Current medical treatment for inflammatory bowel disease (IBD) does not achieve 100% response rates, and a subset of refractory and severely ill patients have persistent active disease after being treated with all possible drug alternatives. Ustekinumab plus vedolizumab and vedolizumab plus anti-TNF were the most used biological therapies (CoT) for Crohn’s disease. For ulcerative colitis, the most used CoTs were vedolizumab plus anti-TNF and vedolizumab plus tofacitinib.
1. Introduction
Currently, there is a reasonable number of useful therapies for IBD. The treatment armamentarium includes small molecules and biological treatments. Small molecules include classical drugs such as mesalazine, corticosteroids, and immunosuppressant treatments; this latter group includes thiopurines, methotrexate for Crohn’s disease (CD), and tofacitinib for ulcerative colitis (UC). Regarding biological treatments, anti-TNF drugs, vedolizumab, and ustekinumab are currently widely used
.
These drugs are mostly used sequentially. Thus, if a drug is ineffective in controlling IBD symptoms, that drug is withdrawn and replaced by another (for example, in patients who have active disease despite receiving an anti-TNF drug, treatment with the anti-TNF drug would be stopped and the patient would begin treatment with an alternative biological drug). The only exception is the combination of an anti-TNF plus azathioprine, which has been widely used in clinical practice since the 2010 SONIC trial showed that this combination achieved higher remission and mucosal healing rates than monotherapy without a clear increase in adverse events
.
Used individually, IBD therapies reach a maximum clinical remission rate of approximately 40–60%
. Therefore, current medical treatment for IBD does not achieve 100% response rates, and a subset of refractory and severely ill patients have persistent active disease after being treated with all the possible drug alternatives. These patients often require aggressive rescue therapies such as major surgery or bone marrow auto-transplant in CD, or proctocolectomy in UC
.
As these rescue treatments have significant risks and may have a negative impact on quality of life, the combination of two biological therapies (CoT) seems a reasonable alternative. In fact, CoT has been increasingly tested in very difficult cases and in two clearly different settings: in patients with uncontrolled IBD, and in patients with controlled IBD but extraintestinal manifestations that did not respond to a single biological therapy
. The safety and efficacy of CoT have mostly been reported as case reports or short series
.
2. Global Efficacy and Safety of CoT
Ribaldone et al. reported seven studies (18 patients) with a combination of TNF inhibitors and vedolizumab as well as vedolizumab and ustekinumab. Clinical improvement was seen in all patients, and endoscopic improvement was reported in 93% of patients. No safety concerns were identified
.
In 2021, Gold et al. reported a review pooling data from 209 CoTs. They included retrospective studies, case reports, and case series. This review suggested that dual biologic therapy may be effective at inducing remission in patients with refractory luminal symptoms and/or extraintestinal manifestations. They reported an efficacy ranging from 67% to 80%. No severe adverse events were described
3. Usefulness and Safety of Biologic Combinations
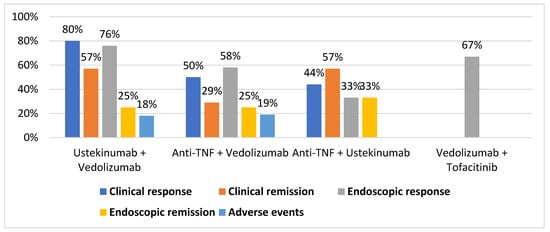
shows the pooled rates of clinical response, clinical remission endoscopic response, endoscopic remission, and adverse event rates for the most used CoTs.
Percentage of clinical response, clinical remission, endoscopic response, endoscopic remission, and adverse events for each combination therapy.
Ustekinumab plus vedolizumab
reported the results of eight patients who received treatment with the combination of ustekinumab and vedolizumab. During follow-up at week 40, five of seven (71%) patients achieved clinical response, four of seven (57%) achieved clinical remission, five of eight (63%) achieved endoscopic improvement, and two of eight (25%) achieved endoscopic remission. The adverse event rate was low—one of eight (13%) patients.
Anti-TNF plus vedolizumab
reported results in six patients. Three of six (50%) patients had a clinical response and three of six had clinical remission (50%) at 6 month follow-up. Only one adverse event was reported in one patient (16.6%), who presented a cutaneous rash.
reported results in four patients on anti-TNF plus ustekinumab; one patient had a clinical response and three had clinical remission at 6 month follow-up.
Glassner et al.
reported eight patients on vedolizumab plus tofacitinib, nine patients on anti-TNF plus tofacitinib, and three patients on tofacitinib plus ustekinumab. However, individual results were not available.
shows the rates of clinical response, clinical remission, endoscopic response, endoscopic remission, and adverse event rates for each CoT.
Results for clinical response, clinical remission, endoscopic response, endoscopic remission, and adverse events for each combination.
| Ustekinumab + Vedolizumab |
| Yang et al. |
5 of 7 |
4 of 7 |
5 of 8 |
2 of 8 |
1 of 8 |
| Kwapisz et al. |
4 of 5 |
|
|
|
0 of 5 |
| Privitera et al. |
3 of 3 |
|
|
|
1 of 3 |
| Glassner et al. |
|
|
11 of 13 |
|
|
| TOTAL |
12 of 15 (80%) |
4 of 7 (57%) |
16 of 21 (76%) |
2 of 8 (25%) |
2 of 11 (18%) |
| Anti-TNF + Vedolizumab |
Yang et al. |
5 of 12 |
4 of 12 |
4 of 12 |
3 of 12 |
2 of 12 |
| Kwapisz et al. |
5 of 8 |
|
|
|
3 of 8 |
| Privitera et al. |
3 of 6 |
3 of 6 |
|
|
1 of 6 |
| Glassner et al. |
|
|
24 of 36 |
|
|
| TOTAL |
13 of 26 (50%) |
7 of 18 (29%) |
28 of 48 (58%) |
3 of 12 (25%) |
5 of 26 (19%) |
| Anti-TNF + Ustekinumab |
Yang et al. |
1 of 3 |
1 of 3 |
1 of 3 |
1 of 3 |
|
| Kwapisz et al. |
2 of 2 |
|
|
|
|
| Privitera et al. |
1 of 4 |
3 of 4 |
|
|
|
| TOTAL |
4 of 9 (44%) |
4 of 7 (57%) |
1 of 3 (33%) |
1 of 3 (33%) |
|
| Secukinumab + Vedolizumab |
Privitera et al. |
2 of 2 |
|
|
|
|
| TOTAL |
2 of 2 (100%) |
|
|
|
|
| Vedolizumab + Apremilast |
Privitera et al. |
1 of 1 |
|
|
|
1 of 1 |
| TOTAL |
1 of 1 (100%) |
|
|
|
1 of 1 (100%) |
| Vedolizumab + Tofacitinib |
Glassner et al. |
|
|
8 of 12 |
|
|
| TOTAL |
|
|
8 of 12 (67%) |
|
The most-used CoTs are shown in Table 2. Of them, vedolizumab plus ustekinumab and vedolizumab plus anti-TNF were the most effective CoTs for CD. Furthermore, vedolizumab plus anti-TNF and vedolizumab plus tofacitinib were the most effective CoTs for UC. The combination of ustekinumab and vedolizumab seems especially attractive because it might combine efficacy, safety, and persistence over time. Very recently, Stone et al. reported similarly good results in a retrospective series of 10 patients [12]. Data are preliminary and, in patients with UC and uncontrolled extraintestinal manifestations, CoT including anti-TNF or tofacitinib might be more effective.
VEDO (vedolizumab), USTE (ustekinumab), TOFA (tofacitinib). * CoTs used for extraintestinal manifestations were excluded. ** Other molecules used: apremilast, cyclosporine, rituximab, secukinumab, leflunomide, and tacrolimus.
CoT has also been explored in other clinical settings, such as the treatment of psoriasis with associated joint manifestations. In these patients, treatment was effective and there was no increase in adverse events . Otherwise, the combinations used for the treatment of rheumatoid arthritis demonstrated good efficacy but an increase in the rate of adverse events
. In IBD, CoT has even been used in pediatric patients with good results and safety
.
In conclusion, IBD treatment is still rapidly evolving. Along with the new therapies that are rapidly becoming available, CoT has demonstrated promising results and may represent a new opportunity to improve both patients’ quality of life and long-term prognosis. However, current data are very limited, and larger studies with longer follow-up are desirable to confirm the safety and efficacy of CoT. In the meantime, CoT seems a real alternative for refractory and severely ill patients who cannot wait for new developments to come.