.A.; Patino, O.J.; Cuca, L.E. In vitro antioxidant and anticholinesterase activities of Colombian plants as potential neuroprotective agents. Res. J. Med. Plants 2018, 12, 9–18.
- Adewusi, E.A.; Steenkamp, V. In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac. J. Trop. Med. 2011, 4, 829–835.
- Saikia, B.; Barua, C.C.; Sarma, J.; Haloi, P.; Tamuli, S.M.; Kalita, D.J.; Purkayastha, A.; Barua, A.G. Zanthoxylum alatum ameliorates scopolamine-induced amnesia in rats: Behavioral, biochemical, and molecular evidence. Indian J. Pharmacol. 2018, 50, 30–38.
- Levinthal, D.J.; DeFranco, D.B. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J. Biol. Chem. 2005, 280, 5875–5883.
- Slutsky, I.; Abumaria, N.; Wu, L.-J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.-G.; Zhuo, M.; et al. Enhancement of learning and memory by elevating brain magnesium. Neuron 2010, 65, 165–177.
- Colucci-D’ Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor BDNF, Physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777.
