Rapid industrialization has led to the pollution of soil and water by various types of contaminants. Heavy metals (HMs) are considered the most reactive toxic contaminants, even at low concentrations, which cause health problems through accumulation in the food chain and water. Remediation using conventional methods, including physical and chemical techniques, is a costly treatment process and generates toxic by-products, which may negatively affect the surrounding environment. Therefore, biosorption has attracted significant research interest in the recent decades. In contrast to existing methods, bacterial biomass offers a potential alternative for recovering toxic/persistent HMs from the environment through different mechanisms for metal ion uptake.
1. Introduction
The rapidly escalating industrial activities release toxic heavy metals (HMs), which pose a serious hazard to ecosystems and human health
[1][2][3]. Environmental HM pollution in soil and water reduces crop production and can be detrimental to health safety through food chains owing to industrial solid waste, and agricultural inputs such as fertilizers and pesticides. These persistent environmental contaminants are non-degradable and can only be transformed into other harmless forms, such as Hg, Cd, As, Cr, Tl, Pb, Mn, and Ni, which cause severe toxic effects in living organisms
[4][5][6][7][8]. Fe, Cu, Co, and Zn are essential HMs that act as coenzymes in biological processes and are less toxic at low concentrations
[9]. Over the last few decades, many conventional treatment methods have been used to remove HMs from polluted environments, including chemical precipitation, ultrafiltration, ion exchange, reverse osmosis, electrowinning, and phytoremediation
[10]. The traditional methods used are described in
Table 1.
Table 1. Conventional methods for heavy metal removal.
| Methods |
Description |
| Chemical precipitation |
The most common method for heavy metal removal from solutions. The ionic metals are converted to insoluble forms by chemical reactions using precipitating reagents (precipitants) and form metal hydroxides, sulfides, carbonates, and phosphates (insoluble solid particles) that can be simply separated by settling or filtration. |
| Electrodialysis (ED) and Electrodialysis Reversal (EDR) |
ED and EDR are considered electro-membrane separation processes as ion-exchange membranes (IEM) that are used to separate different ions present in solution as it permeates owing to electrical potential difference. ED/EDR has been mainly utilized for advanced water deionization, high-efficiency removal of ions in pure and ultrapure water application as well as brackish water desalination. |
| Membrane filtration (MF) |
MF is capable of removing not only suspended solid and organic components but also inorganic contaminants such as metal ions. A membrane is a selective layer used to make contact between two homogenous phases with a porous or non-porous structure for the removal of pollutants. Based on the various sizes of the particle, it is divided into three types as below: |
|
|
- ○
-
Ultrafiltration (UF)
|
|
UF utilizes permeable membrane to separate heavy metals with pore sizes in the range of 0.1–0.001 micron which permeates water and low molecular weight solutes, while retaining the macromolecules, particles, and colloids that are larger in size of 5–20 nm. The removal of Cu (II), Zn (II), Ni (II), and Mn (II) from aqueous solutions is achieved by using ultrafiltration assisted with chitosan-enhanced membrane with a rejection of 95–100% or a copolymer of malic acid and acrylic acid attaining a removal efficiency of 98.8% by forming macromolecular structures with the polymers. |
|
|
- ○
-
Nanofiltration (NF)
|
|
NF is a pressure-driven membrane process that lies between ultrafiltration and reverse osmosis. It is able to reject molecular ionic species by making separation of large molecules possible by small pores when they are within the molecular weight range from 300 to 500 Da with a pore diameter of 0.5–1 nm. A current commercial nanofiltration membrane NF270 is used for removing Cd (II), Mn (II), and Pb (II) with an efficiency of 99, 89, and 74%, respectively. |
|
|
- ○
-
Reverse Osmosis (RO)
|
|
In RO, a pressure-driven membrane process, water can pass through the membrane, while the heavy metal is retained. The removal performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) was investigated for the separation of Cu(II) and Ni(II) ions from both synthetic and real plating wastewater. |
| Microfiltration (MF) |
MF uses the same principle as ultrafiltration. The major difference between the two processes is that the solutes which are removed by MF are larger than those rejected by UF using the pore size of 0.1–10 μm with applied pressure range of 0.1–3 bar. |
| Photocatalysis |
Photocatalysis is based on the reactive properties of electron- hole pairs generated in the semiconductor particles under illumination by light of energy. Metal ions are reduced by capturing the photo-excited conduction band electrons, and water or other organics are oxidized by the balance band holes. Heavy toxic metal ions such as Hg | 2+ | and Ag | + | , and noble metals can be removed from water by photo deposition on Titania surface-trapped photoelectron states, probably Ti(III), and silver deposition could be observed on the same time scale. |
Therefore, the development of remediation treatment methods is essential for mitigating the negative effects on nature. Because of the drawbacks of conventional remediation methods, such as high cost and lack of environmentally friendly solutions, green technologies are growing in the investigation of biosorbents and potential microbial biomass. This is because of their high removal/recovery efficiency, low cost, and safety to restore polluted environments
[11]. However, in such cases, the speed of pollutants released by bacteria is usually low and has a short lifespan because of dead biomass, which limits their feasibility in large-scale applications
[12][13]. As an alternative, numerous studies have confirmed that using enzymes and bio-surfactants produced from microbes is more advantageous than using microbes as a whole to boost remediation efficacy as biocatalysts
[14][15]. Enzymatic degradation requires highly specific and flexible operating conditions. Therefore, exploring enzyme-producing bacterial sources remains attractive. For instance, ChrR and YieF are two soluble enzymes that have been extracted and purified from
Pseudomonas putida MK1 and
Escherichia coli, respectively; these are capable of effectively reducing Cr
6+ to Cr
3+ under both aerobic and anaerobic conditions
[14].
Recently, the use of beneficial microorganisms, such as plant growth-promoting bacteria, which are also capable of reducing HMs, has significantly contributed to agriculture and environmental schemes owing to its outstanding advantages. Numerous reports have demonstrated that several bacteria can adapt to high levels of heavy metal pollution
[16][17][18]. Bacteria use chemical contaminants as an energy source through their metabolic processes; however, excessive amounts of inorganic nutrients pose a risk to their metabolism
[19][20][21]. Bacterial groups that contribute to HM removal include
Bacillus sp.,
Pseudomonas sp.,
Arthrobacter sp.,
Alcaligenes sp.,
Azotobacter sp.,
Rhodococcus sp., and methanogens
[22]. Among them,
Bacillus sp. is considered a potential agent for removing various HMs, especially Gram-positive bacteria
[23]. Oves et al. investigated
Bacillus thuringiensis OSM269 that was tolerant to various concentrations (25–150 mg/L) of HMs, such as Cd, Cr, Cu, Pb, and Ni
[24]. Moreover, because of their diverse enzymatic systems, members of the
Streptomyces genus have recently been evaluated as important producers in the remediation of contaminated environments
[25]. In a previous study, two
Pseudomonas strains were shown to be resistant to As and other HMs such as Ag, Cd, Co, Cr, Cu, Hg, Ni, and Pb
[26]. The biosorption of Al
3+ and Cd
2+ by an extra cellular polymeric substance (EPS) from
Lactobacillus rhamnosus was determined in a previous study
[27]. In another study, one member of the genus
Cellulosimicrobium was found to be a potential bacterium that can protect against six HMs, including Pb, Fe, Cd, Ni, Cu, and Co
[28]. Recently, an exopolysaccharide produced by
Lactiplantibacillus plantarum BGAN8 strain was discovered to have a high Cd-binding capacity and prevent cadmium-induced toxicity
[29].
2. Microbial Remediation: The Mechanisms of Biosorption and Bioaccumulation Using Bacterial Biomass as a Tool in Polluted Environmental Cleanup
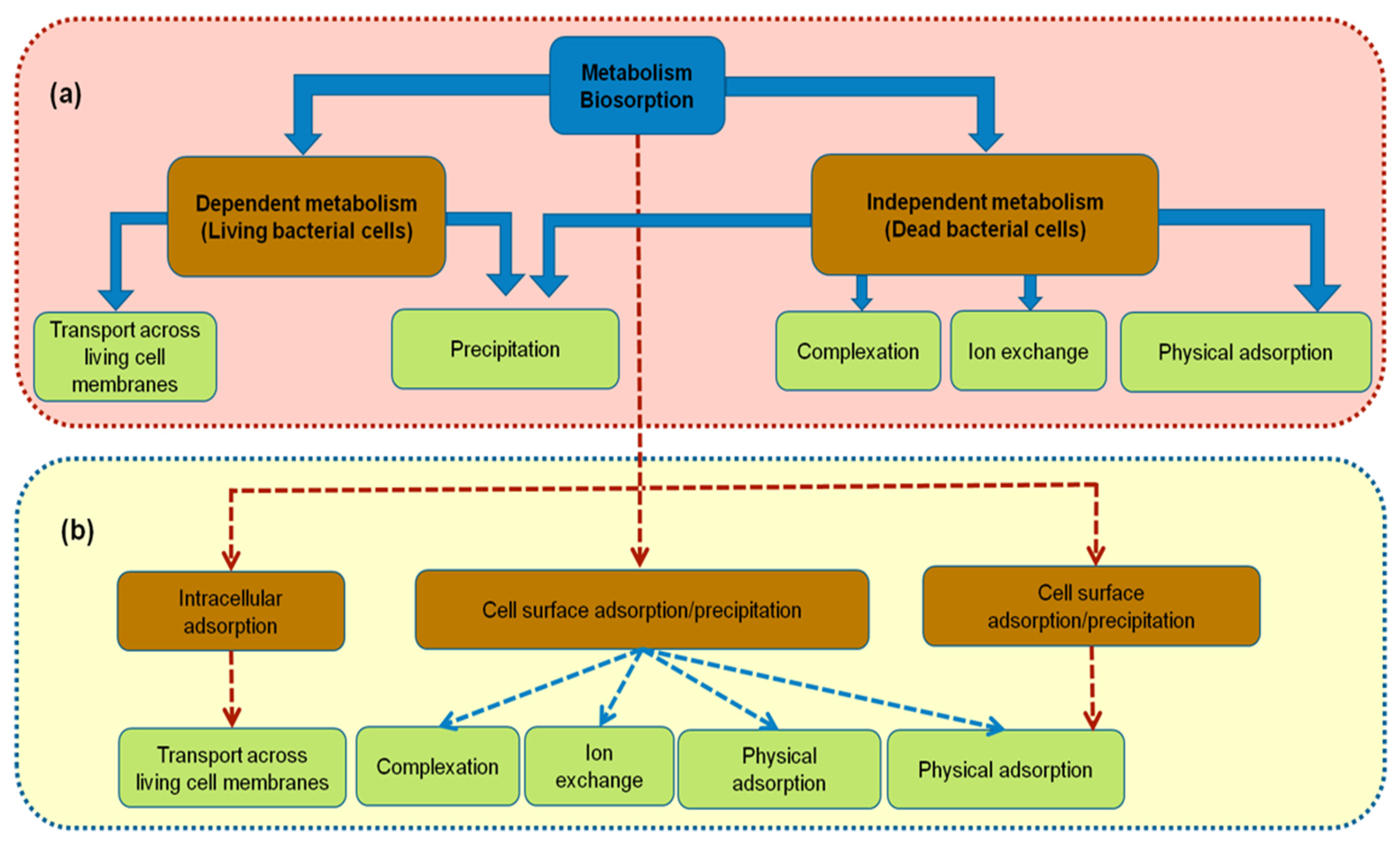
The metabolic diversity and activity of microorganism have provided tremendous potential in the field of waste treatment via cell owners of various metabolic pathways (
Figure 1). Toxic compounds have been used as energy sources for cellular processes through fermentation, respiration, and co-metabolism
[30].
Figure 1. Mechanisms of bacterial biosorbent: (a) on cell surface; (b) within the cell where the HMs are removed.
Owing to the various considerations of each group, as well as under certain experimental conditions, various microbial biomasses have different bioremediation abilities. HMs may disrupt microbial cell membranes, but bacteria possess characteristic enzymatic profiles that are required to overcome toxic effects. The bioremediation process takes place through various mechanisms, including:
-
Alkylation and redox processes in which HMs were transformed. The speciation and mobility of metal (loids) may be different from the initial state. For example, metals generally are less soluble in their oxidation state, whereas the solubility and mobility of metalloids depend on both the oxidation state and the ionic form
[31].
-
Passive adsorption is metabolism-independent, in which metals are on the cell surface via electrostatic attraction with functional groups. This mechanism was explained by the different processes including the precipitation and the surface complexation, ion exchange as only dominating role, or physical adsorption. As protons were addressed by the completion between pH and metal cations on the binding sites, thus, pH is the most strongly effective factor that influences the biosorption process
[32]. The other essential factors include temperature, ionic strength, the concentration and type of the sorbate and biosorbent, the state of biomass: suspension or immobilized and the presence of other anion and cations in the growth medium. Most applications focus on the utilization of dead biomass because the toxicity of bacteria is avoided, no requirement for maintenance, and the storage of biomass is easy and can be kept for long period without loss of effectiveness. Numerous bacterial strains were reported in HMs biosorption that are dominant in
Bacillus, Pseudomonas, Streptomyces [30][33][34].
| 400 |
| >90 |
| [ |
| 63 |
| ] |
| Arthrobacter viscosus |
| Pb |
| 100 |
97 |
[ | 64 | ] |
| Arthrobacter sp. 25 |
Pb |
95.04 |
86.25 |
[65] |
| Pseudomonas sp. I3 |
Pb |
49.48 |
98.96 |
[66] |
| Bacillus badius AK |
Pb |
60 |
60 |
[67] |
| Klebsiellap enumoniae |
Cd |
40.18 |
40.18 |
[68] |
| Rhodotorula sp. |
Cd |
40 |
80 |
[69] |
| Bacillus megaterium sp. |
Cd |
39.5 |
79 |
[69] |
| Bacillus sp. Q3 |
Cd |
108.2 |
93.76 |
[70] |
| Pseudomonas aeruginosa FZ-2 |
Hg |
10 |
99.7 |
[71] |
| Vibrio parahaemolyticus PG02 |
Hg |
5 |
90 |
[72] |
| Pseudomonas aeruginosa |
Cd, Pb |
62.8 (Cd); 73.1 (Pb) |
87 (Cd); 98.5 (Pb) |
[73 |
-
Active adsorption is the metabolism-dependent intracellular accumulation of toxicants in living cells within cytoplasm. HMs were converted to non-bioavailable form by binding with metallothioneins (MTs) as low-molecular mass cystein-rich proteins, and metallo-chaperones. By being bound with HMs, these intracellular proteins can also lower the free ion concentrations within cytoplasm in which the detoxification of metals occurred
[35]. This process is sensitive to environmental conditions depending on each type of bacterial strain such as pH, temperature, salinity. Moreover, it also depends on the biochemical structure, physiological/genetic adaptation, and the toxicity of metal.
CyanobacteriaCyanobacteria, pseudomonads,
pseudomonadsand mycobacteria have been found as the candidates that can synthesize MTs. MTs are usually associated with Zn, Cu, and other toxic metals such as Cd, Hg, and
mycobacteria have been found as the candidates that can synthesize MTsPb [36].
MTs are usually associated with Zn, Cu, and other toxic metals such as Cd, Hg, and Pb [36]. Pseudomonas aeruginosaPseudomonas aeruginosa and Pseudomonas putida were reported as MTs producing bacteria exposed to Ca and Cu contamination [37].
-
The metal ions uptake is carried out by a complex mechanism of releasing EPS, such as proteins, DNA, RNA, and polysaccharides the slippery layer on the outside of the cell wall. These have a crucial role of stopping the penetration of metals into the intracellular environment in which, ion exchange may occur. Numerous bacterial strains were investigated for the commercial production of EPS such as
Stenotrophomonas maltophilia,Stenotrophomonas maltophilia, Azotobacter chroococcum, Bacillus cereus KMS3-1 Azotobacter chroococcum, Bacillus cereus KMS3-1 [38][39][3840]. Bioremediation efficiency by this mechanism relies on the type and amount of carbon source available and other abiotic stress factors like pH, temperature, and the growth phase of each bacterium [3941][40]. Bioremediation efficiency by this mechanism relies on the type and amount of carbon source available and other abiotic stress factors like pH, temperature, and the growth phase of each bacterium [41]..
Biosorption Process
Although bioaccumulation and biosorption are used synonymously and naturally, they differ in the ways they sequester contaminants. Volesky defined biosorption as the adsorption of substances from solution by biological materials using physiochemical pathways of uptake, such as electrostatic forces and ion/proton displacement
[42]. This is based on ionic interactions between the extracellular surface of the dead biomass, living cells, and metal ions. Thus, the amount of contaminants binds to the surface of the cellular structure rather than oxidation through aerobic or anaerobic metabolism. Biosorption has been shown to effectively remove a variety of HMs from aqueous solutions, including highly toxic metal ions, such as Cd, Cr, Pb, Hg, and As
[43][44]. The functional groups in the cell walls of bacteria are responsible for binding tasks, including carboxyl, phosphonate, amine, and hydroxyl groups
[45]. Therefore, the success of biosorption relies on the diversity of cell wall structures. Gram-positive bacteria have been shown to contain a high sorption capacity because of their thicker peptidoglycan layer
[46][47]. Recently, some studies have investigated engineered microorganisms in combination with metal-binding proteins and peptides on the extracellular surface to improve the capacity and specificity of microbial sorbents
[20][48]. Biosorption can remove contaminants using microorganisms (live/dead), agriculture, and other industrial byproducts as biosorbents, providing a fundamental background for sustainable biosorption technology for metal removal and recovery
[49][50]. The effect of several factors such as pH, temperature, shaking speed, initial concentration of pollutants or amount of biosorbent is evaluated to optimize the biosorption efficiency. The binding mechanism depends on the chemical nature of each contaminant, size of the biomass, interaction between different metallic ions, and ionic strength
[51]. Additionally, biosorption is attractive owing to a number of advantages, including the simple requirement for operation, no additional nutrients, operating cost-effectiveness owing to its reversible process, no increase in the chemical oxygen demand (COD), desorption ease, and high adsorption rate. However, it is necessary to consider other factors such as the possible toxicity of the pollutants to bacterial cell in case of using living cell in this process.
Table 2 reports the potential bacterial candidates that are capable of HMs removal by biosorption process.
Table 2. The promising bacterial strains that can remove HMs via biosorption process.
| Bacterial Biosorbents |
Target Metals |
Amount of Heavy Metals Uptake (mg/L) |
Biosorption
Efficiency (%) |
Reference |
| Pseudomonas alcaliphila NEWG-2 |
Cr |
200 |
96.6 |
[52] |
| Pseudomonas sp. strain DC-B3 |
Cr |
55.35 |
41 |
[53] |
| Pseudomonas aeruginosa G12 |
Cr |
10 |
93 |
[54] |
| Cellulosimicrobium funkei AR6 |
Cr |
164.66 |
82.33 |
[55] |
| Stenotrophomonas maltophilia |
Cr |
19.84 |
99.2 |
[56] |
| Acinetobacter sp. WB-1 |
Cr |
6.82 |
68.17 |
[57] |
| Cellulosimicrobium sp. |
Cr |
96.98 |
96.98 |
[58] |
| Stenotrophomonas sp. |
Cr |
270 |
90 |
[59] |
| Cellulosimicrobium sp. |
Pb |
200 |
84.62 |
[58] |
| Methylobacterium sp. |
Pb |
300 |
62.28 |
[60][61] |
| Aeribacillus pallidus MRP280 |
Pb |
86.47 |
96.78 |
[62] |
| Bacillus sp. PZ-1 |
Pb |
| ] |
| Saccharomyces cerevisiae |
| Pb, Cd |
| 0.045 (Pb); 0.47 (Cd) |
| 70.3 (Pb); 76.2 (Cd) |
| [ | 74 | ] |
| Desulfovibrio desulfuricans (immobilize on zeolite) |
Zn |
174 |
100 |
[75] |
| Micrococcus luteus DE2008 |
Pb, Cu |
20.4 (Cu); 98.25 (Pb) |
25.42 (Cu); 36.07 (Pb) |
[76] |
| Bacillus sp. |
Pb, Cu, Cd |
990 (Cd); 970 (Cu); 200 (Pb) |
>90 (Cd, Cu); 20 (Cd) |
[77] |
| Oceanbacillus profundus |
Pb, Zn |
45 (Pb); 1.08 (Zn) |
97 (Pb); 54 (Zn) |
[78] |
| Staphylococcus epidermidis |
Cr, Zn |
118 (Zn); 112 (Cr) |
59 (Zn), 56 (Cr) |
[79] |
| Streptomyces sp. |
Pb, Cd, Cu |
1.43 (Cu); 0.91 (Pb) 3.66 (Cd) |
Pb (83.4); Cu (74.5); Cd (68.4) |
[80][81] |
| Klebsiella sp. USL2S |
Hg, Pb, Cd, Ni |
8500 (Hg); 10,000 (Pb); 1026 (Cd); 8479 (Ni) |
85 (Hg); 97.13 (Pb); 73.33 (Cd) 86.06 (Ni) |
[82] |
| Pseudomonas azotoformans JAW1 |
Cd, Cu, Pb |
24.64 (Cd); 17.44 (Cu); 19.55 (Pb) |
98.57 (Cd); 69.76 (Cu); 78.23 (Pb) |
[83] |