You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Camila Xu.
The concept of “genetically modified organisms” (GMOs) refers to those organisms whose genome has been altered by inserting a gene from another organism, removing a gene, or changing a gene’s function to generate a desirable trait.
- GM crops
- genetic engineering
- Food Safety
1. Introduction
One of the marked public health achievements in modern history has been the acceleration of global food production in recent decades. The Green Revolution of the 1960s led key staple grain crops yields to significantly rise to cover the calorie demands of a growing population worldwide [1][2]. Technological innovations partly motivated this achievement, particularly the introduction of new synthetic chemical fertilizer and pesticide types in the 1940s, the development of higher-yielding grain varieties, agricultural labor being mechanized, and high-yield practices being adopted, including monocropping [3]. Unfortunately, malnutrition is still a leading risk factor for health consequences and death globally. Recent research reveals that 2 billion people are deficient in one micronutrient or more, almost 820 million people go hungry, and approximately 26.4% of the world’s population is affected by moderate and severe food insecurity [4]. Finally, the most recent available analysis reports that undernourishment is associated with 3 million child deaths a year or half of all child deaths in the world [5].
As look forward, facing one of the most formidable challenges of the 21st century: global food demand is expected to steeply continue to rise. A United Nations (UN) report states that the world population is expected to reach 9.8 billion by 2050 [6]. On the one hand, this increase poses colossal food production challenges because crop yield rates do not feed the world’s population [7][8]. On the other hand, economic expansion, globalization, and population growth have brought about structural shifts in consumption patterns worldwide. Surprisingly, meat demand has grown the most in the world, and the cattle industry has been identified to emit the most greenhouse gases. This rising demand has significantly impacted carbon emissions and land use [9]. Moreover, climate change and less cultivable land have resulted in an additional challenge to cover growing food demand [10]. Climate change is associated with rising temperatures and more extreme weather events, such as hurricanes, floods, droughts, and rainstorms. Apart from altered environmental conditions under which food production operates, there are fewer pollinating insects, increasing water scarcity, and alterations to relations linking pests, crops, weeds, and pathogens [1]. The analysis revealed that these situations could be daunting if no action is taken [10]. Here, researchers contemplate the potential of genetically modified (GM) crops to enhance sustainable food production [11].
The concept of “genetically modified organisms” (GMOs) refers to those organisms whose genome has been altered by inserting a gene from another organism, removing a gene, or changing a gene’s function to generate a desirable trait. These genes can come from the same species or a different one. Thus, species boundaries may be crossed to produce novel crops [12]. Depending on the final destination, GMOs can be classified as GM food when the direct consumers are humans and as GM feed when products are intended only for animals. It has been estimated that 70–90% of all GM crops are employed as animal feed [13]. The market share of GM products has grown since the first generations of GM crops were commercialized in the 1990s. The four leading biotech crops are maize, cotton, soybeans, and canola. Based on the global cultivated area for individual crops, 32% of maize, 80% of cotton, 77% of soybeans, and 30% of canola were biotech crops in 2017 (Figure 1). Herbicide tolerance has been a consistent dominant trait which, in 2018, covered 46% of the global area (a 1% drop compared to 2017) [14]. Today, the commercial use of GM crops also extends to other foods, such as sugar beet, papaya, eggplant Solanum melongena, potatoes, and apples [8].
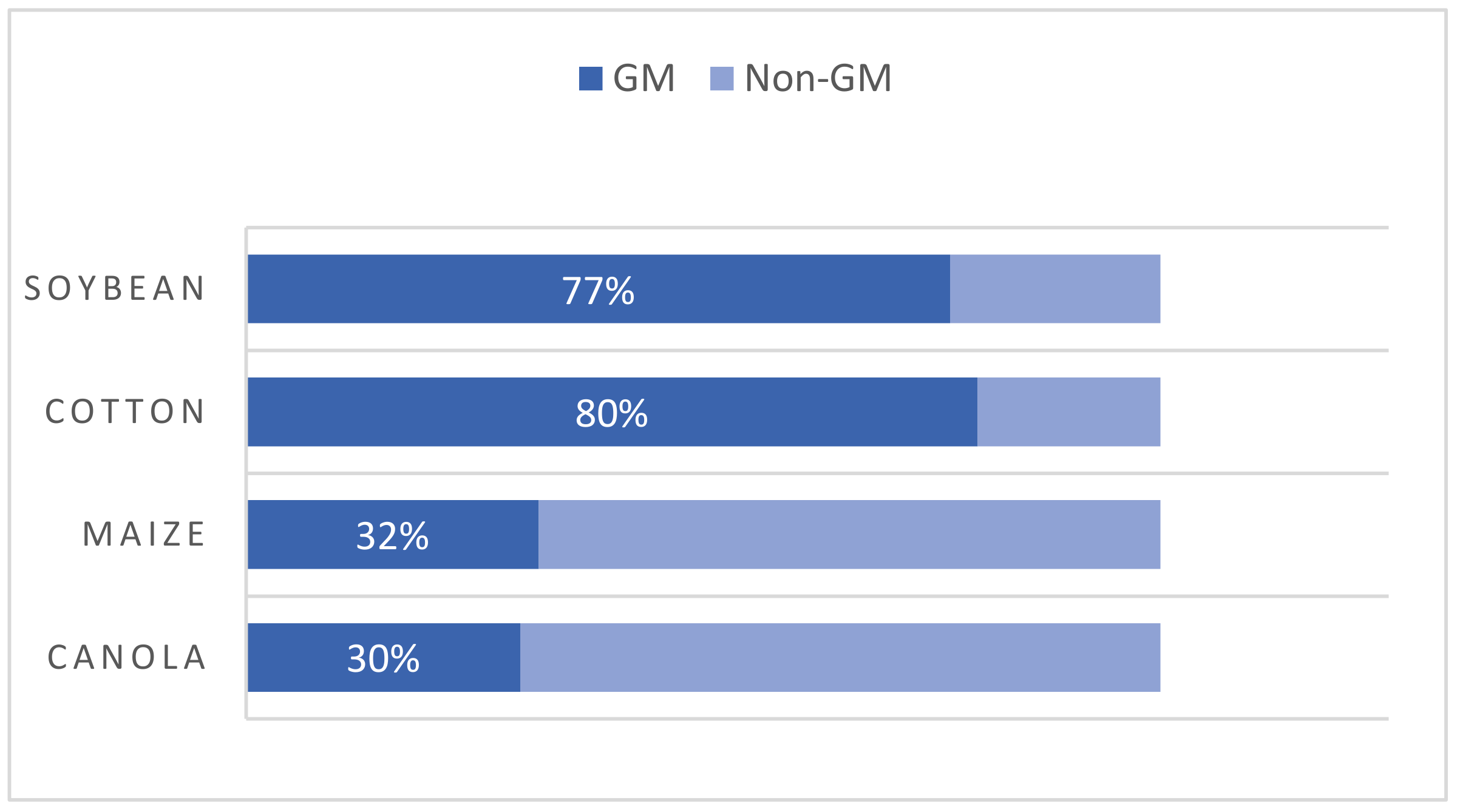 Figure 1. Percentages of the main biotech crops in 2017. Adapted from [14].
Figure 1. Percentages of the main biotech crops in 2017. Adapted from [14].In recent decades, technological molecular biology and genetic engineering (GE) advancements have enabled crops to be developed with improved traits, such as herbicide tolerance, good insect resistance, or better yields [15]. Moreover, interest in developing GM crops with improved nutritional properties is growing, such as higher levels of essential microelements, healthier crops by altering their fatty acids profile, or plants with delayed ripening [16]. Ever since the first GM food was introduced, the debate about the risks of releasing GM crops has been substantial. Lawmakers, scientists, and consumers have been divided on the subject of using GMOs to produce food and feed. Supporters believe that new crop enhancement advances could be a promising solution to ensure food security and cover increasing food demands [17].
2. Legislative Framework of Genetically Modified (GM) Crops
Both GM crops and their products must be rigorously evaluated before being commercially released. The legal framework that regulates GM food and feed attempts to ensure high protection levels for human and animal health and also for the environment [8]. Worldwide, the authorities responsible for evaluating GM products have adopted specific strategies based on the amount of experience and scientific knowledge acquired in the past few decades to assess their safety [18]. These principles were first submitted in 1993 by the Organization for Economic Co-operation and Development (OECD) and were further detailed by an international body jointly established by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) of the United Nations [19][20].
In 1963, the FAO and the WHO created the Codex Alimentarius Commission. The Codex develops international food standards, guidelines, and codes of practice to protect the health of consumers and to ensure fair practices in food trade. Moreover, it promotes the coordination of all food standards work undertaken by international organizations [21]. In 1999, the Codex established the Ad Hoc Intergovernmental Task Force on Foods Derived from Biotechnology (Figure 2). This group is responsible for developing and setting up guidelines, standards, or recommendations for foods that derive from applying modern biotechnology. The Task Force published three documents in 2003 that were adopted by Codex: Principles for the Risk Analysis of Foods Derived from Modern Biotechnology; Guideline for Safety Assessment of Foods Derived from Recombinant-DNA Plants; Guideline for Safety Assessment of Foods Derived from Recombinant-DNA Microbes [19]. The first document is a framework for performing risk analyses on either whole food derived from using biotechnology or the components of those foods. It discusses risk assessment, risk management, and risk communication. The Plant Guideline contains further details about the principles for risk analyses of foods derived from modern biotechnology. Specifically, paragraph 18 describes the framework for making such a safety assessment of food derived from a recombinant-DNA plant. This safety assessment framework follows a stepwise process to address relevant factors [18][19] (Figure 2).
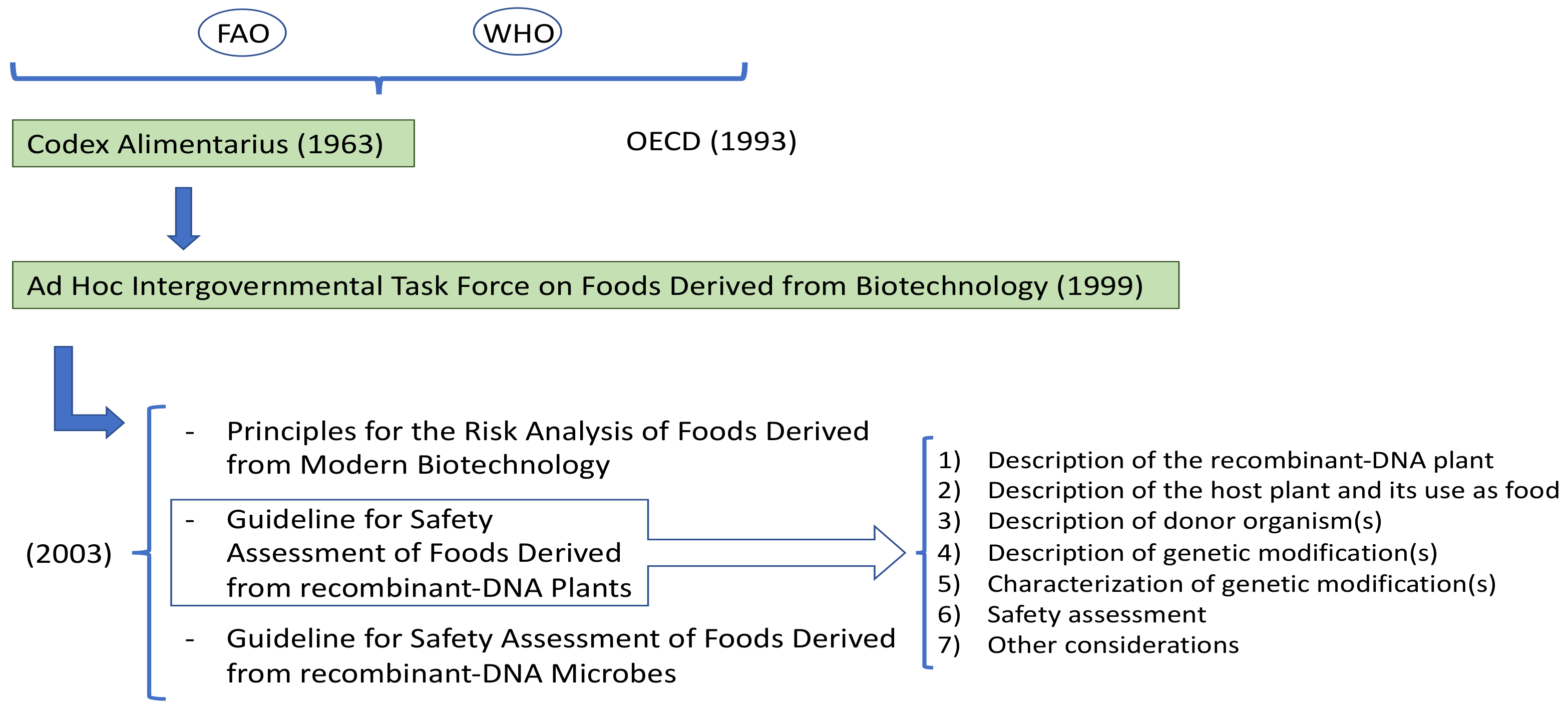 Figure 2. Development of standards and recommendations for products resulting from biotechnology.
Figure 2. Development of standards and recommendations for products resulting from biotechnology.
 Figure 2. Development of standards and recommendations for products resulting from biotechnology.
Figure 2. Development of standards and recommendations for products resulting from biotechnology.Labeling GMOs is also essential for helping consumers to make informed decisions. Consumers care about certain details, such as source, if food is processed, and if it contains additives [22]. GMOs are surrounded by plenty of controversies. Since the first GM products were commercially released, the debate about the real perceived risks of using GMOs has been underway. While dispute rages on, the public’s GM crop concerns have prompted governments to step in and adopt legislation that insists on GM products being labeled [17]. Notwithstanding, no agreement has been reached by government agencies about mandatory labeling, which renders the strategy ineffective and also makes transportation and trade extremely difficult [17][23].
The dispute over GMO labeling is structured on how much information consumers should receive and if this information provides enough knowledge about the contents to help consumers make better decisions. A difference exists between voluntary and mandatory GMO labeling. Voluntary labeling informs consumers that products do not contain bioengineered substances. Mandatory labeling goes much further because it expects all food products that contain GMOs to include warning labels [17]. Both these labeling schemes have their pros and cons. Consumers have the right to know what their food contains, and labeling must help them make purchasing decisions. However, as consumer knowledge about new technology such as GE is limited, they cannot often establish whether GM products spell danger or how to measure any given risk against potential benefits. Hence, individuals rely on those people they perceive as being trustworthy experts to reach informed conclusions, which they might use to form their own views. Consequently, trust is critical in layperson evaluations of GM foods [24].
The first labeling legislation on GM foods was framed by the European Union (EU) in 1997. Later, more modifications were made to legislation on GMO derivatives [25]. In 2007, the Codex Alimentarius Commission made an attempt to produce labeling guidelines for biotech products, but it was prevented because no consensus was reached by different countries [26]. Nonetheless, an increasing number of countries have been involved in labeling with distinct regulatory characteristics [17]. At least 64 countries worldwide, such as the EU countries, China and Australia, expect some form of GMO labeling [24]. Policies and the extent to which they are adopted differ from one country to the next. The Codex Committee has addressed the costs incurred to implement and enforce labeling, as well as potential advantages. Test requirements, agricultural production traceability, processing and distribution, document verification, analytical method feasibility and detection limits, and consumer education are only some of the main concerns being addressed. It would appear unlikely that a worldwide agreement will be reached in the near future because the USA strongly opposes labeling. The US GM labeling position is based on the “substantial equivalence” (SE) premise [17]. This concept first appeared in 1993 and embodies the notion that if a novel food has the same composition and features as conventional food, then it must be taken as safe as conventional food [20]. Proponents of the SE principle state that requiring labeling is not necessary because customers are more concerned about health and safety, functionality, and food usage than about manufacturing processes. Mandatory GMO labeling gives an impression, and even serves as a warning, that these foods differ or are less safe than their non-GM counterparts. The EU takes a “precautionary stance”. As the perceived or true risk of GMOs in long-term exposure remains unknown, labeling is vital for traceability considerations, along with consumer “right to know” legislation. According to the EU, it is no easy task to contemplate and measure the effects of these foods because the population has not been exposed for very long. Lack of evidence does not rule out the time-lag potential between exposure to health/environmental dangers and their consequences [17].
The EU Regulatory Framework of GMOs
The EU has set up a legal framework to disseminate GMOs on markets to ensure high protection levels for human/animal health and the environment. The aim of this regulatory framework is to confer in the authorization process a high degree of transparency. It is based on three basic principles: pre-market authorization based on a prior risk assessment; traceability; labeling [27]. GM foods and feeds in the EU can be approved provided that they successfully pass rigorous safety assessments [28]. The procedures to assess and authorize GM foods and feeds appear in the following documents: (1) Directive 2001/18/EC, which regulates the authorization of deliberate releases and placing GMOs on the market; (2) EC Regulation No. 1829/ 2003, which provides a specific authorization procedure of GM foods and feeds [29][30]. These documents provide rules for GMO safety assessments by regulating GM food and feed production, GMO imports, and the release of GMOs into the environment [18].
A GMO authorization is granted as long as no adverse health or environmental effect is identified. In the EU, the European Food Safety Authority (EFSA) plays the main role in risk assessments [27]. The EFSA’s role is to assess the safety of new GMOs and to provide scientific advice before Europe’s risk managers make decisions (the European Commission and EU Member States). EFSA assessments are made according to applicants’ scientific dossiers and any other relevant scientific data. They bear in mind these aspects: (1) molecular characterization; (2) comparative analysis between the GM plant and its conventional counterpart; (3) evaluation of potential toxicity and allergenicity; (4) evaluation of potential environmental impacts. According to EU legislation, all Member States can decide about cultivating GM crops in their territories. Once a GMO is authorized, it usually receives a 10-year EU market license. Later, it must be re-assessed by the EFSA before any more re-authorization decisions are made [28].
Labeling and traceability rules guarantee operators and consumers access to crucial GMO information [27]. In line with EC Regulation No. 1829/2003 on GM food and feed and No. 1830/2003 on the traceability and labeling of GMOs, products consisting of or containing authorized GMOs or produced from GMOs must be clearly labeled as such. These requirements do not apply to foods containing < 0.9% authorized GM material as long as the GM material is technically or adventitiously unavoidable [27][31]. The EU framework sets a traceability and labeling threshold for approved GMOs, and a “zero tolerance” policy exists for unapproved GMOs. What they mean is that unapproved GMOs in the EU cannot be sold [31]. Several EU Member States have enacted their own national regulations that permit the voluntary labeling of ‘‘GMO-free food or feed” or ‘‘food that originates from animals not fed GM feed” [32].
The EU GMO regulation includes effective enforcement provisions. Those applicants who wish to place GMOs on the EU market must set up and submit an acceptable detection method, positive and negative control samples, and certified reference material. These methods form part of the national authorities’ control and inspection systems to make sure that any GMOs placed on the market have been duly approved and labeled according to legislation [27].
3. Myths and Realities of GM Crops: Risk Assessment
Humans have been altering plant and animal genomes for thousands of years [33]. Since ancient times, selective breeding, also called artificial selection, has been a routine method in agriculture [34]. For instance, humans have driven maize evolution from a plant with many branches and small cobs to a plant with fewer larger stalks and larger kernels, which result from the selective seed selection of plants with highly desirable traits [35]. Something similar has taken place with tomatoes, which are the product of applying careful genetic selection to alter their size, shape, seeds, and taste (Figure 3) [36]. Although the process of creating new traits takes time because it requires spontaneous genetic mutations, the development of GE tools has accelerated the production of GMOs [34].
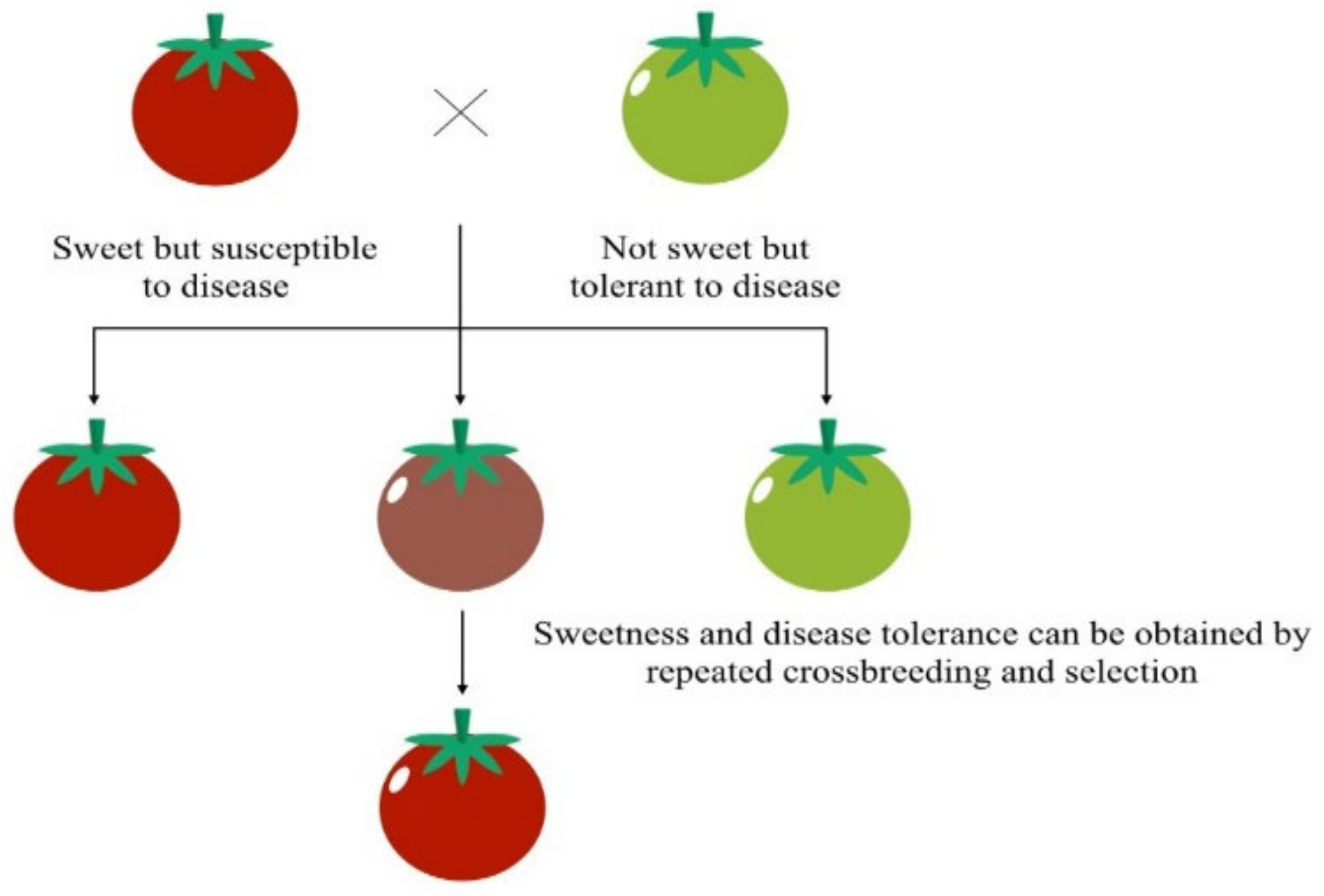 Figure 3. Selective tomato breeding. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [37].
Figure 3. Selective tomato breeding. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [37].
 Figure 3. Selective tomato breeding. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [37].
Figure 3. Selective tomato breeding. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [37].The first transgenic plant was created in 1983: a tobacco plant that resists antibiotics [33]. Nevertheless, the first GM food was not marketed in the USA until 1994: the Flavr SavrTM late-ripening tomato. Although the Flavr SavrTM tomato study was a scientific success, public opinion forged its commercial failure [38]. One reason for its commercial failure was that GM foods inspired moral debate that was closely tied to views on what is considered “unnatural”. Religion is generally associated with opposing GE, and some people believe that it is like “playing God” [34][39]. Nonetheless, there is evidence that gene transfer between species is not as unnatural as researchers think. For instance, Elysia chlorotica is a marine gastropod mollusk that has integrated a gene from an alga into its genome, which confers on it a photosynthetic capability [40].
Anti-GMO activists argue that GM crops occupy much space and are related to intense monoculture systems. Despite monoculture agriculture offering many advantages, it is still a very controversial issue in modern agriculture. Monocropping implies lots of disadvantages, including biological diversity loss. As biodiversity is lacking, crops are more susceptible to pests, and these threats spread more quickly. Consequently, farmers apply more herbicides and pesticides to safeguard crops. These pollutants normally sink into the earth and pollute both soil and groundwater. As monoculture farming exhausts soil by depriving it of biodiversity, farmers will wish to artificially enhance the fertility of their affected fields and apply chemical fertilizers. Artificial nutrients severely impact the natural soil composition and, thus, have a destructive effect on the ecosystem as a whole. Applying herbicides, pesticides, and other chemicals strongly impacts bees and other pollinators (Earth Observing System). Yet monoculture is essential for feeding the world population and has extended nearly 5-fold since 1900. From the agronomic viewpoint, it is the monoculture size (temporal and geographical) that makes it beneficial or harmful. Farmers face the challenge of implementing long-term crop management strategies, crop rotation, and farm management to protect soil health and biodiversity. In relation to GM crops, and based on the history of such practice, they have nothing to do with monocropping’s origins. Farmers have performed monoculture farming worldwide long before GM seeds appeared on the market. Employing GM crops enhances yields and reduces deforestation and other damaging activities carried out to generate farmland. Nowadays, more and diverse fruit and vegetables are available than ever before. GM seed varieties created specially to include genes from other plants and bacteria widened the genetic diversity of large commodity crops in the 1990s [41] (Genetic Literacy Project).
Recently, a survey revealed that consumer GM food views have barely changed since they were introduced in the mid-1990s. Europeans perceive that GMOs are unsafe, and they believe that they can harm not only consumers but also the environment [42]. According to the 2005 Pew Initiative, 50% of Americans are unaware of GM foods and oppose them being introduced into the food system [43]. However, the acceptance of GM foods appears to depend on their development and intended use. For example, modifications made to increase nutritional values are more widely accepted than those made to simply increase crop yields. On the other hand, based on the sort of genetic alteration conducted, “cisgenic” changes are preferred over “transgenic” modifications [44] (Figure 4). The conclusion is that “the more distant the relation between the organisms, the less accepted the modification seems to be” [45].
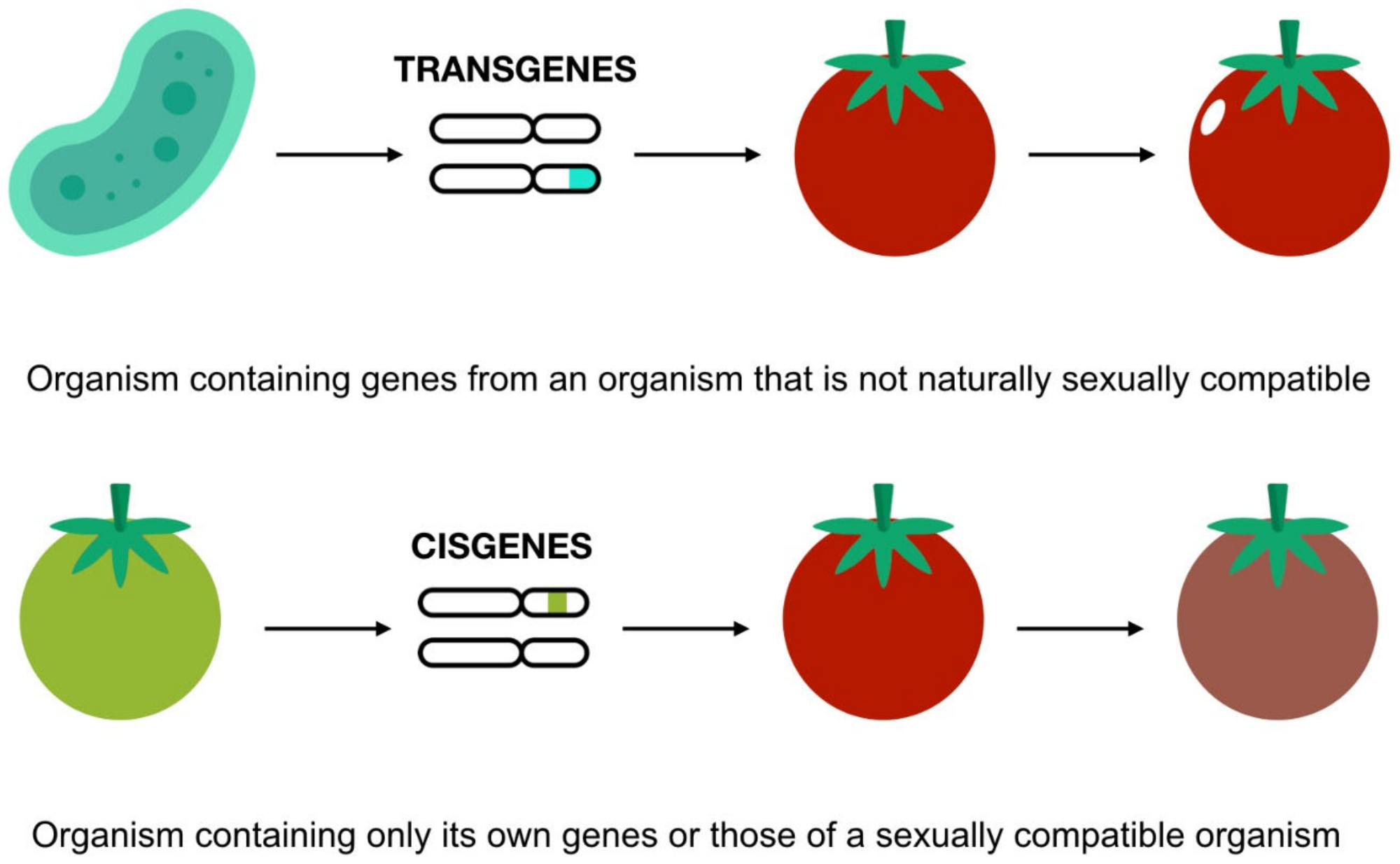 Figure 4. Cisgenic and transgenic modification. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [46].
Figure 4. Cisgenic and transgenic modification. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [46].
 Figure 4. Cisgenic and transgenic modification. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [46].
Figure 4. Cisgenic and transgenic modification. Icons made by Freepik and Smashicons from www.flaticon.com (accessed on 1 December 2021). Adapted from [46].Many prevalent GE myths exist and cover wide-ranging themes, from health and safety to other environmental safety areas. Allergies, toxicity, possible horizontal gene transfer (HGT) to the environment or other species, and several anomalies such as metabolic disturbance, tumor genesis, or infertility have all been related to the consumption of GM crops. Overall, the considerable scientific consensus holds, insofar as currently marketed GM food does not pose a higher risk than traditional food [8][33][47].
References
- Myers, S.; Smith, M.; Guth, S.; Golden, C.; Vaitla, B.; Mueller, N.; Dangour, A.D.; Huybers, P. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu. Rev. Public Health 2017, 38, 259–277.
- Pingali, P. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308.
- Foley, J.; Ramankutty, N.; Brauman, K.; Cassidy, E.; Gerber, J.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342.
- UN News. Over 820 Million People Suffering from Hunger; New UN Report Reveals Stubborn Realities of ‘Immense’ Global Challenge. UN News. 2020. Available online: https://news.un.org/en/story/2019/07/1042411 (accessed on 26 February 2020).
- Black, R.; Victora, C.; Walker, S.; Bhutta, Z.; Christian, P.; de Onis, M.; Ezatti, M.; Grantham-McGregor, S.; Katz, J.; Martorell, J.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451.
- UN News. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. UN News. 2017. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2017.html (accessed on 26 February 2020).
- Bailey-Serres, J.; Parker, J.; Ainsworth, E.; Oldroyd, G.; Schroeder, J. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118.
- Giraldo, P.; Shinozuka, H.; Spangenberg, G.; Cogan, N.; Smith, K. Safety Assessment of Genetically Modified Feed: Is There Any Difference from Food? Front. Plant Sci. 2019, 10, 1592.
- Sharma, R.; Nguyen, T.; Grote, U. Changing Consumption Patterns—Drivers and the Environmental Impact. Sustainability 2018, 10, 4190.
- Hanjra, M.; Qureshi, M. Global water crisis and future food security in an era of climate change. Food Policy 2010, 35, 365–377.
- Barros, J.; Temple, S.; Dixon, R. Development and commercialization of reduced lignin alfalfa. Curr. Opin. Biotechnol. 2019, 56, 48–54.
- Hundleby, P.; Harwood, W. Impacts of the EU GMO regulatory framework for plant genome editing. Food Energy Secur. 2018, 8, e00161.
- Flachowsky, G.; Schafft, H.; Meyer, U. Animal feeding studies for nutritional and safety assessments of feeds from genetically modified plants: A review. J. Verbrauch. Lebensm. 2012, 7, 179–194.
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years. ISAAA Briefs | Brief 53. 2017. Available online: https://www.isaaa.org/resources/publications/briefs/53/download/isaaa-brief-53-2017.pdf (accessed on 15 April 2020).
- Eş, I.; Gavahian, M.; Marti-Quijal, F.; Lorenzo, J.; Mousavi Khaneghah, A.; Tsatsanis, C.; Kampranis, S.; Barba, F.J. The application of the CRISPR-Cas9 genome editing machinery in food and agricultural science: Current status, future perspectives, and associated challenges. Biotechnol. Adv. 2019, 37, 410–421.
- Liu, Q.; Yang, F.; Zhang, J.; Liu, H.; Rahman, S.; Islam, S.; Ma, W.; She, M. Application of CRISPR/Cas9 in Crop Quality Improvement. Int. J. Mol. Sci. 2021, 22, 4206.
- Premanandh, J. Global consensus—Need of the hour for genetically modified organisms (GMO) labeling. J. Commer. Biotechnol. 2010, 17, 37–44.
- Paoletti, C.; Flamm, E.; Yan, W.; Meek, S.; Renckens, S.; Fellous, M.; Kuiper, H. GMO risk assessment around the world: Some examples. Trends Food Sci. Technol. 2008, 19, S70–S78.
- Codex Alimentarius. Biotechnology | CODEXALIMENTARIUS FAO-WHO. 2021. Available online: http://www.fao.org/fao-who-codexalimentarius/thematic-areas/biotechnology/en/ (accessed on 28 January 2022).
- OECD. Safety Evaluation of Foods Derived by Modern Biotechnology, Concepts and Principles. Paris: Organization for Economic Co-Operation and Development. 1993. Available online: https://www.oecd.org/science/biotrack/41036698.pdf (accessed on 28 January 2022).
- Codex Alimentarius. Home | CODEXALIMENTARIUS FAO-WHO. 1963. Available online: http://www.fao.org/fao-who-codexalimentarius/home/en/ (accessed on 28 January 2022).
- Wunderlich, S.; Gatto, K. Consumer Perception of Genetically Modified Organisms and Sources of Information. Adv. Nutr. 2015, 6, 842–851.
- Huffman, W.; McCluskey, J. Food Labels, Information, and Trade in GMOs. J. Agric. Food Ind. Organ. 2017, 15, 1–9.
- Storey, D. Mandatory GMO Labeling: Pros and Cons. 2021. Available online: https://www.tracegains.com/blog/mandatory-gmo-labeling-pros-and-cons (accessed on 28 January 2022).
- The European Parliament. Regulation EC No 258/1997 of the European Parliament and of the council of 27 January 1997 concerning novel foods and novel food ingredients. Off. J. Eur. Communities 1997, L043, 1–7.
- Codex Alimentarius. Report of the Thirty Fifth Session of the Codex Committee on Food Labeling . 2007 . Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FShared%2BDocuments%252FArchive%252FMeetings%252FCCFL%252Fccfl35%252Ffl35_08e.pdf (accessed on 28 January 2022).
- Bruetschy, C. The EU regulatory framework on genetically modified organisms (GMOs). Transgenic Res. 2019, 28, 169–174.
- EFSA. Topics | GMO. 2021. Available online: https://www.efsa.europa.eu/en/topics/topic/gmo#eu-framework (accessed on 9 June 2021).
- The European Parliament. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. Off. J. Eur. Communities 2001, L106, 1–39.
- The European Parliament. Regulation (EC) 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. Off. J. Eur. Communities 2003, L268, 1–23.
- The European Parliament. Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC. Off. J. Eur. Communities 2003, L268, 24.
- The European Parliament. Directive (EU) 2015/1535 of the European Parliament and of the Council of 9 September 2015 laying down a procedure for the provision of information in the field of technical regulations and of rules on Information Society services (codification). Off. J. Eur. Communities 2015, L241, 1–15.
- Yang, Y.; Chen, B. Governing GMOs in the USA: Science, law and public health. J. Sci. Food Agric. 2015, 96, 1851–1855.
- Scott, S.; Inbar, Y.; Wirz, C.; Brossard, D.; Rozin, P. An Overview of Attitudes Toward Genetically Engineered Food. Annu. Rev. Nutr. 2018, 38, 459–479.
- Doebley, J. The Genetics of Maize Evolution. Annu. Rev. Genet. 2004, 38, 37–59.
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094.
- Kato-Nitta, N.; Maeda, T.; Inagaki, Y.; Tachikawa, M. Expert and public perceptions of gene-edited crops: Attitude changes in relation to scientific knowledge. Palgrave Commun. 2019, 5, 137.
- Lee, T.; Ho, H.; Leung, T. Genetically modified foods and allergy. Hong Kong Med. J. 2017, 23, 291–295.
- Rose, K.; Brossard, D.; Scheufele, D. Of Society, Nature, and Health: How Perceptions of Specific Risks and Benefits of Genetically Engineered Foods Shape Public Rejection. Environ. Commun. 2020, 14, 1017–1031.
- Rumpho, M.; Worful, J.; Lee, J.; Kannan, K.; Tyler, M.; Bhattacharya, D.; Moustafa, A.; Manhart, J.R. Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. Proc. Natl. Acad. Sci. USA 2008, 105, 17867–17871.
- Genetic Literacy Project. Do GMOs Encourage Monoculture Cropping and Reduce Biodiversity? 2022 . Available online: https://geneticliteracyproject.org/gmo-faq/do-gmos-encourage-monoculture-cropping-and-reduce-biodiversity/ (accessed on 28 January 2022).
- European Commission. Special Eurobarometer 341/Wave 73.1—TNS Opinion & Social. 2010. Available online: https://ec.europa.eu/commfrontoffice/publicopinion/archives/ebs/ebs_341_en.pdf (accessed on 10 March 2020).
- The Mellman Group. The Pew Initiative on Food and Biotechnology. 2005. Available online: https://www.pewtrusts.org/~/media/legacy/uploadedfiles/%20wwwpewtrustsorg/news/press_releases/food_and_biotechnology/%20pifbpublicsentimentgmfoods2005pdf.pdf (accessed on 28 January 2022).
- Myskja, B. The Moral Difference between Intragenic and Transgenic Modification of Plants. J. Agric. Environ. Ethics 2006, 19, 225–238.
- Ankeny, R.; Bray, H. Genetically Modified Food; Oxford Handbooks Online: Oxford, UK, 2018.
- Schouten, H.J.; Krens, F.A.; Jacobsen, E. Cisgenic plants are similar to traditionally bred plants: International regulations for genetically modified organisms should be altered to exempt cisgenesis. EMBO Rep. 2006, 7, 750–753.
- Touyz, L. Genetically Modified Foods, Cancer, and Diet: Myths and Reality. Curr. Oncol. 2013, 20, 59–61.
More
